Abstract
The ability to regenerate following stress is a hallmark of self-renewing tissues. However, little is known about how regeneration differs from homeostatic tissue maintenance. Here, we study the role and regulation of Wingless (Wg)/Wnt signalling during intestinal regeneration using the Drosophila adult midgut. We show that Wg is produced by the intestinal epithelial compartment upon damage or stress and it is exclusively required for intestinal stem cell (ISC) proliferation during tissue regeneration. Reducing Wg or downstream signalling components from the intestinal epithelium blocked tissue regeneration. Importantly, we demonstrate that Wg from the undifferentiated progenitor cell, the enteroblast, is required for Myc-dependent ISC proliferation during regeneration. Similar to young regenerating tissues, ageing intestines required Wg and Myc for ISC hyperproliferation. Unexpectedly, our results demonstrate that epithelial but not mesenchymal Wg is essential for ISC proliferation in response to damage, while neither source of the ligand is solely responsible for ISC maintenance and tissue self-renewal in unchallenged tissues. Therefore, fine-tuning Wnt results in optimal balance between the ability to respond to stress without negatively affecting organismal viability.
Keywords: Drosophila midgut, enteroblast, intestinal stem cells, regeneration, wingless
Introduction
Homeostasis and regeneration of adult tissues requires a tight balance between the production of new cells and the removal of old or damaged cells. Such an equilibrium is maintained by stem cells that reside at specific locations within the tissue (Nystul and Spradling, 2006). Deregulation of the homeostatic control between stem cell proliferation and/or differentiation has been linked to the initiation and progression of tumours (Fodde, 2009).
Homeostatic turnover in the mammalian intestinal epithelium is achieved through the action of intestinal stem cells (ISCs), which are located at the base of each intestinal crypt (Barker et al, 2007). ISCs also confer a remarkable regenerative capacity to the intestinal epithelium following DNA damage, acute inflammation, surgical resection or knockdown of genes essential for tissue homeostasis (Bach et al, 2000; Ireland et al, 2004; Bernal et al, 2005). The commonalites and differences between the mechanisms regulating intestinal regeneration in response to damage and those involved in homeostatic self-renewal remain largely unknown.
Canonical or β-Catenin-dependent Wnt signalling, which we will refer to as Wnt signalling, is an essential regulator of vertebrate intestinal homeostasis (Korinek et al, 1998; van de Wetering et al, 2002; Ireland et al, 2004). Inactivating mutations in the gene encoding for the Wnt signalling inhibitor, Adenomatous Polyposis Coli (Apc), are detected in 80% of hereditary and sporadic forms of colorectal cancer (CRC) (Kinzler et al, 1991; Korinek et al, 1997). Several lines of evidence suggest that mammalian Wnt signalling might be important for intestinal regeneration: (i) high levels of β-Catenin and the Wnt target gene c-Myc accumulates in regenerating intestinal crypts and (ii) c-myc is required to induce intestinal regeneration in the mouse (Ashton et al, 2010). Nevertheless, the role and regulation of Wnt signalling during intestinal regeneration remains to be directly tested.
Mammalian studies have often been hampered by the absolute requirement of Wnt signalling for normal intestinal homeostasis. Inactivating mutations in the Wnt pathway lead to a very rapid loss of intestinal tissue (Pinto et al, 2003). Furthermore, the presence of multiple vertebrate Wnt ligands and stem cell populations (Tian et al, 2011) makes it difficult to unambiguously identify the source and type of Wnt that composes the ISC niche in homeostatic conditions as well as during regeneration. Work using CRC cell lines favours the presence of a mesenchymal niche (Vermeulen et al, 2010). On the other hand, crypt culture studies propose that Wnt3 secreted from the Paneth cells may represent an intrinsic ISC niche (Sato et al, 2011). Nevertheless, a role for Paneth cells in Wnt-signalling activation and ISC proliferation could not be confirmed in vivo (Durand et al, 2012; Kim et al, 2012), indicating the presence of compensatory signals.
Due to its remarkable resemblances to the vertebrate intestine (Casali and Batlle, 2009) the Drosophila adult midgut is emerging as a useful model to study intestinal homeostasis, regeneration and disease. Importantly, the fly intestinal epithelium is replenished by its own ISCs (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). Drosophila ISCs are randomly scattered along the basal membrane of the intestinal tube and, following division, ISCs give rise to a transcient undifferentiated progenitor—the enteroblast (EB)—which differentiate into either the secretory cell lineage—the enteroendocrine cells (ee)—or the absorptive epithelial cell lineage represented by the enterocytes (ECs).
Genetic studies indicate conservation in the role for the Wnt/Wg signalling in the Drosophila midgut (Lin et al, 2008; Cordero et al, 2009; Lee et al, 2009). Nevertheless, current data have led to the suggestion that the extent to which Wnt signalling is required in the fly and vertebrate intestine may be different (Jiang and Edgar, 2012). Work from the Xi laboratory has shown that the visceral muscle (VM), which surrounds the Drosophila intestinal epithelium expresses the ligand Wg (Lin and Xi, 2008). It has therefore been proposed that Wg secreted from the VM constitutes part of the ISC niche, which is required for ISC proliferation under homeostatic conditions (Lin et al, 2008). However, loss of Wg from the whole intestine or loss of function clones of components of the Wg signalling pathway from the intestinal epithelium show a rather mild, progressive decrease in homeostatic ISC proliferation (Lin et al, 2008). Indeed, later work from the same laboratory suggests that combined loss of Armadillo/β-Catenin, EGFR and JAK/Stat signalling is required for efficient blockade in ISC maintenance during homeostatic self-renewal of the intestinal epithelium (Xu et al, 2011).
Much like its vertebrate counterpart, the Drosophila adult intestine displays a remarkable regenerative response to stressors or damaging agents, which disrupt epithelial integrity. Damage induced by bacterial infection, EC cell death, Bleomycin or dextran sodium sulphate (DSS) treatment leads to activation of ISC proliferation to regenerate the damaged intestinal epithelium (Amcheslavsky et al, 2009; Buchon et al, 2009; Jiang et al, 2009). Pathways such as Jun N-terminal Kinase (JNK), JAK/Stat, Hippo and EGFR signalling have been shown to mediate damage or stress-induced intestinal regeneration in Drosophila (Biteau et al, 2008; Apidianakis et al, 2009; Buchon et al, 2009; Cronin et al, 2009; Jiang et al, 2009, 2011; Karpowicz et al, 2010; Ren et al, 2010; Shaw et al, 2010; Staley and Irvine, 2010; Biteau and Jasper, 2011). JAK/Stat and Hippo signalling have a conserved role in the mammalian intestine (Pickert et al, 2009; Cai et al, 2010). The role and regulation of Wg signalling during intestinal regeneration has not been addressed.
Our results demonstrate that Wg within the intestinal epithelial compartment is induced in response to acute damage/stress in the Drosophila adult midgut. This source of Wg is exclusively required for damage-induced ISC proliferation and subsequent midgut regeneration while it is dispensable for intestinal self-renewal in unchallenged tissues. Importantly, we demonstrate that the EBs represent the source of epithelial Wg, which signals to the ISCs and induces their proliferation in response to damage. Therefore, our results uncover the presence of a ‘regeneration-specific Wg niche’, which is regulated and required for ISC proliferation in conditions of damage or stress.
Results
Wg is induced within the epithelial compartment of the Drosophila midgut in response to damage
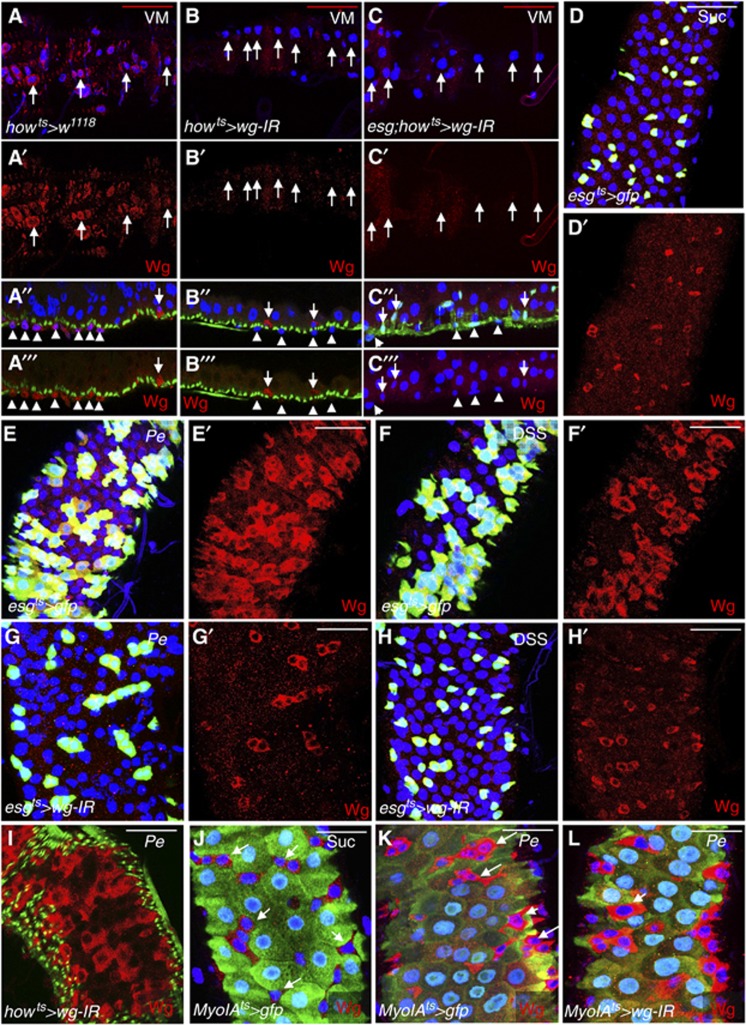
Previous work on the Drosophila midgut indicated that, under homeostatic conditions, endogenous Wg was localized to the VM and at low levels in ISCs/EBs (Lin et al, 2008). Using the previously published antibody staining protocol (Lin et al, 2008) we were able to confirm the localization pattern in both domains (Figure 1A–A″′ and Figure 1D and D′, respectively). To further address the source of Wg in the midgut under basal or ‘steady-state’ conditions, we used RNA interference against wg (wg-IR) to specifically knockdown wg from the VM using the specific drivers how-gal4 (Jiang and Edgar, 2009) (howts>wg-IR; Figure 1B–B″′) and mef2-gal4 (O’Brien et al, 2011) (mef2ts>wg-IR; Supplementary Figure S2C–D′). Wg protein immunostaining confirmed efficient knockdown of Wg in the VM with both drivers (Figure 1B–B″′, compare with Figure 1A–A″′ and Supplementary Figure S2D and D′, compare with Supplementary Figure S2C and C′). Furthermore, RT–qPCR of howts>wg-IR midguts showed almost complete knockdown of basal wg message levels upon VM-specific gene knockdown (Supplementary Figure S1A). We next used the ISC/EB-specific driver escargot-gal4 (esg) (Micchelli and Perrimon, 2006) to knockdown wg within those cells of the epithelial compartment and we were unable to detect a significant knockdown of basal Wg protein or gene mRNA (Supplementary Figure S1B and E–F′). Together, these data would indicate that, in the homeostatic scenario, Wg is almost exclusively produced by the VM. Nevertheless, we observed cells positive for Wg staining within the epithelium of how>wg-IR midguts (Figure 1B″ and B″′, arrows). This raised the posibility of a source of Wg in homeostatic midguts deriving from ISCs/EBs themselves in addition to the VM, which may be underpresented by gene knockdown in individual domains. Therefore, we combined the esg-gal4 and how-gal4 drivers to achieve simultaneous wg knockdown in VM and ISCs/EBs (esg; how>wg-IR) (Figure 1C–C″′; Supplementary Figures S2E–F′ and S3K, L). Unlike how>wg-IR midguts, esg; how>wg-IR midguts showed nearly undetectable Wg staining in ISCs/EBs (Figure 1C″ and C″′; compare with Figure 1B″ and B″′ and Supplementary Figure S2E′ and F′). Together, these data suggest that the VM and ISCs/EBs represent two independent sources of Wg in the midgut under homeostatic or ‘steady-state’ conditions.
Figure 1.
Wg is upregulated in esg+ve cells in response to damage in the adult Drosophila midgut. (A–C″′) Control midguts (A–A″′) and midguts subject to wg knockdown in the visceral muscle (VM) only (howts>wg-IR) (B, B″′) or in VM and ISCs/EBs (esg; howts>wg-IR) (C–C″′) and stained with anti-Wg (red), DAPI (blue) and Phalloidin (green; A″, A″′, B″, B″′) or anti-GFP (C″). Posterior of the midgut is to the right. Panels (A, A′, B, B′, C, C′) show apical confocal sections of the VM and (A″, A″′, B″, B″′, C″, C″′) represent cross-sections of the intestinal tube. Note the loss of Wg staining in the VM after RNAi for Wg (arrows in A, A′, B, B′, C, C′ and arrowheads in remaining panels). Arrows in (A″, A″′, B″, B″′, C″, C″′) point to cells within the midgut epithelium, which either show detectable Wg staining (Wg+ve cells) (A″, A″′, B″, B″′; red) or express esg>gfp (esg+ve cells) (C″; green). Note that Wg+ve cells are still detectable in howts>wg-IR (B″, B″′; compare with A″, A″′) but not in esg; howts>wg-IR midguts (C″, C″′; compare with B″, B″′). (D–L) Wg staining (red) from midguts of the indicated genotypes, treated with Sucrose (Suc, D, D′, J), infected with the bacteria Pseudomonas entomophila (Pe) (E, E′, G, G′, I, K, L), or treated with Dextran sodium sulphate (DSS) (F, F′, H, H′). Note the induction of Wg levels during regeneration (compare E′, F′, I with D′ and K with J). RNAi for Wg suppressed Wg upregulation when expressed in the esg+ve lineage (compare G′, H′ with E′, F′, respectively) but not when expressed in the VM (compare I with E′) or in enterocytes (ECs) (compare L with K). Except otherwise noted posterior midguts were analysed in all cases, and posterior is up in all panels. Scale bars: 40 μm.
We then tested whether Wg was induced in the adult Drosophila midgut in response to acute stress or damage to the midgut epithelium, which triggers a strong regenerative response characterized by a marked increase in ISC proliferation (Biteau et al, 2008; Amcheslavsky et al, 2009; Apidianakis et al, 2009; Buchon et al, 2009; Cronin et al, 2009). We induced damage in the intestine by feeding flies the pathogenic bacteria Pseudomonas entomophila (Pe) (Figure 1E and E′), the detergent DSS (Figure 1F and F′) or the apoptosis-inducing reagent Bleomycin (Supplementary Figure S1G and G′) and stained for Wg. Antibody staining showed a dramatic upregulation of Wg protein within the ISCs/EBs progenitor cell population labelled by esg-gal4, UAS-gfp (esgts>gfp) in response to all the damaging agents used (Figure 1E′ and F′, compare with Figure 1D′; Supplementary Figure S1G′, compare with Supplementary Figure S1E′). RT–qPCR from whole midguts showed an upregulation of the wg transcript upon the different damages (Supplementary Figure S1C). Critically, overexpression of two independent wg-IR constructs under the control of the esg-gal4 driver (esgts>wg-IR and esgts>wg-IRKK) resulted in a complete block in Wg upregulation in the midgut epithelium (Figure 1G′ and H′ and Supplementary Figure S1H′; compare with Figure 1E′ and F′). This was confirmed at the mRNA level by RT–qPCR (Supplementary Figure S1D). Furthermore, esgts>wg-IR midguts showed a clear impairment in the expansion of the esg>gfp domain, which characterizes the regenerating intestinal epithelium (Figure 1G and H; Supplementary Figure S1H; compare with Figure 1E and F). Importantly, driving either RNAi within the VM (howts>wg-IR or howts>wg-IRKK) was not sufficient to prevent upregulation of Wg within the midgut epithelium (Figure 1I; Supplementary Figure S1I; compare with Figure 1E′). To further confirm the identity of the cells showing Wg upregulation in response to damage, we stained midguts from flies carrying the EC marker MyoIA>gfp (Jiang et al, 2009) and fed a control diet (Sucrose) or damaging agents (Figure 1J–L; Supplementary Figure S1J–N′). Consistent with Wg upregulation in ISCs/EBs, control midguts showed exclusive Wg staining in cells with small nuclei and not expressing MyoIA-gfp (MyoIA-gfp negative; MyoIA-gfp−ve) (Figure 1J; Supplementary Figure S1J and J′). In response to damage Wg was consistently upregulated in MyoIA-gfp−ve cells, which were mainly represented by cells with small nuclei (Figure 1K, compare with Figure 1J; Supplementary Figure S1J–K′ to see separate channels). Some MyoIA-gfp−ve cells of intermediate nuclear size that presumably represent newly made immature ECs were also labelled (Figure 1K and L, small arrows). These cells are also labelled with esg>gfp in regenerating midguts due to the increase in ISC proliferation rate and rapid production of new cell lineages (Figure 1E–F′). Importantly, as in the case of regenerating howts-wg-IR midguts, MyoIAts>wg-IR midguts maintained Wg upregulation within the midgut epithelium in response to all damages (Figure 1L; compare with Figure 1K and Supplementary Figure S1M′ and N′; compare with Supplementary Figure S1K′). Together, these data show that the main source of upregulated Wg within the epithelial compartment of regenerating adult Drosophila midguts is the esg+ve progenitor cell population.
Wg from ISCs/EBs but not the VM is required for damage-induced ISC proliferation in regenerating adult Drosophila midguts
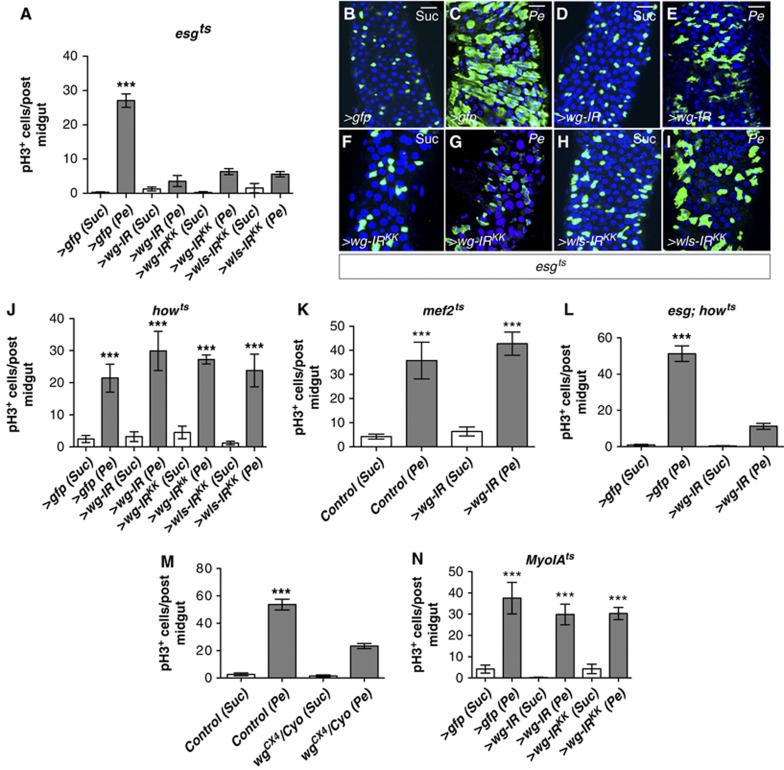
To unambigously identify the functional source of Wg during intestinal regeneration, we measured ISC proliferation in response to damage in intestines where wg was knocked down from either ISCs/EBs (esgts>wg-IR) (Figure 2A–I), the VM (howts>wg-IR and mefts>wg-IR) (Figure 2J and K) or ECs (MyoIAts>wg-IR) (Figure 2N). We confirmed our results by the use of two independent wg RNAis (wg-IR and wg-IRKK) as well as by knocking down Wntless/Evi (Wls/Evi) (wls-IR), a conserved regulator of Wg secretion, which acts specificaly within the ligand-producing cells (Banziger et al, 2006; Bartscherer et al, 2006).
Figure 2.
Wg from the ISC/EB population but not the VM is required for ISC proliferation during regeneration. (A) Quantification of pH3+ve cells from posterior midguts of the indicated genotypes (wg-IR and wg-IRKK are independent RNAi transgenes for wg) after Sucrose (Suc) or bacterial (Pe) feeding. (B–I) Immunofluorescence of midguts as in (A) stained with anti-GFP (green) to visualize esg+ve cells and Dapi (blue) to label all cell nuclei. (J–N) Quantification of pH3+ve cells in posterior midguts of the indicated genotypes. Note that only RNAi for Wg or Wls involving the esg+ve lineage (L), or heterozygosity for the null allele wgCX4 (M) significantly inhibits ISC proliferation in response to damage. In contrast, no significant effect was seen by wg knockdown in the VM (J, K) or the ECs (N) (***P<0.0001 one-way ANOVA with Bonferroni’s multiple comparison test). Scale bars: 20 μm.
We found that midguts with knockdown of wg in ISCs/EBs (esgts>wg-IR) showed a consistently impaired regenerative response to all the damaging agents used (Figure 2A–I; Supplementary Figure S2A). Interestingly, wg heterozygote midguts (wgCX4/Cyo) showed a 50% reduction in the rate of ISC proliferation in response to damage (Figure 2M). Conversely, knocking down wg from the VM only or ECs did not significantly affect intestinal regeneration (Figure 2J, K and N; Supplementary Figure S2B and G). The combined knockdown of wg from ISCs/EBs and the VM (esg; howts>wg-IR) resulted in a block in midgut regeneration comparable to that observed in esgts>wg-IR midguts (Figure 2L). Together, these results indicate that Wg produced by the progenitor cell population expressing escargot (escargot positive; esg+ve) but not the VM or ECs (the latter expressing MyoIA; MyoIA+ve) is required for the acute proliferative response of ISCs to damage in the Drosophila adult midgut.
Wg from the epithelial compartment is essential for damage-induced ISC proliferation but dispensable for homeostatic self-renewal
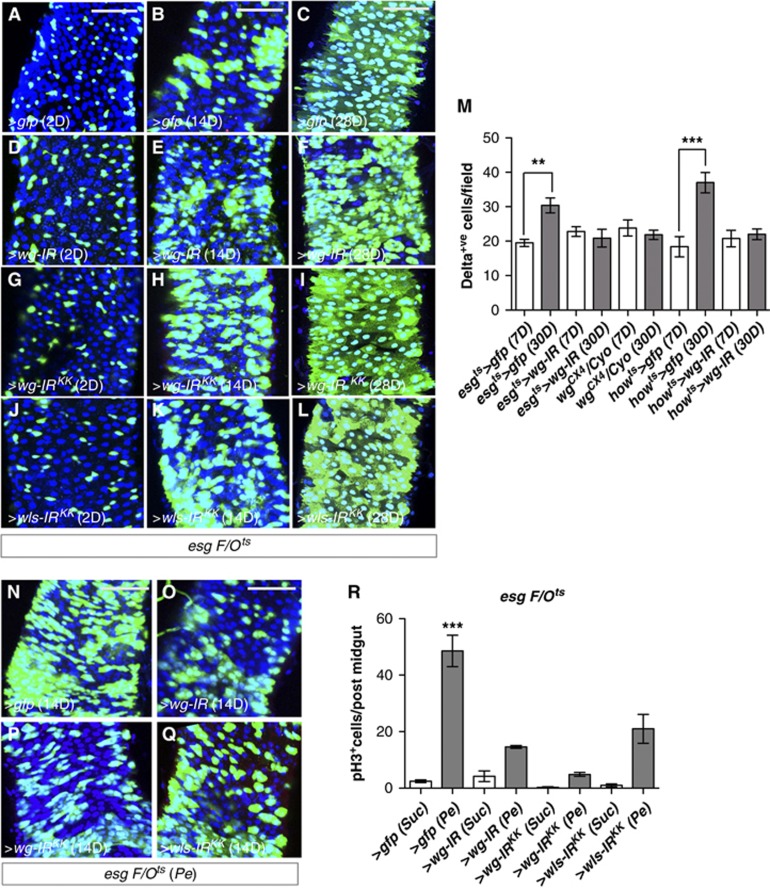
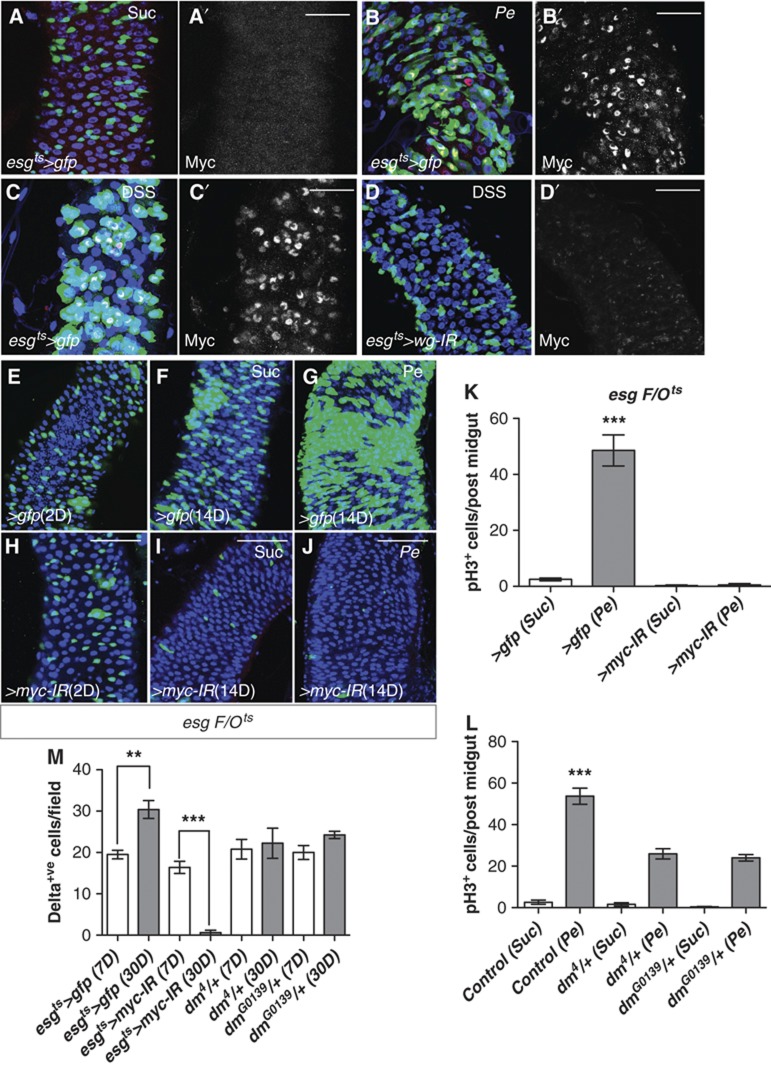
We next tested whether Wg within the epithelial compartment was required for homeostatic self-renewal of the midgut. We reasoned that loss of function clones of wg would probably produce inconclusive results due to potential non-autonomous effect from surrounding wild-type ISCs. Therefore, we knocked down wg from the epithelial compartment by RNAi using the inducible ‘escargot flip out’ system (esgts F/O>gfp) (Jiang et al, 2009), in which every progenitor cell and its new progeny will express Gal4 and UAS-gfp in addition to our UAS-RNAi of interest. We made use of two, independent wg-IRs and a wls-IR transgenes and visualized the newly produced esg cell lineage 2, 14 and 28 days after transgene induction (Figure 3A–L; see Materials and methods). Our results indicated that knocking down wg or wls from the epithelium of undamaged midguts had no effect on homeostatic self-renewal (Figure 3D–L; compare with Figure 3A–C). Furthermore, we observed no significant decrease in the number of Delta+ve ISCs even after long-term (30 days) knockdown of wg in either esgts>wg-IR or howts>wg-IR midguts (Figure 3M; Supplementary Figure S3A–D and G–J). Note the similar number of ISCs in 7- and 30-day-old esgts>wg-IR and howts>wg-IR midguts, when compared to 7-day-old control counterparts. This suggested no requirement for Wg in either the VM or the epithelial compartment alone for ISC maintenance. Midguts from wgCX4 heterozygote animals also showed a stable number of ISCs over time (Figure 3M; Supplementary Figure S3E and F) in spite of showing deficient regeneration (Figure 2M). Corresponding with the previously described ageing phenotype (Biteau et al, 2008), 30-day-old control midguts (esgts>gfp and howts>gfp) had increased number of ISCs when compared to younger counterparts (Figure 3M; Supplementary Figure S3A, B, I and J). Importantly, damage induced by Pe feeding in esg>ts F/O midguts 14 days after transgene induction confirmed the essential requirement of epithelium-induced Wg for midgut regeneration (Figure 3N–Q; compare with Figure 3B, E respectively and Figure 3R). Together, these results demonstrate that Wg from the epithelial compartment is specifically required for ISC proliferation in response to damage to the midgut epithelium but it is dispensable for tissue self-renewal and ISC maintenance under homeostatic conditions.
Figure 3.
Epithelial Wg is dispensable for homeostatic self-renewal. (A–L) esgts>F/O (esg-gal4, UAS-FLP, actin5C>stop>gal4, UAS-GFP, tub-gal80ts) clones from guts with the indicated genotypes after 2, 14 and 28 days of incubation at 29°C to induce clones. There was no change in esg-derived clones in homeostatic conditions upon loss of wg or wls (compare A–C with D–F, G–I, J–L). (M) Quantification of Delta+ve cells in posterior midguts of the indicated genotypes. Note that Delta+ve ISCs numbers were not decreased by knockdown of wg or wls or by heterozygosity for the null allele wgCX4. However, these conditions prevented the mild increase in ISCs observed in aged guts (M; ***P<0.0001, **P<0.005 Student’s t-test). (N–Q) esgts>F/O midguts from the indicated genotypes after 14 days of clone induction and treated with Pe (compare with B, E, H, K, respectively). Note the requirement of epithelial-derived wg and wls for damage-induced proliferation. (R) Quantification of pH3+ve cells in posterior midguts of the indicated genotypes and treatments. Note the suppression of damage-induced proliferation when wg or wls is knocked down (***P<0.0001 one-way ANOVA with Bonferroni’s multiple comparison test). Scale bars: 60 μm.
Wg signalling is required for intestinal regeneration
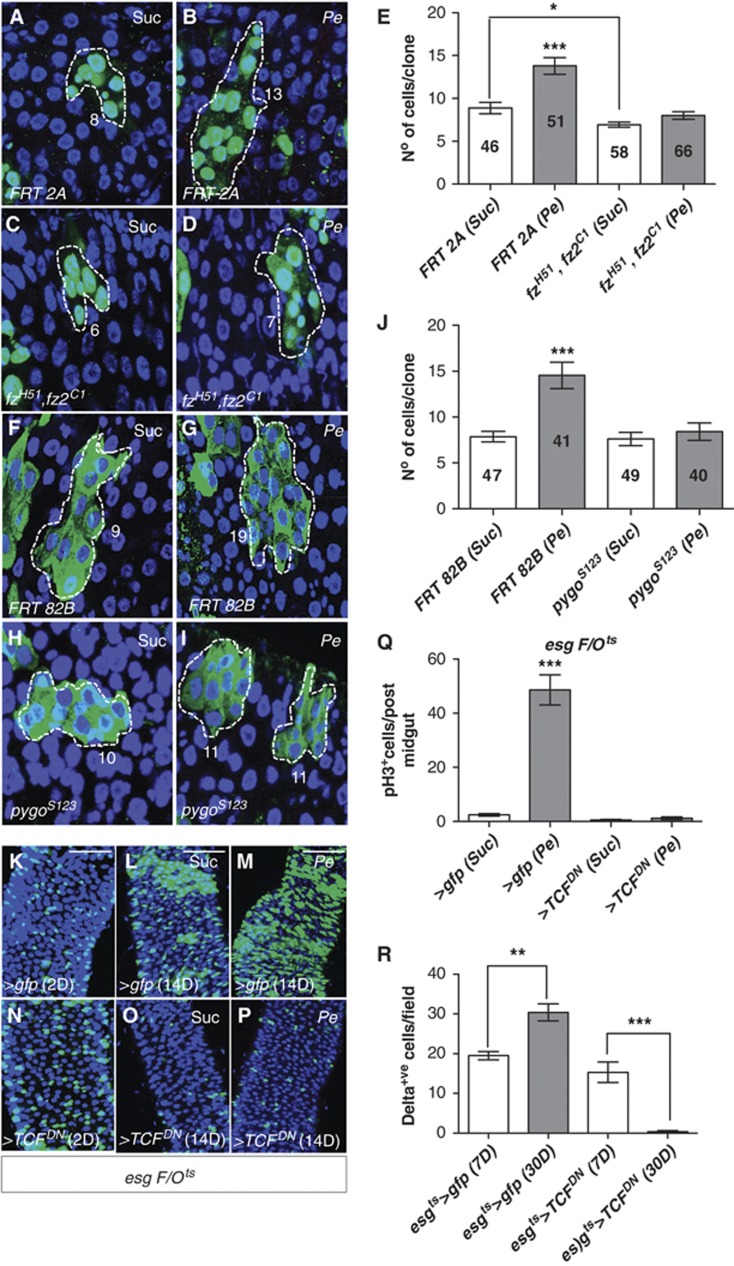
We next tested components of canonical Wg signalling for their role in the ISC proliferative response to damage during regeneration. We first used the MARCM system (Lee and Luo, 2001) to make clones of combined loss of function alleles of the genes encoding for the Fz1 and Fz2 receptors (fzH51, fz2C1) (Chen and Struhl, 1999; Figure 4A–E) and the β-Catenin/TCF co-activator Pygopus (pygoS123) (Kramps et al, 2002; Parker et al, 2002; Thompson et al, 2002; Figure 4F–J). Control (FRT 2A and FRT 82B) and loss of function clones were induced in adult animals and allowed to age at 25°C for 13 days followed by 1 day sucrose (Suc) or bacterial (Pe) feeding. We assessed ISC proliferation during regeneration by scoring the number of cells per clone in sucrose-treated and damaged midguts. Control clonal size increased significantly in response to Pe infection (Figure 4A, B) while the size of fzH51, fz2C1 and pygoS123 clones remained unchanged after damage (Figure 4C–E and H–J). We also compared the size of control and loss of function midgut clones from sucrose fed animals to assess the requirement for Fz and Pygo in homeostatic ISC proliferation. Previous work reported a mild-progressive decrease in the number of fz, fz2 loss of function clones in the midgut indicating a requirement in homeostatic ISC maintenance (Lin et al, 2008). Indeed, our data showed a marginal decrease in the size of sucrose fzH51, fz2C1 clones when compared with equally treated control clones (Figure 4A, C and E) while no significant difference was detected between the size of sucrose, pygoS123 and control clones (Figure 4F, H and J). Differences in the requirements for Pygo in intestinal homeostasis versus regeneration may be due to the partial requirement for Pygo in Wnt signalling (Parker et al, 2002; Jessen et al, 2008). To further address the role of canonical Wg signalling in intestinal regeneration, we next overexpressed a dominant-negative isoform of the β-Catenin co-factor Tcf/Pangolin (van de Wetering et al, 1997) using the esgts F/O system (esgtsF/O>TcfDN) (Figure 4K–P). esgtsF/O>TcfDN midguts showed a complete block in ISC proliferation upon Pe infection (Figure 4O and P; compare with Figure 4L and M and Figure 4Q). We confirmed these results in esgts>TcfDN midguts with the use of different damaging agents (Supplementary Figures S4A–H and M). Furthermore, esgtsF/O>TcfDN midguts showed a severe impairment in homeostatic self-renewal as evidenced by an almost complete loss in the esg cell lineage after 14 days of transgene expression (Figure 4N and O; compare with Figure 4K and L). This result was consistent with the loss of Delta+ve ISCs observed in esgts>TcfDN midguts after 30 days of transgene expression (Figure 4R; Supplementary Figure S4I–L). Together, these data indicate that canonical Wg pathway is essential for damage-induced ISC proliferation in regenerating Drosophila midguts. In contrast, the role of this pathway in homeostasis was component dependent. Blocking all signalling via the dominant-negative Tcf transgene completely inhibited homeostatic self-renewal, while other pathway components had mild or no effect in homeostasis. In contrast, all pathway components tested were essential for midgut regeneration. Thus, a marked difference could be observed between the requirement of Wg signalling in intestinal homeostasis and regeneration.
Figure 4.
Canonical Wnt pathway components are required for intestinal regeneration. (A–I) Fourteen-day-old control MARCM clones (A, B and F, G) or MARCM loss of function clones of frizzled 1 and 2 (C, D) or pygopus (H, I) treated with either Suc (A, C, F, H) or Pe (B, D, G, I). Numbers in (A–I) refer to the number of cells in each of the clones shown, which represent average size clones for each of the conditions depicted. (E, J) Quantifications of cell number per clone from (A–I). The numbers inside bars represent the total number of clones scored. Note that frizzled or pygopus mutant clones failed to increase in size in response to damage when compared to control clones (***P<0.0001 one-way ANOVA with Bonferroni’s multiple comparison test). On the other hand, only frizzled clones showed decreased size when compared to control ones in homeostatic conditions (*P=0.005 Student’s t-test). (K–P) Posterior esgts>F/O midguts expressing gfp (K–M) or a dominant-negative Tfc (N–P) at 2 or 14 days after clone induction, treated with Suc or Pe. Note the reduced number of esg-derived clones in homeostatic conditions after 14 days of TcfDN expression (compare O with L) and blockade in regeneration (compare P with M). (Q) Quantification of pH3+ve cells in posterior midguts as in (K–P) (***P<0.0001 one-way ANOVA with Bonferroni’s multiple comparison test). (R) Quantification of Delta+ve ISCs in posterior midguts of the indicated genotypes. Note the loss of ISCs in 30-day-old esgts>TcfDN midguts when compared to 7-day-old ones (**P<0.005, ***P<0.0001 Student’s t-test). Since the scoring of Delta+ve cells was done in parallel for all the genotypes analysed, controls (esgts>gfp) presented here for reference are the same as shown in Figure 3M. Scale bars: 50 μm.
Myc is activated downstream of Wg during regeneration of the adult Drosophila midgut
Myc proteins are conserved proto-oncogenes and regulators of normal growth and proliferation (Johnston et al, 1999; Trumpp et al, 2001). Furthermore, c-Myc is a well-known Wnt target, which mediates Apc-driven hyperplasia (Sansom et al, 2007) and damage-induced regeneration in the mouse intestine (Ashton et al, 2010). We have also demonstrated that Myc has a conserved role as mediator of Apc-driven ISC hyperproliferation in the Drosophila midgut (Cordero et al, under review). We next examined the role of Myc in ISC proliferation in response to damage as well as in tissue homeostasis. First, we assessed the levels of Myc in midguts under homeostatic conditions and in response to damage. Antibody staining showed a dramatic increase in Myc levels within esg+ve cells in response to damage (Figure 5A–C′; Supplementary Figure S5A–B′). Importantly, Myc induction in response to damage was diminished in esgts>wg-IR midguts (Figure 5D and D′; compare with Figure 5C and C′) indicating that Myc upregulation during midgut regeneration is at least in part dependent on Wg. We next knocked down myc by RNAi using the esgts F/O system (esgtsF/O>myc-IR) (Figure 5E–J). esgtsF/O>myc-IR midguts showed a complete block in ISC proliferation in response to Pe infection after 14 days of RNAi transgene expression (Figure 5I and J; compare Figure 5F and G and Figure 5K). We confirmed these results in esgts>myc-IR midguts and by the use of different damaging agents (Supplementary Figure S5C). Previous work in the Drosophila midgut reported that concomitant 2-day RNAi knockdown of myc and DSS treatment did not affect ISC proliferation (Amcheslavsky et al, 2011). This discrepancy with our results is likely due to the kinetics of myc knockdown, as it would take time for the RNAi to reduce Myc mRNA levels. It is therefore possible that RNAi was not efficient to overcome Myc upregulation and prevent regeneration as per the protocol used in that study. As in the case of TcfDN, esgtsF/O>myc-IR midguts showed impaired homeostatic self-renewal, which was clearly evident after 14 days of transgene expression (Figure 5H and I; compare with Figure 5E and F). Consistently, we observed an almost complete loss of Delta+ve ISCs in esgts>myc-IR midguts after 30 days of transgene expression (Figure 5M; Supplementary Figure S5H–M). Importantly, heterozygote dm4/+ and dmG0139/+ midguts showed a 50% reduction in the number of ISCs proliferating in response to damage (Figure 5L) in spite of maintaining a constant number of ISC over time (Figure 5M; Supplementary Figure S5F and G). This suggests that the requirement for Myc in midgut regeneration is not simply due to a general permissive role in ISC maintenance. Together, our results indicate that Myc has an instructive role in damaged-induced ISC proliferation in regenerating Drosophila midguts as well as a permissive role in ISC proliferation during normal tissue homeostasis.
Figure 5.
Myc is activated downstream of Wg during regeneration. (A–D′) Midguts of the indicated genotypes treated with Suc (A, A′), Pe (B, B′) or DSS (C–D′) and stained with anti-MYC (red in left panels and grey in right panels). Note the upregulation of Myc during regeneration (compare B′, C′ with A′), which is prevented by Wg knockdown in esg+ve cells (compare D′ with C′). (E–J) esgts>F/O midguts expressing gfp (E–G) or RNAi for myc (H–J) at 2 and 14 days after clone induction and treated with Suc or Pe. Note the reduced number of esg-derived clones upon time during homeostatic conditions (compare I with F) and the complete block in the response to damage when myc is knocked down (compare J with G). (K, L) Quantification of pH3+ve cells in posterior midguts of the indicated genotypes and treatments. Note the complete block in proliferation by myc knockdown (K), and the halved proliferation rates in regenerative conditions by myc heterozygosity (L) (***P<0.0001 one-way ANOVA with Bonferroni’s multiple comparison test). (M) Quantification of Delta+ve ISCs in posterior midguts of the indicated genotypes and age. Note the loss of Delta+ve ISCs after 30 versus 7 days of myc knockdown in progenitor cells (esgts>myc-IR) but not in age-matched myc heterozygotes (***P<0.0001 Student’s t-test). As indicated in the previous figure, Delta+ve values from control (esgts>gfp) midguts presented here for reference are the same as shown in Figure 3M. Unlike controls, myc heterozygotes midguts did not display increase in number of ISC over time (**P<0.005 Student’s t-test). Sale bars: 40 μm (A–D′); 50 μm (E–J).
Wg from EBs is required for Myc-dependent ISC proliferation in regenerating midguts
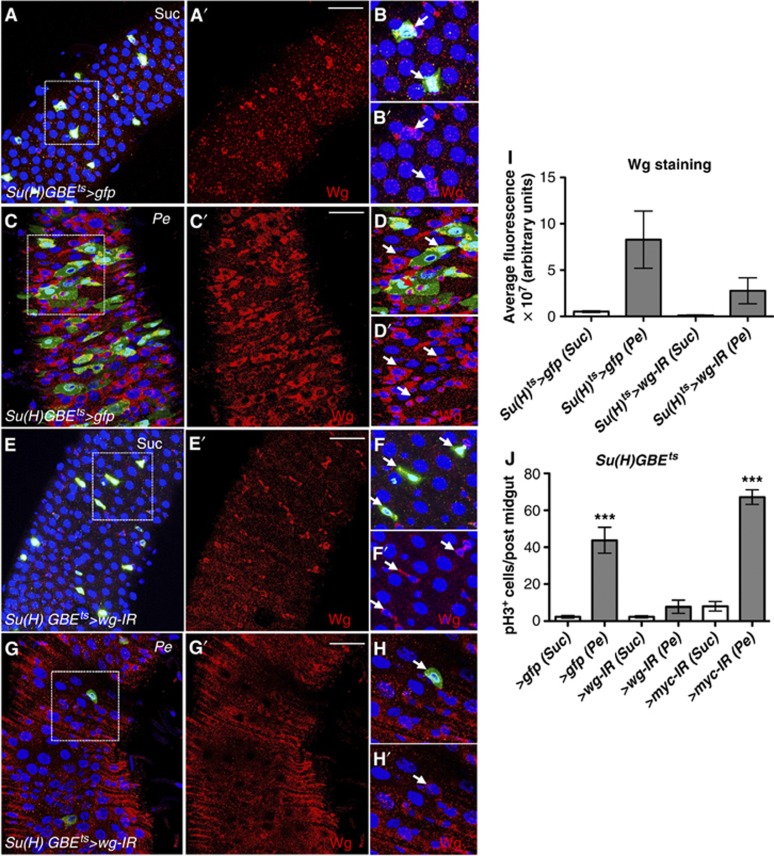
Using the esg-gal4 driver we have demonstrated that Wg from stem/progenitor cells (ISCs/EBs) is largely responsible for midgut regeneration upon multiple damages (Figures 1 and 2). To further identify the source of Wg in regenerating midguts, we used the Su(H)GBE-gal4, UAS-gfp driver to express wg RNAi (Su(H)ts>wg-IR) (Figure 6). This allows wg knockdown within EBs only (Zeng et al, 2010). As expected from our previous localization data (Figure 1), Wg was found in all small nuclei cells expressing Su(H)>gfp (Su(H)>gfp+ve), even though some cells showing Wg staining did not express Su(H)>gfp (Su(H)>gfp−ve) (Figure 6A–B′). Consistent with the knockdown in the esg+ve cells we were unable to detect a significant knockdown of basal Wg in Su(H)ts>wg-IR versus Su(H)ts>gfp midguts (Figure 6E′ and F′; compare with Figure 6A′ and B′). Pe infection caused high ISC hyperproliferation (Figure 6J) and expansion of the Su(H)>gfp expression domain to include cells of intermediate nuclear size and thought to be immature ECs (Figure 6C and D). As expected, Wg was highly upregulated in the epithelium of damaged midguts (Figure 6C′ and D′; compare with Figure 6A′ and B′; Figure 6I). Critically, Su(H)ts>wg-IR midguts showed diminished Wg upregulation (Figure 6G′ and H′; compare with Figures 6C′ and D′; Figure 6I) and strong reduction in ISC proliferation in response to damage (Figure 6J). Furthermore, we observed a drastic decrease in Su(H)>gfp+ve cells in damaged Su(H)ts>wg-IR midguts (Figure 6G), which is likely due to prevention of ISC proliferation and tissue regeneration after damage. Importantly, knocking down myc from EBs (Su(H)ts>myc-IR) did not affect ISC proliferation during midgut regeneration (Figure 6J). Thus, Myc is not required in the cells that produce Wg and instead is required in ISCs to drive regeneration. Together, these data suggest that Wg from the undifferentiated progenitor of ISCs, the EBs, is required for ISCs proliferation during regeneration of the Drosophila midgut.
Figure 6.
Wg from enteroblasts (EBs) is required for Myc-dependent ISC proliferation during regeneration. (A–H′) Wg staining (red) from midguts expressing the EB driver Su(H)GBE-gal4 gfp only (A–D′) or in combination with RNAi for wg (E–H′). Note the impairment in Wg upregulation in response to damage in midguts with wg knockdown in EBs. Panels (B, B′, D, D′, F, F′, H, H′) show high magnification views from the boxed areas in the left panels. Arrows point to Wg+ve cells. (I) Wg levels represented as the average fluorescence intensity from three independent samples for each of the conditions and genotypes analysed. (J) Quantification of pH3+ve cells in the indicated genotypes. Note that when driven with Su(H)-gal4, and in contrast to myc knockdown, midguts with wg knockdown failed to proliferate in response to damage (***P<0.0001 one-way ANOVA with Bonferroni’s multiple comparison test). Scale bars: 40 μm.
JNK activation induces Wg and Myc in the Drosophila adult midgut
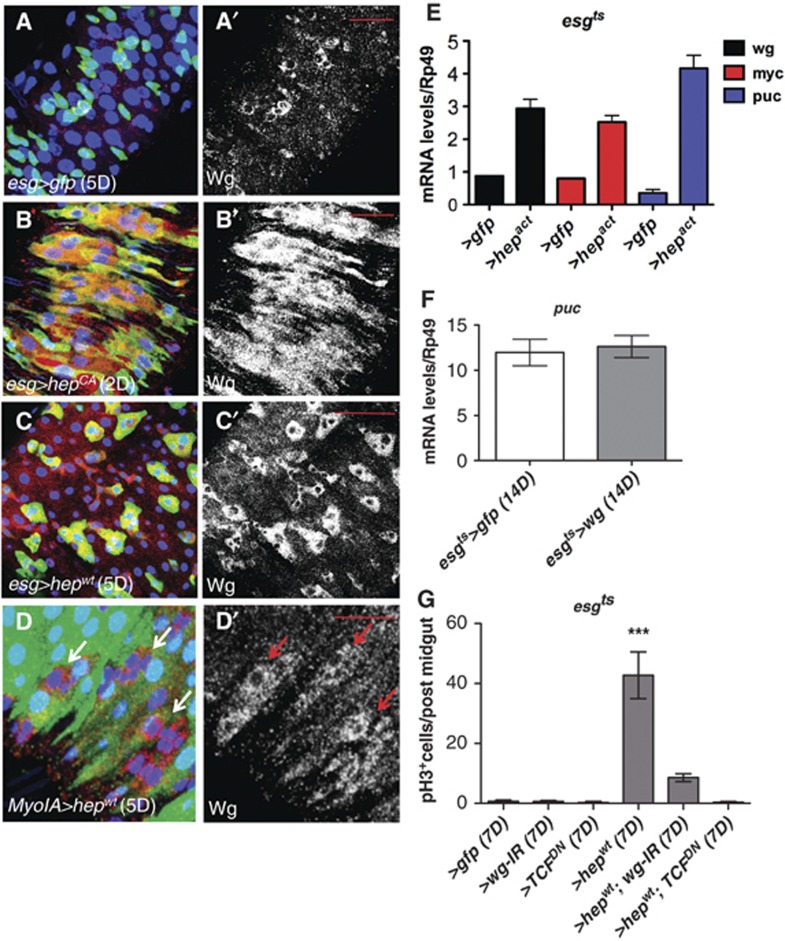
The JNK pathway is activated in response to different types of cellular stresses (Weston and Davis, 2007). We observed that damage in the intestine resulted in activation of JNK as assessed by phosphorylation (pJNK) predominantly in ECs and big nuclei esg+ve cells (Supplementary Figure S6A and B, arrows and not shown). This is consistent with previous reports on the localization of puckered as a readout of JNK activity (Biteau et al, 2008). Importantly, JNK activation drives ISC proliferation in the Drosophila adult midgut (Biteau et al, 2008, 2011; Jiang et al, 2009). We activated JNK signalling by overexpressing activated or wild-type hemipterous (hep) in progenitor cells (esgts>hepCA) (Figure 7B and B′); (esgts>hepwt) (Figure 7C and C′), and observed significant upregulation of Wg (Figure 7B′ and C′; compare with Figure 7A′ and Figure 7E) and Myc (Figure 7E; Supplementary Figure S6C–D′). Activation of JNK in ECs under the MyoIA driver (MyoIAts>hepwt) resulted in upregulation of Wg in MyoIA−ve cells (Figure 7D and D′). Together, these data suggest that JNK activation is sufficient to drive Wg and Myc upregulation in progenitor cells in the Drosophila adult midgut. Overexpression of wg under the esg-gal4 driver (esgts>wg) showed no detectable regulation of JNK signalling as assesed by puckered (puc) expression levels (Figure 7F) and pJNK staining (not shown). Importantly, downregulation of Wg singnalling by overexpressing wg RNAi (esgts>wg-IR) or TcfDN (esgts>TcfDN) significantly suppressed the hyperplastic phenotype of esgts>hepwt midguts (Figure 7G; Supplementary Figure S6E–I). This demonstrates that Wg signalling is an important effector of JNK activity in the Drosophila adult midgut.
Figure 7.
JNK activation induces Wg in the Drosophila adult midgut. (A–D′) Control midguts (A, A′) or after forced activation of JNK in ISCs/EBs via activated (B, B′) or wild-type (C, C′) JNKK hemipterous (hep) for the indicated times and stained with anti-Wg antibody (red in left panels and grey in right ones). JNK activation resulted in drastic increase in the levels of Wg as assessed by staining (compare B–C′ with A, A′). (D) Forced activation of JNK in ECs labelled with MyoIAts>gfp (D; green) resulted in Wg upregulation in non-MyoIA expressing cells (arrows in D, D′). (E) qPCR quantification of wg, myc and puckered (puc) transcript levels in control midguts and midguts overexpressing activated hemipterous under the esgts>gfp driver (esgts>hepact). (F) qPCR quantification of puckered (puc) transcript levels in control midguts and midguts overexpressing Wg under the esgts>gfp driver. (G) Quantification of pH3+ve in the genotypes indicated. Note that knockdown of wg or Tcf repressed JNK-driven ISC proliferation (***P<0.0001 one-way ANOVA with Bonferroni’s multiple comparison test). Scale bars: 20 μm.
Wg and Myc are upregulated in ageing Drosophila midguts
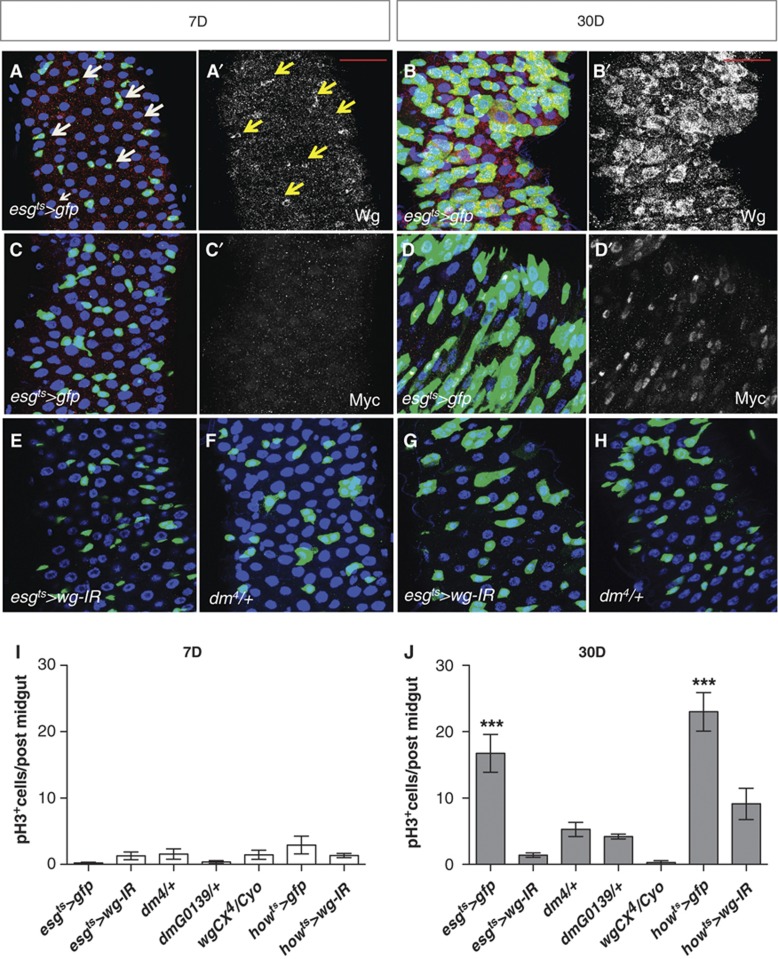
Ageing Drosophila midguts display high levels of JNK activity followed by ISC hyperproliferation and differentiation defects (Biteau et al, 2008, 2011; Choi et al, 2008; Supplementary Figure S7A–B′ and E). Ectopic Delta and Notch activity seem responsible for misdifferentiation in old midguts (Supplementary Figure S7E; Biteau et al, 2008). As in young damaged midguts, ageing tissues showed pJNK in ECs and big nuclei esg+ve cells (Supplementary Figure S7B and B′, arrows). We noticed that midguts where wg was knocked down showed an impaired age-dependent increase in ISC number (Figure 3; Supplementary Figure S3). To assess the role of Wg in ageing midguts, we first looked at the levels of Wg in young versus old midguts. Remarkably, ageing Drosophila midguts displayed a significant increase in Wg (Figure 8A–B′) and Myc (Figure 8C–D′) within the esg+ve cells when compared to young tissues. RT–qPCR quantification confirmed upregulation of both transcripts (Supplementary Figure S7F). Furthermore, Myc levels were largely dependent on Wg in old intestines (Supplementary Figure S7H and not shown). This suggests that ageing Drosophila midguts upregulate Wg and Myc in a similar fashion to young regenerating tissues.
Figure 8.
Wg and Myc are upregulated in ageing Drosophila midguts. (A–D′) Midguts expressing gfp under the control of the esg-gal4 driver stained with anti-Wg (A–B′) or anti-Myc (C–D′) at 7 and 30 days after adult eclosion. Arrows in (A, A′) point to a subset of esg+ve cells that also express Wg. Note the increase in esg+ve cells and upregulation of Wg and Myc in aged guts. (E–H) Posterior midguts from 7- or 30-day-old adults, expressing RNAi for wg under the control of the esg-gal4 driver (E, G) and midguts from age-matched flies heterozygous for a loss of function allele of myc (F, H). (I, J) Quantification of pH3+ve cells in the genotypes indicated at 7 (I) or 30 (J) days (***P<0.0001 one-way ANOVA with Bonferroni’s multiple comparison test). Partial loss of wg or myc suppressed the age-dependent ISC proliferation hyperproliferation. Scale bars: 20 μm.
Wg and Myc mediate ISC hyperproliferation in the ageing Drosophila midgut
High Wnt signalling has been associated with age-dependent phenotypes in stem cell-based systems such as the epidermis and the haematopoietic lineage in a mouse model of accelerated ageing (Liu et al, 2007; Castilho et al, 2009). Nevertheless, little is known about its impact on ISCs upon ageing of the intestinal epithelium. We next examined the role of Wg and Myc in ageing Drosophila midguts. Downregulation of wg from progenitor cells (esgts>wg-IR) almost completely suppressed ISC hyperproliferation in ageing midguts (Figure 8I and J). JNK signalling was still upregulated in old esgts>wg-IR intestines (Supplementary Figure S7C–D′; compare with Supplementary Figure S7A–B′ and Supplementary Figure S7G) consistent with Wg being downstream of JNK activation in ageing midguts. Strikingly, midguts from old, wg and myc heterozygote animals showed no ISC hyperproliferation and looked essentially like young wild-type ones (Figure 8F, H–J). Interestingly, we observed that—even though not to the same extent as esgts>wg-IR midguts—old howts>wg-IR midguts showed significant suppression of age-dependent ISC hyperproliferation (Figure 8I and J).
Discussion
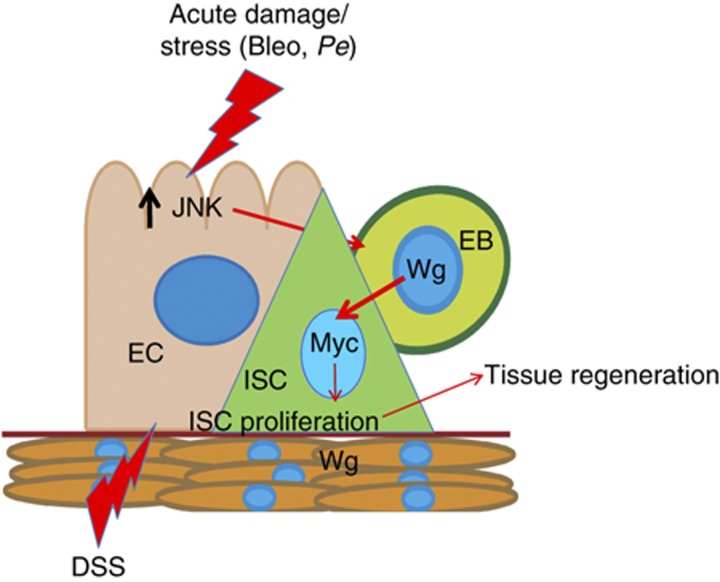
Here, we use the posterior adult Drosophila midgut to address the role of Wg and its downstream signalling pathway during the proliferative response of ISCs to acute damage of the intestinal epithelium. Our results suggest that, in response to stress or damage, Wg production is induced in EBs, which stimulates ISC proliferation and subsequent midgut regeneration in a Myc-dependent manner (Figure 9). Our results place Wg induction downstream of the damage/stress activated kinase JNK.
Figure 9.
Damage-inducible Wg from EBs is required for ISC proliferation during midgut regeneration. Model of interactions in the ISC niche during damage. Intestinal damage or stress results in JNK activation in ECs. This results in upregulation and secretion of Wg by EBs, which in turn activates canonical Wnt signalling and Myc upregulation in its progenitor ISC to drive proliferation and tissue regeneration. The visceral muscle niche expresses Wg but is dispensable for regeneration. See the text for details.
Wnt ligands and the ISC niche
The VM that surrounds the Drosophila midgut was proposed to constitute the Wg niche and be the sole source of the ligand required for ISC maintenance and homeostatic tissue self-renewal (Lin et al, 2008). Our results suggest that Wg produced within the intestinal epithelium and not the VM is essential for intestinal regeneration in response to acute damage. Furthermore, neither source of the ligand seems solely responsible for homeostatic self-renewal. Apparent discrepancies with the previous study could have different explanations. One possibility is that the ISC phenotype of whole wg mutants (Lin et al, 2008) may be the result of combined Wg loss in VM and midgut epithelium. Alternatively, minimal levels of wg may be sufficient to maintain ISCs during homeostasis while a higher threshold of ligand production and subsequent signalling activation may be required for regeneration. In such a scenario, wg knockdown by RNA interference may still leave enough Wg to maintain the tissue under homeostatic conditions. We favour the latter possibility over the former one since combined wg knockdown in esg;how>wg-IR midguts did not show significant loss of ISCs (Supplementary Figure S3K–Q). Dose-dependent roles of Wnt signalling have been previously reported to regulate haematopoietic stem cells and tumourigenesis (Gaspar et al, 2009; Luis et al, 2011). Another possible explanation to the mild (Lin et al, 2008) or absent role of Wg in homeostatic self-renewal may be compensation by other Wnt ligands. Therefore, while Wg is the main ligand required for regeneration, different ligands may compensate for each other during homeostatic maintenance of the tissue. Recent work in the murine small intestine has shown that one of the differentiated progeny of ISCs, the Paneth cell, expresses multiple growth factors such as EGFs and Wnt3 and are sufficient to drive the formation of ‘crypt-like’ structures from single Lgr5-expressing ISCs in vitro (Sato et al, 2011). Paneth cells have therefore been proposed as an important component of the Wnt ISC niche. In vivo work involving ablation of Paneth cells suggests the presence of potential compensatory mechanisms during homeostasis (Durand et al, 2012; Kim et al, 2012), while their role in intestinal regeneration has been suggested (Shroyer et al, 2007). Our work demonstrates that Wg from the transient daughter of the ISC, the EBs, is essential for efficient ISC proliferation during regeneration of damaged midgut epithelium. Therefore, Drosophila EBs could be seen as a functional homologue of the vertebrate Paneth cell and represent an essential component of the ISC niche. In the particular case of Wg, we propose that EBs represent a ‘regeneration-specific ISC niche’. Growth factors such as IL-6/Upds and EGF-like ligands are important components of the Drosophila ISC niche (Beebe et al, 2010; Buchon et al, 2010; Biteau and Jasper, 2011; Jiang et al, 2009, 2011). Intriguingly, the EGF-like ligand Spitz has been shown to be expressed in the small progenitor cells (ISCs/EBs) in the midgut, which is characterized by the expression of escargot (Jiang et al, 2011). Even though direct functional assesment of the role of Spitz and other growth factor from EBs remains to be performed our results strongly point to these cells as a potential general source of factors essential for ISC proliferation.
Previous work suggests that midgut regeneration involves an intricate crosstalk between multiple signalling pathways. JAK/Stat signalling seems to be a central component of this response (Buchon et al, 2009; Jiang et al, 2009; Ren et al, 2010; Shaw et al, 2010; Staley and Irvine, 2010; Jiang et al, 2011). Current work from our group suggests that JAK/Stat signalling is an important mediator of the hyperplastic phenotype resulting from loss of Apc in the Drosophila midgut (Cordero et al, under review). Our results presented here suggest that damage to the midgut results in parallel activation of Wg/Myc and JAK/Stat (Supplementary Figure S8). Knockdown of either pathway does not affect upregulation of the other pathway in response to damage even though midguts are still unable to regenerate. A similar scenario has been reported in the interplay between EGFR and JAK/Stat signalling (Jiang et al, 2011). Therefore, activation of multiple pathways is a necessary condition for proper midgut regeneration (Supplementary Figure S8). Likewise, midgut hyperproliferation in response to ectopic Wg signalling requires Myc and involves concerted activation of EGFR and JAK/Stat (Cordero et al, under review). Consistently, forced overexpression of ectopic Myc only is not sufficient to drive ISC proliferation (Cordero et al, under review) and cannot overcome the absence of other proliferating signals such as JAK/Stat (Supplementary Figure S8) or Wg (not shown) during midgut regeneration.
Homeostasis and regeneration: separation of work?
One important question in the stem cell arena is whether tissue homeostasis and regeneration are controlled by similar mechanisms. Work in the mammalian intestine has shown examples where genes redundant for normal homeostasis are required for intestinal regeneration and Apc-driven intestinal hyperplasia (Ashton et al, 2010). Therefore, the regenerative process cannot be interpreted as a simple acceleration of tissue self-renewal. Consistent with this concept, our work shows that partial reduction of the levels of Wg and Myc prevents ISC hyperproliferation during regeneration and ageing but does not lead to long-term loss of ISCs. Furthermore, components of Wnt signalling such as Pygo are required for intestinal regeneration upon damage but dispensable for ISC proliferation in homeostatic conditions. Therefore, modulating Wg levels could lead to controlled ISC proliferation in conditions of hyperplasia without crossing a threshold that affects tissue integrity.
The Wnt signalling pathway is a central regulator of homeostasis in the mammalian intestine. Inactivating mutations in Wnt pathway components lead to a very rapid loss of intestinal tissue (Korinek et al, 1998; Pinto et al, 2003; Ireland et al, 2004). Our results show that, with the exception of Tcf, knocking down Wnt/Wg signalling has a rather mild and component-dependent role in homeostatic self-renewal of the Drosophila midgut. This is indeed consistent with previous reports (Lin et al, 2008; Xu et al, 2011). Although this scenario may appear at odds with that of the mammalian intestine there are indeed many similarities. In contrast, to genetic ablation of β-Catenin or Tcf4, the impact of Wnt inhibitors such as LRP6 blocking antibodies or Frizzled traps, which partially decrease Wnt signalling is much more subtle with little impact on intestinal homeostasis (DeAlmeida et al, 2007; Ettenberg et al, 2010). In addition, it is also possible that β-Catenin signalling that is independent of ligand may explain the strong phenotype of TcfDN midguts. For example, it is known that the phosphorylation of β-Catenin by AKT/PKB has important roles in mammalian intestinal homeostasis (He et al, 2007; Lee et al, 2010).
Although the similarities between the fly gut and the intestinal systems are often highlighted, intrinsic differences in the rates of homeostatic proliferation are observed between the two systems. The mouse intestine shows a high rate of homeostatic proliferation and undergoes complete self-renewal in 3–4 days (Radtke and Clevers, 2005) while the homeostatic fly midgut is a much more quiescent tissue. Basal proliferation rates in young, undamaged Drosophila midguts are very low and essentially undetectable by simple pH3 staining. Our lineage-tracing experiments show it takes almost a month to achieve complete self-renewal of the midgut epithelium (Figure 3). Therefore, the homeostatic vertebrate intestine could be more comparable to the regenerating fly midgut. On the other hand, the homeostatic Drosophila midgut resembles a lowly proliferative epithelium like that of the mammalian urinary bladder or liver, which both show remarkable regenerative potential and shift from an almost quiescent to a hyperproliferative state in response to injury (Hung et al, 2009; Greenbaum and Wells, 2011). Recent work uncovering an inducible role of Wg in regeneration of the bladder epithelium (Shin et al, 2011) suggests potential general implications of our work. Additionally, one can expect that the intestinal epithelium of flies living in the wild, which is subject to constant challenges, might show higher basal proliferation than that of laboratory animals. Therefore, similarities between the mammalian and Drosophila intestine are likely to out weight their differences.
Materials and methods
Fly maintenance and genetics
Crosses were maintained at 18°C or 22°C in standard medium. Only posterior midguts of female flies were analysed in this study. Animals of the desired genotypes were collected within 48 h of eclosion, and then kept at either 25 or 29°C in incubators with controlled 12 h light-dark cycles. Flies were changed into new food every 2 days. See Supplementary data for further details regarding fly stocks and genotypes used in this study.
Transgene activation by temperature-inducible gal4 and flip out systems
For transgene expression under the gal4/gal80ts system, animals of the desired genotype were selected and allowed to age at 18°C. Three- to five-day-old animals were switched to 29°C to allow Gal4 activity and subsequent expression of the ‘UAS-transgenes’ of interest. The same protocol was applied for initial collection and ageing of animals carrying the inducible ‘escargot flip out’ system (esgts F/O>gfp) (Jiang et al, 2009). Three- to five-day-old animals were then switched to 29°C and their midguts were analysed 2, 14 and 28 days after transgene induction to visualize the newly produced esg cell lineage at 29°C. Additional details on transgene expression times are indicated in the corresponding figure legends.
Clonal analysis
Recombinant clones were generated using the MARCM system (Lee and Luo, 2001). Crosses were maintained at 25°C. Three- to five-day-old adults of the desired genotypes were selected and subject to three 30 min heat shocks at 37°C in 1 day. Flies were then incubated at 25°C for different periods of times.
Histology and tissue analysis
Immunofluorescence. Tissues were dissected in PBS and fixed 30–45 min in 4% para-formaldehyde (Polysciences, Inc.). After fixation, samples were washed three times in PBS+0.1% Triton X-100 (PBST) and incubated in primary antibodies overnight at 4°C. Samples were then washed as described and subjected to secondary antibody staining for 2 h at room temperature followed by washing and mounting on Vectashield containing DAPI (Vector Laboratories, Inc.). Primary and secondary antibodies were incubated in PBST+0.5% BSA. Wg staining was performed as previously described (Lin et al, 2008).
Primary antibodies. Primary antibodies used were chicken anti-GFP 1:4000 (Abcam); mouse anti-Delta 1:20 (Developmental Studies Hybridoma Bank; DSHB), mouse anti-Wg 1:10 (DSHB); rabbit anti-pH3 S10 and S28 1:100 (Cell Signalling); guinea pig anti-Myc 1:100 and pre-absorbed (from G Morata); mouse p-JNK 1:100 (Cell Signalling).
Secondary antibodies. Secondary antibodies used were Alexa 488 1:200 and Alexa 594 1:100 (Invitrogen) and Cy5 1:50 (Jackson Laboratories). Confocal images were captured using the Zeiss 710 Confocal microscope and processed with Adobe photoshop CS to adjust brightness and contrast. Images represent maximal intensity projections of a stable number of Z-Stacks.
Regeneration assays
Experimental flies were collected within 48 h of eclosion at 18°C and moved to 29°C for 14 days on standard media. Flies were then transferred to 5% sucrose (vehicle), 5% sucrose−3% DSS (Fisher Scientific), 5% sucrose–25 μg/ml Bleomycin (Sigma) or 10 × overnight (Pe) culture on filter-paper discs (Whatman) for the last 3, 2 or 1 day of the incubation period. Media was changed daily. Guts were then dissected and analysed using immunofluorescence and confocal imaging. Regeneration experiments including control and all experimental transgenes driven under a common gal4 driver were done in parallel for each of the damaging agents used.
Ageing assay
Experimental flies were collected within 48 h of eclosion at 22°C and moved to 29°C for 30 days on standard media. Adults were moved to new food every 2–3 days. Guts were then dissected and analysed by immunofluorescence and confocal imaging.
Quantifications and statistics
Between 5 and 15 midguts were analysed in each experiment. Results were presented in bar graphs created using Graphpad Prism 5. A combination of t-test and one-way ANOVA with Bonferroni’s multiple comparison test was used to calculate statistical significance.
Quantification of the Delta+ve cells. We counted the total number of Delta+ve cells present within a consistent region of the posterior midgut, which was imaged with a × 63 lens and comprised a field of 0.015 mm2.
Quantification of Wg staining levels. We used Image J to quantify integrated fluorescent intensity from three independent samples for each of the conditions and genotypes analysed. We took all images under a × 63 lens and using identical microscope settings (Figure 6I).
Quantification of ISC proliferation. Total number of pH3+ve cells per posterior midgut was quantified. In the case of fzH51, fz2C1 and pygoS123 clones, ISC proliferation was assessed by the number of cells per clone. To score the number of cell per clone, we determined clonal boundaries and counted the number of nuclei (stained with DAPI) inside each clone.
RNA quantification
Total RNA was extracted from 6 to 10 midguts according to Sanz et al (2010). cDNA synthesis was performed using the High-Capacity cDNA reverse transcription kit (Applied Biosystems). Transcript levels were measured using the primer pairs shown in Supplementary data. RNA extractions were performed from three biological replicates. In the case of regenerating midguts, 5–6 biological replicates were analysed. DNA was analysed in triplicate using the Applied Biosystems 7500. Expression of the target genes was measured relative to that of RpL32 (rp49). A series of 10-fold dilutions of an external standard was used in each run to produce a standard curve. MAXIMA SYBR GREEN Master Mix (Fermentas) was used for qPCR following manufacturer’s instructions. Data were extracted and analysed using Applied Biosystems 7500 software version 2.0 and Prism GraphPad software.
Supplementary Material
Acknowledgments
We are grateful to Bruce Edgar, Peter Gallant, Jean Paul Vincent, Konrad Basler, Gines Morata, Shigeo Hayashi, Steven X Hou, Jun Wu, Marek Mlodzik, Aaron Diantonio, Mariann Bienz and David Bilder for generously sharing fly lines and reagents. We thank VDRC and Bloomington stock centers and the Developmental Studies Hybridoma Bank for providing fly lines and reagents. We apologize to those whose work could not be cited due to space restrictions. MV and OJS are Cancer Research UK investigators. This work was partly funded by an NC3Rs grant. JBC is funded by Marie Curie and EMBO fellowships.
Author contributions: JBC designed, performed and analysed most experiments and wrote the manuscript. RKS optimized damaging protocols for the intestinal regeneration experiments, contributed to regeneration assays, performed qRT–PCRs and wrote the manuscript. AS contributed to regeneration assays and sample collection for qRT–PCRs. MV and OJS designed and supervised experiments and wrote the manuscript. All authors critically read and discussed the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Amcheslavsky A, Ito N, Jiang J, Ip YT (2011) Tuberous sclerosis complex and Myc coordinate the growth and division of Drosophila intestinal stem cells. J Cell Biol 193: 695–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amcheslavsky A, Jiang J, Ip YT (2009) Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell 4: 49–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apidianakis Y, Pitsouli C, Perrimon N, Rahme L (2009) Synergy between bacterial infection and genetic predisposition in intestinal dysplasia. Proc Natl Acad Sci USA 106: 20883–20888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton G, Morton J, Myant K, Phesse T, Ridgway R, Marsh V, Wilkins J, Athineos D, Muncan V, Kemp R, Neufeld K, Clevers H, Brunton V, Winton D, Wang X, Sears R, Clarke A, Frame M, Sansom O (2010) Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c-Myc signaling. Dev Cell 19: 259–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach SP, Chinery R, O’Dwyer ST, Potten CS, Coffey RJ, Watson AJ (2000) Pyrrolidinedithiocarbamate increases the therapeutic index of 5-fluorouracil in a mouse model. Gastroenterology 118: 81–89 [DOI] [PubMed] [Google Scholar]
- Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K (2006) Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell 125: 509–522 [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007 [DOI] [PubMed] [Google Scholar]
- Bartscherer K, Pelte N, Ingelfinger D, Boutros M (2006) Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell 125: 523–533 [DOI] [PubMed] [Google Scholar]
- Beebe K, Lee WC, Micchelli CA (2010) JAK/STAT signaling coordinates stem cell proliferation and multilineage differentiation in the Drosophila intestinal stem cell lineage. Dev Biol 338: 28–37 [DOI] [PubMed] [Google Scholar]
- Bernal NP, Stehr W, Zhang Y, Profitt S, Erwin CR, Warner BW (2005) Evidence for active Wnt signaling during postresection intestinal adaptation. J Pediatr Surg 40: 1025–1029discussion 1029 [DOI] [PubMed] [Google Scholar]
- Biteau B, Hochmuth CE, Jasper H (2008) JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell 3: 442–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, Jasper H (2011) EGF signaling regulates the proliferation of intestinal stem cells in Drosophila. Development 138: 1045–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, Karpac J, Hwangbo D, Jasper H (2011) Regulation of Drosophila lifespan by JNK signaling. Exp Gerontol 46: 349–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Chakrabarti S, Lemaitre B (2009) Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev 23: 2333–2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Kuraishi T, Lemaitre B (2010) Drosophila EGFR pathway coordinates stem cell proliferation and gut remodeling following infection. BMC Biol 8: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Zhang N, Zheng Y, de Wilde RF, Maitra A, Pan D (2010) The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev 24: 2383–2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casali A, Batlle E (2009) Intestinal stem cells in mammals and Drosophila. Cell Stem Cell 4: 124–127 [DOI] [PubMed] [Google Scholar]
- Castilho RM, Squarize CH, Chodosh LA, Williams BO, Gutkind JS (2009) mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell 5: 279–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CM, Struhl G (1999) Wingless transduction by the Frizzled and Frizzled2 proteins of Drosophila. Development 126: 5441–5452 [DOI] [PubMed] [Google Scholar]
- Choi N-H, Kim J-G, Yang D-J, Kim Y-S, Yoo M-A (2008) Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging Cell 7: 318–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero J, Vidal M, Sansom O (2009) APC as a master regulator of intestinal homeostasis and transformation: from flies to vertebrates. Cell Cycle 8: 2926–2931 [PubMed] [Google Scholar]
- Cronin SJF, Nehme NT, Limmer S, Liegeois S, Pospisilik JA, Schramek D, Leibbrandt A, RdM Simoes, Gruber S, Puc U, Ebersberger I, Zoranovic T, Neely GG, von Haeseler A, Ferrandon D, Penninger JM (2009) Genome-wide RNAi screen identifies genes involved in intestinal pathogenic bacterial infection. Science 325: 340–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeAlmeida VI, Miao L, Ernst JA, Koeppen H, Polakis P, Rubinfeld B (2007) The soluble wnt receptor Frizzled8CRD-hFc inhibits the growth of teratocarcinomas in vivo. Cancer Res 67: 5371–5379 [DOI] [PubMed] [Google Scholar]
- Durand A, Donahue B, Peignon G, Letourneur F, Cagnard N, Slomianny C, Perret C, Shroyer NF, Romagnolo B (2012) Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1). Proc Natl Acad Sci USA 109: 8965–8970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg SA, Charlat O, Daley MP, Liu S, Vincent KJ, Stuart DD, Schuller AG, Yuan J, Ospina B, Green J, Yu Q, Walsh R, Li S, Schmitz R, Heine H, Bilic S, Ostrom L, Mosher R, Hartlepp KF, Zhu Z et al. (2010) Inhibition of tumorigenesis driven by different Wnt proteins requires blockade of distinct ligand-binding regions by LRP6 antibodies. Proc Natl Acad Sci USA 107: 15473–15478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodde R (2009) The stem of cancer. Cancer Cell 15: 87–89 [DOI] [PubMed] [Google Scholar]
- Gaspar C, Franken P, Molenaar L, Breukel C, van der Valk M, Smits R, Fodde R (2009) A targeted constitutive mutation in the APC tumor suppressor gene underlies mammary but not intestinal tumorigenesis. PLoS Genet 5: e1000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum LE, Wells RG (2011) The role of stem cells in liver repair and fibrosis. Int J Biochem Cell Biol 43: 222–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XC, Yin T, Grindley JC, Tian Q, Sato T, Tao WA, Dirisina R, Porter-Westpfahl KS, Hembree M, Johnson T, Wiedemann LM, Barrett TA, Hood L, Wu H, Li L (2007) PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet 39: 189–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung CS, Dodson KW, Hultgren SJ (2009) A murine model of urinary tract infection. Nat Protoc 4: 1230–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland H, Kemp R, Houghton C, Howard L, Clarke A, Sansom O, Winton D (2004) Inducible Cre-mediated control of gene expression in the murine gastrointestinal tract: effect of loss of beta-catenin. Gastroenterology 126: 1236–1246 [DOI] [PubMed] [Google Scholar]
- Jessen S, Gu B, Dai X (2008) Pygopus and the Wnt signaling pathway: a diverse set of connections. Bioessays 30: 448–456 [DOI] [PubMed] [Google Scholar]
- Jiang H, Edgar BA (2009) EGFR signaling regulates the proliferation of Drosophila adult midgut progenitors. Development 136: 483–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Edgar BA (2012) Intestinal stem cell function in Drosophila and mice. Curr Opin Genet Dev (advance online publication, 18 May 2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Grenley MO, Bravo MJ, Blumhagen RZ, Edgar BA (2011) EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell Stem Cell 8: 84–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA (2009) Cytokine/JAK/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 137: 1343–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LA, Prober DA, Edgar BA, Eisenman RN, Gallant P (1999) Drosophila myc regulates cellular growth during development. Cell 98: 779–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpowicz P, Perez J, Perrimon N (2010) The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development 137: 4135–4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Escudero S, Shivdasani RA (2012) Intact function of Lgr5 receptor-expressing intestinal stem cells in the absence of Paneth cells. Proc Natl Acad Sci USA 109: 3932–3937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hedge P, McKechnie D, Finniear R, Markham A, Groffen J, Boguski MS, Altshul FS, Horii A, Ando H, Miyoshi Y, Miki Y, Nishisho I et al. (1991) Identification of FAP locus genes from chromosome 5q21. Science 253: 661–665 [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H (1998) Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet 19: 379–383 [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H (1997) Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science 275: 1784–1787 [DOI] [PubMed] [Google Scholar]
- Kramps T, Peter O, Brunner E, Nellen D, Froesch B, Chatterjee S, Murone M, Zullig S, Basler K (2002) Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell 109: 47–60 [DOI] [PubMed] [Google Scholar]
- Lee G, Goretsky T, Managlia E, Dirisina R, Singh AP, Brown JB, May R, Yang GY, Ragheb JW, Evers BM, Weber CR, Turner JR, He XC, Katzman RB, Li L, Barrett TA (2010) Phosphoinositide 3-kinase signaling mediates beta-catenin activation in intestinal epithelial stem and progenitor cells in colitis. Gastroenterology 139: 869–881881.e861–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Luo L (2001) Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci 24: 251–254 [DOI] [PubMed] [Google Scholar]
- Lee W-C, Beebe K, Sudmeier L, Micchelli CA (2009) Adenomatous polyposis coli regulates Drosophila intestinal stem cell proliferation. Development 136: 2255–2264 [DOI] [PubMed] [Google Scholar]
- Lin G, Xi R (2008) Intestinal stem cell, muscular niche and Wingless signaling. Fly (Austin) 2: 310–312 [DOI] [PubMed] [Google Scholar]
- Lin G, Xu N, Xi R (2008) Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature 455: 1119–1123 [DOI] [PubMed] [Google Scholar]
- Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T (2007) Augmented Wnt signaling in a mammalian model of accelerated aging. Science 317: 803–806 [DOI] [PubMed] [Google Scholar]
- Luis TC, Naber BA, Roozen PP, Brugman MH, de Haas EF, Ghazvini M, Fibbe WE, van Dongen JJ, Fodde R, Staal FJ (2011) Canonical wnt signaling regulates hematopoiesis in a dosage-dependent fashion. Cell Stem Cell 9: 345–356 [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N (2006) Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439: 475–479 [DOI] [PubMed] [Google Scholar]
- Nystul T, Spradling A (2006) Breaking out of the mold: diversity within adult stem cells and their niches. Curr Opin Genet Dev 16: 463–468 [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A (2006) The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439: 470–474 [DOI] [PubMed] [Google Scholar]
- O’Brien LE, Soliman SS, Li X, Bilder D (2011) Altered modes of stem cell division drive adaptive intestinal growth. Cell 147: 603–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker DS, Jemison J, Cadigan KM (2002) Pygopus, a nuclear PHD-finger protein required for Wingless signaling in Drosophila. Development 129: 2565–2576 [DOI] [PubMed] [Google Scholar]
- Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, Lehr HA, Hirth S, Weigmann B, Wirtz S, Ouyang W, Neurath MF, Becker C (2009) STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med 206: 1465–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D, Gregorieff A, Begthel H, Clevers H (2003) Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev 17: 1709–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke F, Clevers H (2005) Self-renewal and cancer of the gut: two sides of a coin. Science 307: 1904–1909 [DOI] [PubMed] [Google Scholar]
- Ren F, Wang B, Yue T, Yun E-Y, Ip YT, Jiang J (2010) Hippo signaling regulates Drosophila intestine stem cell proliferation through multiple pathways. Proc Natl Acad Sci USA 107: 21064–21069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom OJ, Meniel VS, Muncan V, Phesse TJ, Wilkins JA, Reed KR, Vass JK, Athineos D, Clevers H, Clarke AR (2007) Myc deletion rescues Apc deficiency in the small intestine. Nature 446: 676–679 [DOI] [PubMed] [Google Scholar]
- Sanz A, Soikkeli M, Portero-Otín M, Wilson A, Kemppainen E, McIlroy G, Ellilä S, Kemppainen KK, Tuomela T, Lakanmaa M, Kiviranta E, Stefanatos R, Dufour E, Hutz B, Naudí A, Jové M, Zeb A, Vartiainen S, Matsuno-Yagi A, Yagi T et al. (2010) Expression of the yeast NADH dehydrogenase Ndi1 in Drosophila confers increased lifespan independently of dietary restriction. Proc Natl Acad Sci USA 107: 9105–9110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, van Es J, Snippert H, Stange D, Vries R, van den Born M, Barker N, Shroyer N, van de Wetering M, Clevers H (2011) Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469: 415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RL, Kohlmaier A, Polesello C, Veelken C, Edgar BA, Tapon N (2010) The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Development 137: 4147–4158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K, Lee J, Guo N, Kim J, Lim A, Qu L, Mysorekar IU, Beachy PA (2011) Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature 472: 110–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroyer NF, Helmrath MA, Wang VY, Antalffy B, Henning SJ, Zoghbi HY (2007) Intestine-specific ablation of mouse atonal homolog 1 (Math1) reveals a role in cellular homeostasis. Gastroenterology 132: 2478–2488 [DOI] [PubMed] [Google Scholar]
- Staley BK, Irvine KD (2010) Warts and Yorkie mediate intestinal regeneration by influencing stem cell proliferation. Curr Biol 20: 1580–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B, Townsley F, Rosin-Arbesfeld R, Musisi H, Bienz M (2002) A new nuclear component of the Wnt signalling pathway. Nat Cell Biol 4: 367–373 [DOI] [PubMed] [Google Scholar]
- Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ (2011) A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478: 255–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumpp A, Refaeli Y, Oskarsson T, Gasser S, Murphy M, Martin GR, Bishop JM (2001) c-Myc regulates mammalian body size by controlling cell number but not cell size. Nature 414: 768–773 [DOI] [PubMed] [Google Scholar]
- van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, Peifer M, Mortin M, Clevers H (1997) Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88: 789–799 [DOI] [PubMed] [Google Scholar]
- van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis A, Tjon-Pon-Fong M, Moerer P, van den Born M, Soete G, Pals S, Eilers M, Medema R, Clevers H (2002) The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111: 241–250 [DOI] [PubMed] [Google Scholar]
- Vermeulen L, De Sousa EMF, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, Sprick MR, Kemper K, Richel DJ, Stassi G, Medema JP (2010) Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol 12: 468–476 [DOI] [PubMed] [Google Scholar]
- Weston CR, Davis RJ (2007) The JNK signal transduction pathway. Curr Opin Cell Biol 19: 142–149 [DOI] [PubMed] [Google Scholar]
- Xu N, Wang SQ, Tan D, Gao Y, Lin G, Xi R (2011) EGFR, Wingless and JAK/STAT signaling cooperatively maintain Drosophila intestinal stem cells. Dev Biol 354: 31–43 [DOI] [PubMed] [Google Scholar]
- Zeng X, Chauhan C, Hou SX (2010) Characterization of midgut stem cell- and enteroblast-specific Gal4 lines in Drosophila. Genesis 48: 607–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.