Abstract
As antibody-based diagnosis and therapy grow at an increased pace, there is a need for methods which rapidly and accurately determine antibody-antigen interactions. Here, we report a method for the multiplex determination of antibody epitopes using bacterial cell-surface display. A protein-fragment library with 107 cell clones, covering 60 clinically-relevant protein targets, was created and characterized with massively parallel sequencing. Using this multi-target fragment library we determined simultaneously epitopes of commercial monoclonal and polyclonal antibodies targeting PSMA, EGFR, and VEGF. Off-target binding was observed for one of the antibodies, which demonstrates the method´s ability to reveal cross-reactivity. We exemplify the detection of structural epitopes by mapping the therapeutic antibody Avastin. Based on our findings we suggest this method to be suitable for mapping linear and structural epitopes of monoclonal and polyclonal antibodies in a multiplex fashion and could find applicability in serum profiling as well as other protein-protein interaction studies.
Antibodies are invaluable tools both as research reagents and for medical purposes, in diagnostics and treatment of disease. Approximately 300 antibody-based therapeutics are currently in clinical trials1 and antibodies have been generated towards more than half of the human proteome for cataloging protein expression in tissues and organs in the Human Protein Atlas project2. The key feature defining an antibody's utility is its unique ability to selectively recognize its epitope on the target protein.
There are several methods for determining antibody epitopes. The most comprehensive is structure determination of the binding complex using X-ray crystallography3,4 or NMR spectroscopy5,6. Although extremely informative when successful, particularly for conformational epitopes, these methods are laborious and may not be suitable for polyclonal antibodies. The most common epitope mapping approach is the generation of consecutive, overlapping synthetic peptides which cover the complete primary sequence of the protein antigen7. Screening for antibody binding is typically done in ELISA wells, on cellulose membranes8, on glass arrays slides9, or with Luminex suspension bead arrays10. While peptide arrays accelerate the epitope mapping process by encompassing many antigens and provide high-resolution epitopes, they are limited by relatively short peptide lengths (usually <15 aa), which may preclude secondary structure formation and thus limit the use of peptide arrays to the mapping of linear epitopes.
Mapping of epitopes using cell-surface display provides an advantage over peptide array-based epitope mapping platforms by presenting large antigen fragments, which can potentially fold on the cell surface. Several display systems have been described, most notably systems based on bacteriophage11, E. coli12, S. cerevisiae13 and Staphylococcus carnosus14. Recently, the entire human proteome was displayed on the surface of bacteriophage as short peptides11. Human serum was assayed for antibody reactivity and phages selected via immunoprecipitation. The three latter microbial systems have the advantage of compatibility with flow cytometry and sorting. E.coli exhibits high transformation frequencies, but secretion through the double membrane is suboptimal. The eukaryotic yeast host can display large and complex antigens15 but may impart undesired glycosylation. The Gram-positive Staphylococcus exhibits lower transformation frequencies than E. coli and yeast, but possesses an efficient secretion and cell-wall insertion mechanism based on the staphylococcal protein A16. The staphylococcal display system also allows for expression normalization during flow sorting from albumin-binding protein (ABP), an albumin-binding region of streptococcal protein G17. This normalization tag minimizes surface-expression bias during epitope mapping as it allows for detection and enrichment of cells which display only small amounts of antigen on the surface.
Here, we have used the staphylococcal display system to construct a multi-target fragment library (MTF library) for epitope mapping. The library is comprised of 60 antigens and includes the majority of the human protein targets with antibody therapeutics either on the market or in Phase 3 clinical trials. In this way, the main bottleneck of cell-surface display for epitope mapping is avoided, namely the time-consuming construction of individual antigen libraries. The MTF library was used to determine the epitopes of monoclonal and polyclonal antibodies simultaneously. The use of this new multiplex method for detection of structural epitopes and potential cross-reactivity is discussed. The platform has great flexibility with regards to antigen size, number of antigens, and detection of linear or conformational binding modes. The platform can be useful in studies relating antibody therapeutic efficacy with antigen affinity, as well as to elucidate antibody-antigen structure-function relationships and other protein-protein interactions.
Results
Construction and characterization of a multi-target fragment library
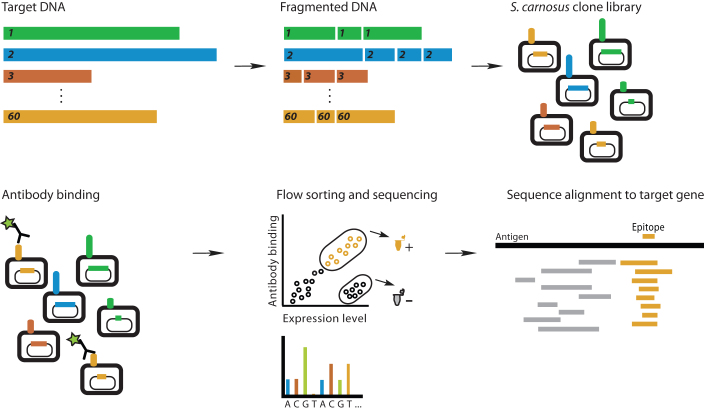
We chose 60 disease-related human proteins for incorporation into the multi-target fragment (MTF) library (Table 1). The library thus comprises potential therapeutic targets that belong to several structural families and exhibit a wide range of function. Several members are targets of approved therapeutic antibodies18. For membrane-associated proteins, we incorporated the ectodomains (ECDs), as these are relevant for antibody binding assays in therapeutic applications. Coding DNA for each target was amplified by PCR (total library size 65 kbp), pooled, fragmented by sonication, and subcloned into a S. carnosus surface-display vector. Transformation into S. carnosus yielded a library with approximately 107 members, of which 6% (6*105) contained in-frame gene fragments and displayed protein fragment on the cell surface (data not shown). The average fragment length was adjusted with sonication time to be 150 bp. During epitope mapping, fragments ranging from 30 bp to 400 bp were enriched. This diversity of size within a single fragment library allows for detection of both small and large epitopes and highlights the flexibility of the platform. The overall layout of the method is outlined in Figure 1.
Table 1. Antigens in the S. carnosus multi-target fragment (MTF) library. The library has 6*105 expressing members and comprises 60 target antigens (65 kbp coding DNA). For membrane-bound antigens, only the ectodomains were included. Details can be found in SI.
| Integrins | Angiogenesis | Interleukins | TNF | Other B Cell Antigens | Other T Cell Antigens | Enzymes | Other | ||
|---|---|---|---|---|---|---|---|---|---|
| αIIβ | EGFR | HGF | IL-1 | IL-15 | TNFα | CD19 | CLTA4 | PSMA | AMOT |
| α4 | HER-2 | HGFR | IL-2 | IL-17 | TRAIL | CD20 | CD2 | GAD65 | APPI |
| αL | HER-3 | DLL4 | IL-5 | IFNα | RANKL | CD33 | CD4 | CA9 | ADD3 |
| β3 | HER-4 | MSTN | IL-9 | CSF2Rα | BLyS | CD80 | CD3γ | HSP90 | PSCA |
| VEGF | ANGPT2 | IL-12α | CSF2Rβ | DR-5 | CD86 | CD3δ | MYO1A | ||
| VEGFR-1 | TIE-1 | IL-12β | IL-2Rα | TNFRSF9 | ICOSLIG | CD3ε | |||
| VEGFR-2 | IL-13 | IL-5Rα | TREM1 | CD3ζ | |||||
| CD52 | |||||||||
Figure 1. Multiplex epitope mapping using cell-surface display of a multi-target fragment library.
A library of 60 antigen genes is pooled, fragmented by sonication, and ligated into a bacterial surface display vector. This multi-target fragment (MTF) library contains approximately 6*105 functionally expressing members. The MTF library is incubated with a mixture of antibodies and positive cells are sorted for DNA sequencing. Inserts are sequenced and aligned to reference genes to determine the antibody epitope.
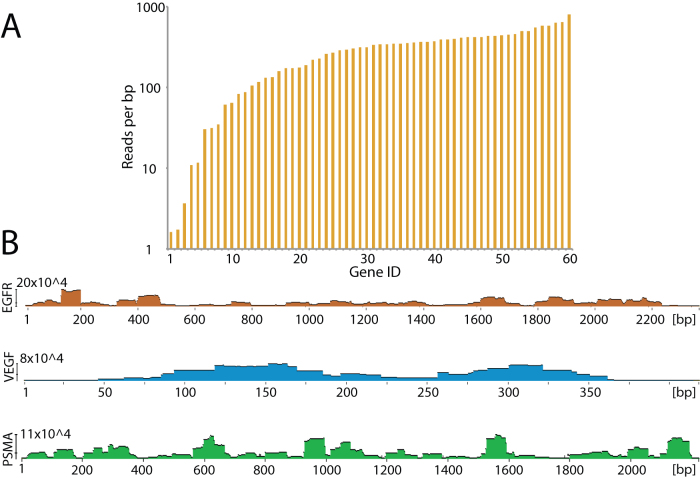
To ensure representation of all target proteins, we characterized the MTF library through massively parallel sequencing. We sorted approximately 106 clones expressing functional in-frame gene fragments (based on expression of the normalization tag ABP on the cell surface) directly into liquid media and sequenced these using massively parallel sequencing on the Illumina Hiseq platform. Figure 2a summarizes the coverage of each antigen in the library. Approximately 95% percent of library members were represented with at least 10 reads per base pair, and 75% were represented with at least 100 reads per base pair. Figure 2b shows in-detail sequence alignments for three antigens, prostate-specific membrane antigen (PSMA), epidermal growth factor receptor (EGFR), and vascular endothelial growth factor A (VEGF). The ruggedness of such alignment plots may indicate susceptible breakage spots within the gene, leading uneven distribution of gene fragments. This is particularly pronounced within the PSMA antigen. However, the sharp discontinuities are not enriched or deficient in GC content (data not shown). It is also possible that certain passenger fragments were weakly expressed on the cell surface and are thus underrepresented in the sequenced library. However, all antigens were represented by multiple overlapping gene -fragments, ensuring complete coverage of every antigen.
Figure 2. Characterization of the multi-target fragment library through deep sequencing of 106 library members.
(A) Sequencing coverage of the 60 target genes (Gene ID 1–60). Approximately 95% of the target genes were detected at over 10 reads per base pair. (B) Detailed coverage for three antigens shows distinct enrichment patterns.
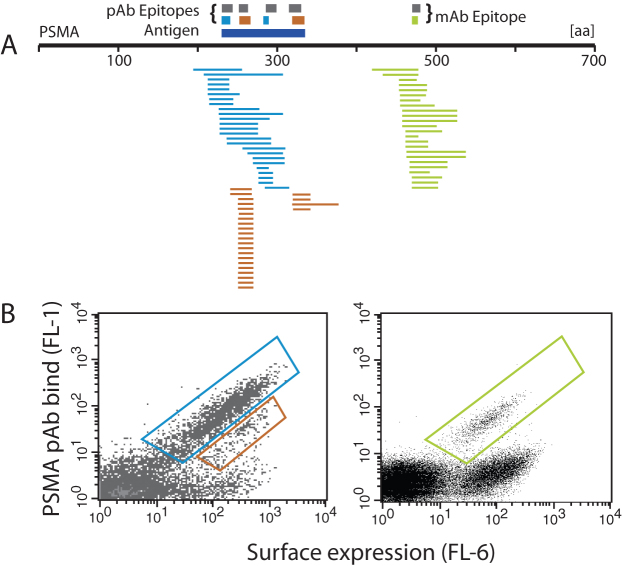
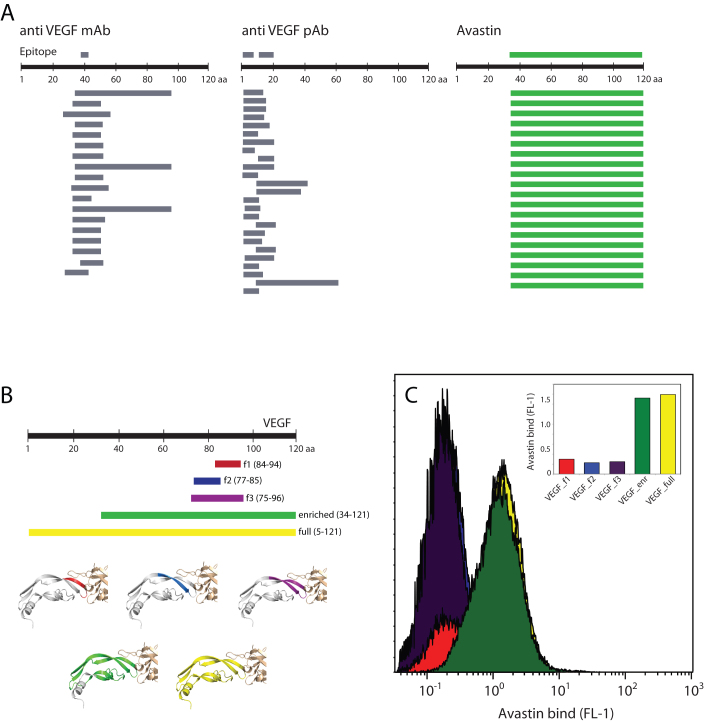
We next tested the utility of the MTF library for FACS-based epitope mapping of monoclonal and polyclonal antibodies. We chose antibodies directed towards PSMA, EGFR, and VEGF. To validate the MTF library we also determined the epitopes of each antibody using target-specific fragment libraries (2*105 expressing members each). After two rounds of FACS, we picked approximately 100 colonies of enriched cells for DNA sequencing. DNA sequences of enriched clones were aligned to the reference antigen sequence and epitopes were identified based on regions of sequence overlap (for details, see Supplemental Information Figure S1). Figure 3 shows the mapping results for monoclonal and polyclonal anti-PSMA antibodies using the MTF library. Gene fragments from sorted cells are aligned to the PSMA reference sequence. Epitopes determined from these MTF library alignments are shown as colored boxes and the epitopes determined using the PSMA-specific fragment library are shown for comparison in grey boxes. Epitope resolution is dependent on the size of surface-displayed protein fragments, antigen coverage of the fragment library, and the number of enriched clones that are sequenced. In the case of polyclonal antibodies (such as Figure 3A), multiple epitopes may exist on the same fragment, and dissection of these epitopes requires overlapping shorter fragments enriched containing only one epitope and not the other.
Figure 3. Epitope mapping of polyclonal and monoclonal antibodies using the multi-target fragment library.
(A) Consensus epitopes for the pAb and mAb determined using the MTF agree with those determined using PSMA-dedicated fragment libraries (gray bars). The anti-PSMA pAb showed four epitopes from two gates (blue and red bars) while the anti-PSMA mAb showed one (green bar). The dark blue bar under the pAb epitopes is the antigen used in immunization. (B) Second-round sorting with gates shown. Left: HPA anti-PSMA pAb, Right: anti-PSMA mAb.
In the case of the PSMA mAb, both fragment libraries gave a single epitope in the region specified as immunogen by the manufacturer. Surprisingly, the MTF library resulted in a shorter epitope (6 residues) than the PSMA fragment library (9 residues). In the PSMA pAb assay, clones were sorted from two gates, indicating epitopes of high and low apparent affinity. The PSMA fragment library gave four pAb epitopes, spanning the immunogen. All four of these epitopes were detected with the MTF library, although with lower resolution (i.e. larger epitopes). From this data it is clear that high-resolution epitope mapping of polyclonal antibodies requires sequencing of more enriched clones.
Epitopes determined for other antibodies are summarized in Table 2 and in detail in Supplemental Information Table S1 and Figures S1–S3. For all antibodies there was a clear concordance in the position of epitopes between the target-specific and MTF libraries, though in general the MTF library gave slightly larger epitopes. In the case of EGFR pAb, the MTF library did not detect an N-terminal epitope, which was detected by the EGFR-specific fragment library. We attribute this to an insufficient number of picked clones in post-sorting sequencing. These results show that the multi-target fragment library can be used for epitope mapping of monoclonal and polyclonal antibodies in a manner similar to target-specific fragment libraries, with multiple epitopes found from sequencing a small number of sorted cells14,10.
Table 2. Summary of MTF and target-specific fragment epitope mappings for three pAbs and two mAbs. In most cases the MTF shows the same epitope as target-specific fragment libraries. N-terminal epitopes for EGFR and PSMA were not detected multiplex MTF. In general, epitopes determined with MTF are larger than those determined with dedicated fragment libraries.
| Antibody | Target Library | Epitope 1 | Epitope 2 | Epitope 3 | Epitope 4 |
|---|---|---|---|---|---|
| FOLH mAb | FOLH fragment | 473-491 | |||
| MTF | 474-489 | ||||
| VEGF mAb | VEGF fragment | 45-50 | |||
| MTF | 42-70 | ||||
| FOLH pAb | FOLH fragment | 231-245 | 253-264 | 287-301 | 317-331 |
| MTF | 231-242 | 254-268 | 284-291 | 321-336 | |
| MTF (multiplex) | N.D. | 256-265 | 289-298 | 314-328 | |
| EGFR pAb | EGFR fragment | 344-352 | 381-398 | 425-442 | |
| MTF | N.D. | 381-393 | 404-446 | ||
| MTF (multiplex) | 346-366 | 372-386 | N.D. | ||
| VEGF pAb | VEGF fragment | 3-8 | 11-16 | ||
| MTF | 1-10 | 13-26 | |||
| MTF (multiplex) | 3-9 | 13-25 |
Antibody epitopes determined using target-specific fragment libraries, the multi-target fragment (MTF) library, and the MTF library in multiplex. mAb – Monoclonal antibody. pAb- polyclonal antibody. N.D. – Not detected.
Multiplex epitope mapping using the multi-target fragment library
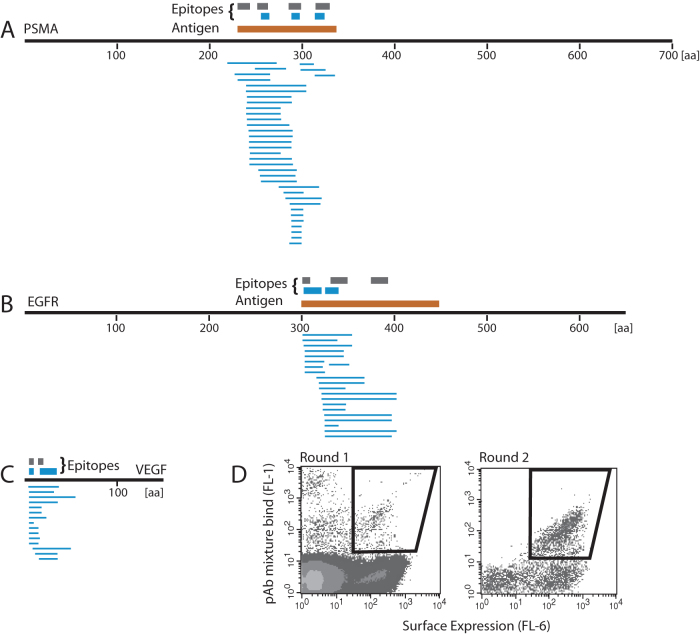
A primary motivation for developing the MTF library was to allow the simultaneous determination of epitopes of many antibodies. Multiplex epitope mapping of this kind could greatly increase the throughput of cell-based epitope mapping platforms. We sought to map the epitopes of the PSMA, EGFR, and VEGF pAbs using the MTF library. Furthermore, we tested the multiplex capability of the platform by mapping the epitopes of the three pAbs simultaneously. The three pAbs were pooled at equimolar amounts and incubated with the MTF library. After the second round of sorting, 96 colonies were picked and their inserts sequenced for alignment. Epitopes from the multiplex epitope mapping were compared to those determined by individual mappings of single pAbs.
Figure 4 shows that the enriched clones were distributed among the three antigens, indicating that no single epitope was preferentially enriched. All epitopes from the multiplex MTF mapping were in agreement with those from individual mapping experiments using target-specific libraries, although some of those epitopes were not detected in the multiplex assay. The N-terminal epitope on PSMA and the C-terminal epitope on EGFR were not found in the multiplex mapping experiment but were detected using PSMA-specific and EGFR-specific fragment libraries, respectively (Table 2). In both of these cases the multiplex epitope mapping experiment enriched clones covering the potential epitope, but there were not enough unique shorter clones for the algorithm to identify the region as an epitope. These results show that multiplex epitope mapping with the MTF library is possible for polyclonal antibodies, provided an adequate number of clones are sequenced. The use of massively parallel sequencing, instead of colony picking followed by Sanger sequencing, should allow for detection of all epitopes and will allow for even more antibodies to be assayed simultaneously.
Figure 4. Multiplex epitope mapping of a pAb mixture using the multi-target fragment library.
An equimolar mixture of three pAbs was assayed. After two rounds of FACS, 96 clones (blue lines) were sequenced and gene inserts aligned to reference sequences (A–C). All three pAbs bind to multiple epitopes (blue bars) as detected by the MTF library. Epitopes of each antibody determined using dedicated antigen fragment libraries are shown as gray bars over reference sequences. (D) Enrichment of positive clones in the first and second round of FACS.
S. carnosus fragment display for detection of conformational epitopes
To demonstrate the ability to display and detect conformational epitopes, we used a VEGF-specific fragment library to attempt to map the epitope of Avastin, a therapeutic, anti-VEGF monoclonal antibody with a known conformational epitope19. The VEGF library contained approximately 105 fragment-expressing clones with an average insert length of 100 bp (data not shown).
FACS epitope mapping of Avastin enriched a single clone encoding an 88-residue VEGF fragment, VEGF34–121 (Figure 5). The enrichment of a single protein fragment from the myriad smaller fragments was in contrast to the pattern observed when mapping other anti-VEGF antibodies and is in agreement with a discontinuous epitope. The crystal structure of the VEGF:Avastin Fab complex shows the Fab contacts two beta strands of VEGF (notated as B5 and B6 in20), and forms multiple, discontinuous contacts (see inset Figure 5B). A discontinuous Avastin epitope is also supported by alanine scanning studies, which showed that multiple, non-sequential VEGF residues contribute substantially to the binding free energy21. All of these critical VEGF residues are present in the enriched fragment VEGF34–121.
Figure 5. S. carnosus displays folded VEGF which is recognized by Avastin.
(A) Epitope mapping of anti-VEGF antibodies using S. carnosus surface display of VEGF fragments. Commercial mAbs and pAbs bind to small VEGF fragments, allowing for fine epitope mapping. In the Avastin assay, a single, large VEGF fragment is enriched, indicating a conformational epitope. (B) VEGF fragments containing potential Avastin epitopes. The fragments are shown colored on the VEGF:Avastin Fab co-crystal structure (PDB 1BJ1), which shows a conformational epitope. (C) Avastin binding to S. carnosus clones of VEGF fragments (colored as in (B)). Avastin does not bind to smaller epitope fragments but requires large and presumably folded VEGF fragments.
We hypothesized that VEGF34–121 represents a minimal VEGF sequence required for high-affinity Avastin binding. This fragment encompasses three strands of the four-stranded beta sheet core of the VEGF protein, as well as two of the three disulfide bonds. To test this hypothesis we constructed three smaller VEGF fragments for cell-surface display and Avastin affinity assays: (i) F1 VEGF84–94 comprises B5 and contains the critical Avastin contact residues G88, Q89 and M94, (ii) F2 VEGF77–85, comprises B6 and contains weak contact residues, and (iii) F3 VEGF75–96 which includes B5 and B6 and comprises all significant Avastin contact residues. Although VEGF exists as a homo-dimer in solution, Avastin makes no contact with the second monomer21.
Figure 5C shows the results of binding assays to the VEGF fragment clones. Avastin exhibited appreciable affinity to the VEGF34–121 fragment and the whole VEGF1–121 protein, but showed low or negligible affinity to the constructed VEGF fragments, despite their coverage of all VEGF-Avastin contacts. These results confirm that the Avastin epitope is conformational, in that the presence of all critical contact residues in VEGF75–96 was not enough for appreciable affinity. The additional residues in VEGF34–121 were necessary, presumably for the epitope to adopt the proper cysteine-knot conformation. These results indicate that it is possible to detect a conformational epitope using cell-surface fragment libraries, and that in such cases the mean fragment size could be increased in order to allow for more accurate mapping.
Epitope mapping with a multi-target fragment library reveals antibody cross-reactivity
In addition to epitope mapping, the multi-target fragment library allows for detection of antibody cross-reactivity via FACS. Such cross-reactivity is undesirable for affinity-based purification or diagnostic reagents as well as therapeutics. We investigated the cross-reactivity of two polyclonal antibodies (PSMA pAb and EGFR pAb, used above) by sequencing 96 gated clones after one round of FACS with the MTF library, instead of two rounds typically used in our epitope mapping procedure, and aligning clone sequences to library members.
All sequenced clones from the PSMA pAb FACS assay corresponded to PSMA fragments, indicating no cross-reactivity of this antibody to other proteins in the target library and demonstrating the possibility of epitope mapping with only one round of cell sorting. Of the 96 clones sequenced from the EGFR pAb FACS assay, we found three identical clones encoding a 77-residue ITGA2b fragment (residues 877–956). All other clones aligned to the EGFR antigen (EGFR299–447). Figure 6 shows FACS assays of the EGFR pAb to this clone as well as to a fragment containing an EGFR epitope (EGFR335–505). The enrichment of the ITGA2b877–956 fragment by the anti-EGFR pAb occurred in the background of other fragments from the growth factor receptor family (HER2, HER3, and HER4) which exhibit homology with EGFR. ITGA2b877–956 contains a 36-residue stretch with 28% sequence identity to the EGFR antigen (Supplemental Information Figure S4), which may explain the observed cross reactivity of the EGFR pAb. It was recently reported that anti-sera raised toward the carboxy-terminus of soluble EGFR were cross-reactive with a member of the integrin family (ITGAV)22. While the cross-reactive sequences reported there are different than what we observed, that study highlights the close biological connection between cell-surface growth-factor receptors and integrins. We also tested the PSMA pAb antibody for affinity to these clones, and this antibody again showed affinity only to its epitope (Figure 6 inset). While the MTF library is useful for identifying off-target binding of antibodies, it is important to note that multiplex epitope mapping of several pAbs simultaneously may obscure cross-reactivity.
Figure 6. Antibody cross-reactivity revealed using the combined fragment library.
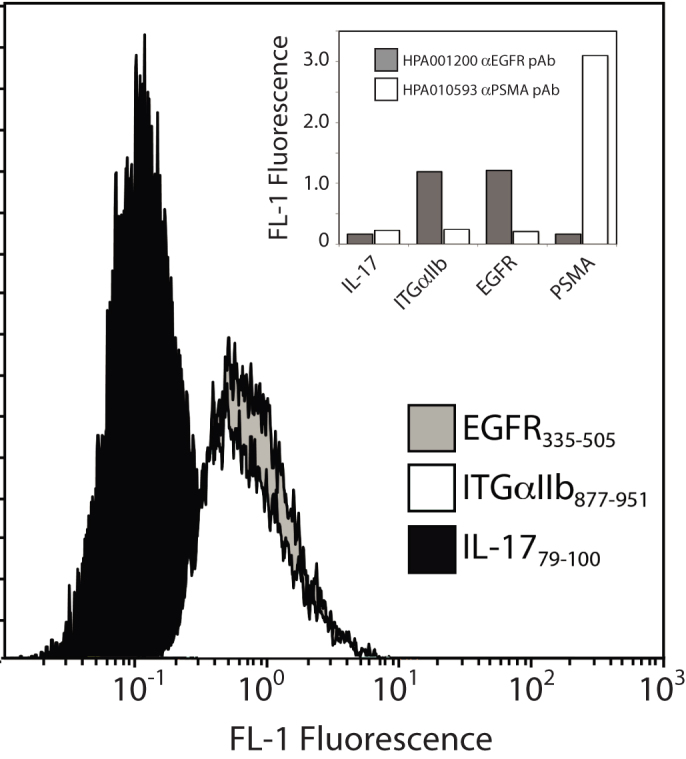
HPA001200 anti-EGFR pAb binds to a S. carnosus clone displaying a fragment of integrin protein ITGA2b. Also shown is strong reactivity to a fragment of EGFR containing the antigen and nil reactivity to a negative control (a fragment not detected in the first round), IL-17. Insert: Binding of anti-EGFR pAb to selected fragments shows cross reactivity to the ITGA2b clone while anti-PSMA antibody does not.
Discussion
Here, we show that bacterial cell-surface display can be used for epitope mapping in a multiplex fashion using a library containing fragments spanning 60 therapeutic protein targets. The presence of multiple protein targets allowed us to perform simultaneous epitope mapping of polyclonal antibodies and also allowed us to detect off-target binding of an anti-EGFR polyclonal antibody to an integrin fragment.
The epitope resolution provided by cell-surface display is dependent on the fragmentation pattern of antigen genes. Deep sequencing of the MTF library showed an overall complete coverage of all genes, although an uneven distribution of fragments within a gene (Figure 2B). We chose sonication for gene fragmentation because it is more likely to provide overlapping fragments of uniform size than enzyme digestion. It has been shown that GC-rich DNA stretches are more resistant to fragmentation at short sonication times23. However, we do not observe a correlation between GC content and prevalence in the MTF library. There is strong evidence that these regions of enrichment do not preclude suitable coverage of other antigen regions. For example, the MTF library was able to detect small epitopes on the N-terminus of VEGF (Figure 4 and Table 2), despite underrepresentation of this region among VEGF fragments (Figure 2B center).
The MTF library accelerated the epitope mapping process by allowing us to detect multiple high and low-affinity epitopes from three polyclonal antibodies simultaneously. The presence of 7 epitopes from 96 sequenced clones indicates no growth bias after the second round of sorting. Amplification bias can occur when selecting ligands from phage-display libraries, leading to loss of positive clones24. To sequence enriched S. carnosus clones, we picked colonies of sorted cells from agar plates. This is suitable for determining epitopes of monoclonal antibodies, but becomes a throughput bottleneck and may limit epitope resolution if multiple polyclonal antibodies are assayed simultaneously. For example, the multiplex epitope mapping assay failed to detect some polyclonal epitopes when an insufficient number of clones were sequenced (Figure 4). We expect that throughput, and thus epitope resolution, could be easily increased by coupling FACS to a high-throughput sequencing platform. To demonstrate compatibility of FACS-based epitope mapping with high-throughput sequencing, we sorted 106 cells directly into liquid culture based on in-frame gene expression. This cell slurry was used directly for PCR and sequencing. The high coverage of all library members indicates no major growth bias among library members.
The enrichment of VEGF34–121 by Avastin from the VEGF-fragment library emphasizes the utility of cell-surface display for detecting conformational epitopes and suggests that heterogeneity in fragment size after sonication can be beneficial. The 88-residue fragment is beyond the current limits of conventional peptide synthesis. Cell-surface display is thus a means to present both linear and structured protein fragments and functional enzymes up to 46 kDa have been displayed on the surface of S. carnosus25,26. Furthermore, the average size of cell-surface displayed fragments can be adjusted with sonication time during DNA fragmentation. We envision small and large fragment libraries could be used in tandem to uncover conformational epitopes. Fine mapping of discovered conformational epitopes could then be done using cell-surface alanine scanning. Cell-surface display protein fragment libraries could also find application in “interactome” studies, where protein-protein or protein-DNA binding interfaces may be distributed over several residues non-adjacent in sequence. Based on the transformation efficiencies and library coverage observed for this focused library, an S. carnosus fragment-display library comprising the 20,000 proteins of the human proteome is feasible, albeit at a reduced coverage depth (see Methods for an estimate). Such a bacterial cell-surface displayed human proteome would be a valuable tool also in serum profiling.
Cell-surface display-based binding assays utilizing fluorescence detection are sensitive and agree with other biophysical characterization methods such as surface-plasmon resonance27. Using flow cytometry of S.carnosus-presented proteins, interactions of KD = 1.5 µM are clearly visible above background28. Furthermore, flow cytometry allows for high-accuracy separation of library members within a range of affinities29. The ability to detect low-affinity interactions is particularly useful for antibody characterization; while therapeutic antibodies often exhibit high affinity toward their antigen (KD = 2 nM for Avastin used here21), low affinity, off-target binding can lead to poor pharmokinetics30.
In summary, the cell-surface display epitope mapping method is suitable for mapping of both linear and structural epitopes of monoclonal, as well as polyclonal antibodies, in a multiplex fashion. It is versatile in that it bridges the fine epitope mapping of linear epitopes associated with peptide arrays with the large, folded proteins typical of protein microarrays. The compatibility with high-speed multi-color FACS circumvents the background problems associated with immunoprecipitation31. The high coverage of target genes in our 60-target library indicates that cell-surface display multiplex epitope mapping can be extended to libraries of more antigens.
Methods
MTF library construction
The multi-target fragment library was constructed from the coding DNA of 60 human proteins. The Novo Nordisk Foundation Center for Protein Research at the University of Copenhagen, Denmark kindly provided bacterial clones containing the full-length genes in various shuttle vectors. A list of clones can be found in Supplemental Information file TargetList.xls. Gene-specific PCR primers for amplification of target ectodomains were from Thermo Fischer (MA, USA). The primers contained NotI (forward primer) and AscI (reverse primer) restriction sites for insertion into the E. coli pAff8c vector. A full primer list can be found in Supplemental Information. PCR products were cleaved with NotI and AscI, ligated into pAff8c, and used to transform E. coli in 96-well format. Successful transformants were verified by DNA sequencing. The pAff8c-gene plasmids were purified via 96-well miniprep (Millipore Montage). To construct the multi-target fragment library, target genes were PCR amplified (50 μL each) with pAff8c-specific primers (U2 and N5) which annealed 30 bp up- and downstream of the vector MCS. After PCR amplification, the amplicons were pooled, fragmented by sonication for 4 h, treated with a DNA-fragment repair mixture (Fermentas) and blunt-end-ligated into EcoRV-digested pSCEM2. The ligation product was extracted via phenol:chloroform treatment, precipitated and washed with EtOH, and used for electroporation (10 µg DNA) of E. coli. Cells were plated on ampicillin agar plates. E. coli transformants (5*107) were scraped and cultured overnight. Vector DNA was purified with maxiprep (Qiagen) and 20 µg was used for electroporation of S. carnosus according to published protocols32. Library size (typically 5*105/µg DNA) was determined by counting transformants on chloramphenicol agar plates. Colonies were scraped from the plates, centrifuged, and the pellets re-suspended in 1 mL TSB with 20% glycerol. Fragment display libraries were stored at −80°C. Target-specific libraries were prepared similarly but with only one amplicon (2.0 mL PCR volume). We performed six S. carnosus transformations33 to generate the MTF library of 107 members. This library covered 45 proteins at over 100 reads per bp (58 at over 10 reads per bp). An extension of this to 20,000 proteins of the human proteome would require approximately 2500 transformations (for 100 reads per bp) or 250 transformations (for 10 reads per bp).
Antibodies used in this study
Anti-PSMA mAb (mouse IgG) was from Invitrogen (ID 3739000). Anti-VEGF mAb (mouse IgG) was from AbCam (ID ab36424). Anti-VEGF mAb (Avastin, human IgG) was a kind gift from Professor Bengt Glimelius (Uppsala Academic Hospital, Uppsala, Sweden).
Polyclonal antibodies anti-EGFR HPA001200 and anti-PSMA HPA010593 (both rabbit IgG) were from Atlas Antibodies (Stockholm, Sweden). Anti-VEGF pAb A-20 was from Santa Cruz Biotechnologies.
FACS-based epitope mapping
Culture stabs (5-10 mL) from frozen fragment libraries were used to inoculate 10 mL of TSB media containing chloramphenicol. Cultures were grown overnight at 37°C to an OD600 of 14. Aliquots (10 mL, 107 cells) were washed twice with 500 mL PBS-P (PBS buffer pH 7.4, 1 g/L Pluronic acid (BASF)) and re-suspended in 100 mL PBS-P containing primary antibody (pAb or mAb) at 1 mg/mL. After 45 min the cells were pelleted and washed with PBS-P, then re-suspended in 100 mL PBS-P. Secondary antibody (AlexaFluor488-conjugate) was added at 1:50,000 dilution (0.02 ng/mL) and AlexaFluor647-conjugate human serum albumin (HSA) was added to 40 nM. The mixture was incubated at 4°C for 45 min. Cells were then pelleted, washed once with 500 mL ice-cold PBS-P, and re-suspended in 300 mL ice-cold PBS-P for FACS. Cells were sorted with a FACS Vantage SE (BD Biosciences).
Two rounds of sorting were performed for each epitope mapping analysis, except for the off-target binding study, where only one round was performed. Sorting gates were drawn based on high 647 nm fluorescence in channel FL-4 (indicating HSA-AlexaFluor647 binding to the albumin-binding protein ABP scaffold) and high 488 nm fluorescence in channel FL-1 (indicating primary antibody binding to the peptide fragment). Approximately 5,000,000 cells were queried in the first round and between 500 and 5,000 were sorted. Approximately 1,000 cells were sorted in the second round. After the second round, 96 colonies were picked and analyzed via PCR using the pSCEM2 primers SAPA23 and SAPA24. PCR products were sequenced using Sanger sequencing (ABI). Alignment of reads were performed using BLAST through an in-house Bioperl / Matlab script as described previously14.
Deep sequencing of library members
For characterization of the MTF library, cells were treated as described above but with only HSA-647 incubation. Cells expressing ABP (high 647 nm fluorescence) and those not expressing ABP (low 647 nm fluorescence) were sorted separately (106 cells each). The sorted cells were pelleted and re-suspended in 10 µL PBS. The cell suspensions were used as PCR template with primers PYRO_F and PYRO_R. The PCR products were purified (Qiagen) and sequenced using the Illumina sequencing platform (Science for Life Laboratory, Royal Institute of Technology, Stockholm, Sweden) yielding 87 million reads. Alignment of reads was performed using BWA and plotting done using Geneious34.
Author Contributions
EPH, MU, and JR designed experiments. EPH and JR performed experiments. All authors wrote and reviewed the manuscript.
Supplementary Material
Supplemental Information
Supplementary Dataset 1
Acknowledgments
We would like to acknowledge ProNova VINN Excellence Centre for Protein Technology for funding this project and Novo Nordisk Foundation Centre for Protein Research for supply of cDNA clones.
References
- Strohl W. R. & Knight D. M. Discovery and development of biopharmaceuticals: current issues. Current opinion in biotechnology 20, 668–72 (2009). [DOI] [PubMed] [Google Scholar]
- Uhlen M. et al. Towards a knowledge-based Human Protein Atlas. Nature biotechnology 28, 1248–50 (2010). [DOI] [PubMed] [Google Scholar]
- Cho H.-S. et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 421, 756–60 (2003). [DOI] [PubMed] [Google Scholar]
- Du J., Yang H., Guo Y. & Ding J. Structure of the Fab fragment of therapeutic antibody Ofatumumab provides insights into the recognition mechanism with CD20. Molecular Immunology 46, 2419–2423 (2009). [DOI] [PubMed] [Google Scholar]
- Hoyer W., Gronwall C., Jonsson A., Stahl S. & Hard T. Stabilization of a beta-hairpin in monomeric Alzheimer's amyloid-beta peptide inhibits amyloid formation. Proceedings of the National Academy of Sciences 105, 5099–5104 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen O. & Anglister J. Epitope Mapping of Antibody-Antigen Complexes by Nuclear Magnetic Resonance Spectroscopy. Methods in Molecular Biology 524, 37–57 (2009). [DOI] [PubMed] [Google Scholar]
- Kazim A. & Atassi M. Haemoglobin binding with haptoglobin. Biochemical Journal 197, 507–510 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otvos L. et al. In situ stimulation of a T helper cell hybridoma with a cellulose-bound peptide antigen. Journal of immunological methods 233, 95–105 (2000). [DOI] [PubMed] [Google Scholar]
- F.H. Winkler D. Chemistry of SPOT Synthesis for the Preparation of Peptide Macroarrays on Cellulose Membranes. Mini-Reviews in Organic Chemistry 8, 7 (2011). [Google Scholar]
- Hjelm B. et al. Exploring epitopes of antibodies toward the human tryptophanyl-tRNA synthetase. New biotechnology 27, 129–37 (2010). [DOI] [PubMed] [Google Scholar]
- Larman H. B. et al. Autoantigen discovery with a synthetic human peptidome. Nature Biotechnology 1–9 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christmann A., Wentzel A., Meyer C., Meyers G. & Kolmar H. Epitope mapping and affinity purification of monospecific antibodies by Escherichia coli cell surface display of gene-derived random peptide libraries. Journal of immunological methods 257, 163–73 (2001). [DOI] [PubMed] [Google Scholar]
- Chao G., Cochran J. R. & Wittrup K. D. Fine epitope mapping anti-epidermal growth factor receptor antibodies through random mutagenesia and yeast surface display. Journal Of Molecular Biology 342, 539–550 (2004). [DOI] [PubMed] [Google Scholar]
- Rockberg J., Lofblom J., Hjelm B., Uhlen M. & Stahl S. Epitope mapping of antibodies using bacterial surface display. Nature Methods 5, 1039–1045 (2008). [DOI] [PubMed] [Google Scholar]
- Gai S. A. & Wittrup K. D. Yeast surface display for protein engineering and characterization. Current opinion in structural biology 17, 467–73 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson M. et al. Expression of recombinant proteins on the surface of the coagulase-negative bacterium Staphylococcus xylosus. Journal of bacteriology 174, 4239–45 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson P. et al. Cell surface display of recombinant proteins on Staphylococcus carnosus. Journal of Bacteriology 177, 1470–1476 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. J. Potent antibody therapeutics by design. Nature Reviews Immunology 6, 343–357 (2006). [DOI] [PubMed] [Google Scholar]
- Chen Y. et al. Selection and analysis of an optimized anti-VEGF antibody: crystal structure of an affinity-matured Fab in complex with antigen. Journal of molecular biology 293, 865–81 (1999). [DOI] [PubMed] [Google Scholar]
- Muller Y. A. et al. Vascular endothelial growth factor: Crystal structure and functional mapping of the kinase domain receptor binding site. Proceedings Of The National Academy Of Sciences Of The United States Of America 94, 7192–7197 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuh G. et al. Structure-function studies of two synthetic anti-vascular endothelial growth factor Fabs and comparison with the AvastinTM Fab. Journal of Biological Chemistry 281, 6625 (2006). [DOI] [PubMed] [Google Scholar]
- Wilken J. A., Baron A. T., Foty R. A., Mccormick D. J. & Maihle N. J. Identification of Immunoreactive Regions of Homology between Soluble Epidermal Growth Factor Receptor and alpha5-Integrin. Biochemistry 4309–4321 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deininger P. Random subcloning of sonicated DNA: Application to shotgun DNA sequence analysis. Analytical Biochemistry 129, 216–223 (1983). [DOI] [PubMed] [Google Scholar]
- Derda R. et al. Diversity of phage-displayed libraries of peptides during panning and amplification. Molecules 16, 1776–803 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss A. & Götz F. In vivo immobilization of enzymatically active polypeptides on the cell surface of Staphylococcus carnosus. Molecular Microbiology 21, 491–500 (1996). [DOI] [PubMed] [Google Scholar]
- Lehtio J., Wernerus H., Samuelson P., Teeri T. T. & Stahl S. Directed immobilization of recombinant staphylococci on cotton fibers by functional display of a fungal cellulose-binding domain. FEMS Microbiology Letters 195, 197–204 (2001). [DOI] [PubMed] [Google Scholar]
- Lofblom J., Sandberg J., Wernérus H. & Ståhl S. Evaluation of staphylococcal cell surface display and flow cytometry for postselectional characterization of affinity proteins in combinatorial protein engineering applications. Applied and environmental microbiology 73, 6714–21 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilvebrant J., Alm T., Hober S. & Löfblom J. Engineering Bispecificity into a Single Albumin-Binding Domain. PLoS ONE 6, e25791 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofblom J., Wernerus H. & Stahl S. Fine affinity discrimination by normalized fluorescence activated cell sorting in staphylococcal surface display. Fems Microbiology Letters 248, 189–198 (2005). [DOI] [PubMed] [Google Scholar]
- Bumbaca D. et al. Highly specific off-target binding identified and eliminated during the humanization of an antibody against FGF receptor 4. mAbs 3, 376–386 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidder B. L., Hu G. & Zhao K. ChIP-Seq: technical considerations for obtaining high-quality data. Nature Publishing Group 12, 918–922 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronqvist N., Lofblom J., Jonsson A., Wernerus H. & Stahl S. A novel affinity protein selection system based on staphylococcal cell surface display and flow cytometry. Protein Engineering Design & Selection 21, 247–255 (2008). [DOI] [PubMed] [Google Scholar]
- Rockberg J., Löfblom J., Hjelm B., Ståhl S. & Uhlén M. Epitope mapping using gram-positive surface display. Current protocols in immunology/edited by John E. Coligan ... [et al.] Chapter 9, Unit9.9 (2010). [DOI] [PubMed] [Google Scholar]
- Drummond A. J., Ashton B., Buxton S., Cheung M., Cooper A., Duran C., Field M., Heled J., Kearse M., Markowitz S., Moir R., Stones-Havas S., Sturrock S., Thierer T. & Wilson A. (2012) Geneious v5.6, Available from http://www.geneious.com.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Information
Supplementary Dataset 1