Abstract
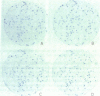
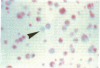
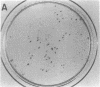
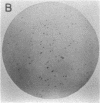
Single somatic cells, including fibroblasts, myelomas, and hybridomas, proliferate normally when trapped between a plastic dish and a disc of polyester cloth. Contact between the overlay and the plastic for 8-16 days results in identical colony patterns on the cloth and the plate. When several cloth discs are simultaneously stacked over Chinese hamster ovary cells, three or four-high resolution colony copies can be generated from a single master dish. The colonies on the cloth can be analyzed by radiochemical methods [Esko, J. D. & Raetz, C. R. H. (1978) Proc. Natl. Acad. Sci. USA 75, 1190-1193] or by "replica plating" to a new disc. The use of polyester cloth, singly or in stacks, has several major advantages over previous techniques for somatic cell replica plating, including: (i) broad applicability to diverse cell lines such as fragile membrane mutants of Chinese hamster ovary cells and relatively nonadherent myelomas or hybridomas; (ii) the possibility of generating multiple copies of the same colony population, allowing simultaneous analysis for several enzymes or cellular components; and (iii) superior resolution and transfer efficiency in copying colony patterns from one surface to another. The remarkable capacity of animal cell colonies to proliferate upward through "polyester stacks" may reflect chemotropic movement of individual cells and opens new approaches to somatic cell genetics.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Busch D. B., Cleaver J. E., Glaser D. A. Large-scale isolation of UV-sensitive clones of CHO cells. Somatic Cell Genet. 1980 May;6(3):407–418. doi: 10.1007/BF01542792. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esko J. D., Raetz C. R. Autoradiographic detection of animal cell membrane mutants altered in phosphatidylcholine synthesis. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5192–5196. doi: 10.1073/pnas.77.9.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esko J. D., Raetz C. R. Mutants of Chinese hamster ovary cells with altered membrane phospholipid composition. Replacement of phosphatidylinositol by phosphatidylglycerol in a myo-inositol auxotroph. J Biol Chem. 1980 May 25;255(10):4474–4480. [PubMed] [Google Scholar]
- Esko J. D., Raetz C. R. Replica plating and in situ enzymatic assay of animal cell colonies established on filter paper. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1190–1193. doi: 10.1073/pnas.75.3.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esko J. D., Wermuth M. M., Raetz C. R. Thermolabile CDP-choline synthetase in an animal cell mutant defective in lecithin formation. J Biol Chem. 1981 Jul 25;256(14):7388–7393. [PubMed] [Google Scholar]
- Firestone G. L., Heath E. C. The cyclic AMP-mediated induction of alkaline phosphatase in mouse L-cells. J Biol Chem. 1981 Feb 10;256(3):1396–1403. [PubMed] [Google Scholar]
- Hirschberg C. B., Baker R. M., Perez M., Spencer L. A., Watson D. Selection of mutant Chinese hamster ovary cells altered glycoproteins by means of tritiated fucose suicide. Mol Cell Biol. 1981 Oct;1(10):902–909. doi: 10.1128/mcb.1.10.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JERNE N. K., NORDIN A. A. Plaque formation in agar by single antibody-producing cells. Science. 1963 Apr 26;140(3565):405–405. [PubMed] [Google Scholar]
- Jerne N. K., Henry C., Nordin A. A., Fuji H., Koros A. M., Lefkovits I. Plaque forming cells: methodology and theory. Transplant Rev. 1974;18:130–191. doi: 10.1111/j.1600-065x.1974.tb01588.x. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- Polokoff M. A., Wing D. C., Raetz C. R. Isolation of somatic cell mutants defective in the biosynthesis of phosphatidylethanolamine. J Biol Chem. 1981 Aug 10;256(15):7687–7690. [PubMed] [Google Scholar]
- Robb J. A. Microcloning and replica plating of mammalian cells. Science. 1970 Nov 20;170(3960):857–858. doi: 10.1126/science.170.3960.857. [DOI] [PubMed] [Google Scholar]
- Robbins A. R. Isolation of lysosomal alpha-mannosidase mutants of Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1911–1915. doi: 10.1073/pnas.76.4.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon J., Morrison S. L., Kabat E. A. Formation of hybridoma clones in soft agarose: effect of pH and of medium. Somatic Cell Genet. 1980 May;6(3):435–441. doi: 10.1007/BF01542794. [DOI] [PubMed] [Google Scholar]
- Siminovitch L. On the nature of hereditable variation in cultured somatic cells. Cell. 1976 Jan;7(1):1–11. doi: 10.1016/0092-8674(76)90249-x. [DOI] [PubMed] [Google Scholar]
- Thompson L. H. Mutant isolation. Methods Enzymol. 1979;58:308–322. doi: 10.1016/s0076-6879(79)58147-6. [DOI] [PubMed] [Google Scholar]