Abstract
Background. The nephroprotective effect of vitamins E and C or losartan against cisplatin (CP)- induced nephrotoxicity when they are accompanied by estrogen was investigated. Methods. The ovariectomized rats received estradiol valerate for two weeks. At the end of the first week, a single dose of CP (7 mg/kg, IP) was also administered, and they received placebo (group 1), vitamin E (group 2), vitamin C (group 3), or losartan (group 4) every day during the second week, and they were compared with another three control groups. Results. CP alone increased the serum levels of blood urea nitrogen (BUN), creatinine (Cr), and kidney tissue damage score (KTDS), significantly (P < 0.05), however at the presence of estradiol and CP, vitamin C, vitamin E, or losartan not only did not decrease these parameters, but also increased them significantly (P < 0.05). The serum level of superoxidase dismutase (SOD) was reduced by CP (P < 0.05), but it was increased when estradiol or estradiol plus vitamin C or losartan were added (P < 0.05). Conclusion. The particular pharmacological dose of estrogen used in this study abolish the nephroprotective effects vitamins C and E or losartan against CP-induced nephrotoxicity.
1. Introduction
Nephrotoxicity is the major adverse effect of cisplatin (CP) in about 30 percent of patients during chemotherapy in clinic due to tubular toxicity, inflammation, oxidative stress, and change in the renal circulation [1–4], and it is a limitation for CP use. To avoid this side effect and based on different experimental designs, protective role of administration of many synthetic or herbal agents as supplementation has been investigated against CP-induced nephrotoxicity [5–34], including vitamins E and C and losartan as antioxidant agents [5, 6, 22, 23]. During CP chemotherapy, plasma antioxidant concentrations decrease, and in human subject coadministrations of vitamin C, vitamin E, and selenium markedly increase plasma antioxidant levels [35]. In addition, the protective role of vitamin E supplementation against CP-induced peripheral neuropathy in patients was evaluated, and its neuroprotective role was reported [36, 37]. Although vitamins C and E and losartan have antioxidant effects [22, 23, 38, 39], losartan itself acts as a blocker of angiotensin II receptor 1 (ATR1), which facilitates renal circulation [40, 41]. The protective effect of losartan against CP-induced nephrotoxicity was reported before [5, 6], and it has no effect on CP uptake by the kidney [6]. However, acute administration of losartan does not affect the CP-induced kidney toxicity [5].
The protective role of estrogen in cardiovascular system in women before menopause is well documented [42–47]. The incidence of chronic renal diseases is also gender related and is potentially higher in males [48, 49], which may relate to estrogen-mediated vascular endothelial growth factor [48]. It is reported that estrogen receptors may be disturbed by CP, the steroid hormone increases the potency of CP [50], and estrogen may enhance oxidative stress in the kidney [51]. Therefore, not only the protective role of estrogen against CP-induced nephrotoxicity may be failed, but also its adverse side effect in CP-induced kidney toxicity became a question. Recently, we have compared the CP-induced kidney damage in male and female rats, and demonstrated that the CP-induced nephrotoxicity is gender related [52]. The protective role of losartan against CP-induced nephrotoxicity also indicates that losartan as an antioxidant is nephroprotectant in males but not in females [53]. So, one question is raised about the role of female sex hormone in this gender-based difference. Accordingly, we hypothesized that administration of estrogen and CP together promotes the potency of CP-induced nephrotoxicity, which abolishes the nephroprotective effects of antioxidants such as vitamins C and E and losartan. To test this hypothesis and in the first step, the effect of estrogen and its combination with vitamin E, vitamin C, or losartan in CP-induced nephrotoxicity should be determined. Therefore, ovariectomized rats were treated with the pharmacological dose of estradiol valerate. Then, particular doses of vitamin C, vitamin E, or losartan plus single dose of CP were administered, and the results were compared with those obtained from the control groups.
2. Materials and Methods
2.1. Animals
Forty-two adult female Wistar rats (Animal Centre, Ahvaz University of Medical Sciences, Ahvaz, Iran) with the mean weight of 182 ± 2.7 g were used. The rats were individually housed at a temperature of 23–25°C. Rats had free access to water and chow. The experimental procedures were in advance approved by the Isfahan University of Medical Sciences Ethics Committee.
2.2. Drugs
CP (cis-diammineplatinum (II) dichloride, code P4394), vitamin E, and vitamin C were purchased from Sigma (St. Louis, MO, USA). Estradiol valerate was obtained from Aburaihan Co. (Tehran, Iran). Losartan was provided by Darupakhsh Pharmaceutical Company (Tehran, Iran).
2.3. Experimental Protocol
The animals were anesthetized by injection of 75 mg/kg, IP ketamine. An incision was made in the subabdominal region. The abdominal muscles were opened. The uterus tube and vascular base of ovaries were twisted, and the ovaries were removed. The muscles and skin were stitched backed into the place. After recovery, the animals were allowed to acclimatize to the same diet at least for one week. Then, they were randomly divided into seven experimental groups as follows.
Group 1: 2.5 mg/kg/week estradiol valerate in sesame oil was injected intramuscular for two weeks, and at the end of the first week, blood sample was obtained and a single dose of CP (7 mg/kg, IP) was also administered. Then, the animals received 0.5 mL/rat/day vehicle (saline) during the second week.
Groups 2 to 4: the groups had regimen the same as group 1, except that vitamin E (1 g/kg/day, group 2), vitamin C (250 mg/kg/day, group 3), or losartan (10 mg/kg/day, group 4) was administered instead of vehicle during the second week. Blood samples were also obtained at the end of the first week.
Group 5 (positive control) received saline for two weeks, and at the end of the first week, blood sample was obtained. At the end of the first week, a single dose of CP alone was administered. Similarly, group 6 was treated by estradiol alone (2.5 mg/kg/week estradiol valerate for two weeks), and group 7 (negative control) received vehicle alone during the experiment.
At the end of the experiment (one week after CP injection), blood samples were obtained again and the rats were sacrificed by anaesthesia drug overdose. Kidneys and uterus were removed and weighted immediately, and the left kidney was prepared for histopathological procedures. Summary of the experimental protocol for each group of experiment is provided in Table 1.
Table 1.
Summary of the treatment in the groups. Estradiol was injected at the beginning of each week. CP was administrated as a single dose at the beginning of the second week. The vitamins and losartan were injected daily during the second week.
| Group | First week | Second week |
|---|---|---|
| 1 | Estradiol | Estradiol + CP + saline |
| 2 | Estradiol | Estradiol + CP + vitamin E |
| 3 | Estradiol | Estradiol + CP + vitamin C |
| 4 | Estradiol | Estradiol + CP + losartan |
| 5 | Saline | CP |
| 6 | Estradiol | Estradiol |
| 7 | Saline | Saline |
2.4. Measurements
Body weight of the animals was recorded daily. The levels of serum creatinine (Cr), blood urea nitrogen (BUN), and magnesium (Mg) were determined using quantitative diagnostic kits (Pars Azmoon, Tehran, Iran). Serum level of nitrite (stable NO metabolite) was measured using a colorimetric ELISA kit (Promega Corporation, USA) that involves the Griess reaction. Serum level of estradiol was measured using enzyme immunoassay ELISA kit (Diagnostics Biochem Canada Inc., Canada). The serum level of superoxidase dismutase (SOD) was determined by a double-antibody sandwich enzyme-linked immunosorbent assay ELISA kits (Glory Science Co., USA). For each of the above-mentioned parameters, the concentration difference (Δ) was defined and calculated as (end of the second week (one week after CP administration) concentration − end of the first week (before CP administration) concentration).
2.5. Histopathological Procedures
The removed kidneys were fixed in 10% neutral formalin solution and embedded in paraffin for hematoxylin and eosin staining to examine the tubular damage. The damage was evaluated by two independent pathologists who were totally blind to the study. Based on the intensity of tubular lesions (hyaline cast, debris, vacuolization, flattening and degeneration of tubular cells, and dilatation of tubular lumen), kidney tissue damage score (KTDS) was graded from 1 to 4, while score zero was assigned to normal tubules without any damage.
2.6. Statistical Analysis
Data are expressed as mean ± SEM. The percentage of changes in the body weight, the difference (after-before, Δ) of BUN, Cr, Mg, SOD, estradiol, and nitrite serum levels, and the kidney and the uterus weights in groups 1–4, 6, and 7 were compared with group 5 (the positive control group) using Student's t-test. The same comparison was applied to the KTDS using Mann-Whitney analysis. The P values ≤0.05 were considered statistically significant.
3. Results
The number of animals in each group and animals survived in the seven experiment groups are shown in Table 2. The mortality rate was more than 50 percent in groups 3 and 4 (Table 1). No mortality rate was observed in others groups.
Table 2.
The mortality rate of animals in each group during the second week of the experiment.
| Group | N | Day | n | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| Number 1: estradiol + CP | 6 | — | — | — | — | — | — | — | 6 |
| Number 2: estradiol + CP + vitamin E | 7 | — | — | — | — | — | — | — | 7 |
| Number 3: estradiol + CP + vitamin C | 7 | — | — | — | — | 1 | 2 | 1 | 3 |
| Number 4: estradiol + CP + losartan | 7 | — | — | — | — | 1 | 1 | 2 | 3 |
| Number 5: CP + vehicle (positive control) | 5 | — | — | — | — | — | — | — | 5 |
| Number 6: estradiol + vehicle (negative control) | 5 | — | — | — | — | — | — | — | 5 |
| Number 7: vehicle (negative control) | 5 | — | — | — | — | — | — | — | 5 |
N: total number of animals at the beginning of experiment. All animals were ovariectomized, n: number of animals at the end of the experiment, CP: cisplatin. CP was administered at the beginning of the second week.
3.1. Effect of CP: Comparison between Positive (Group 5) and Negative Control (Group 6 and 7) Groups
The serum difference levels (one week after CP administration-before CP administration; Δ) of BUN, Cr, Mg, SOD, estradiol, and nitrite; the kidney and the uterus weights; the KTDS in vehicle alone, estradiol alone, or CP alone (the positive control group) treated ovariectomized groups are demonstrated in Figure 1.
Figure 1.
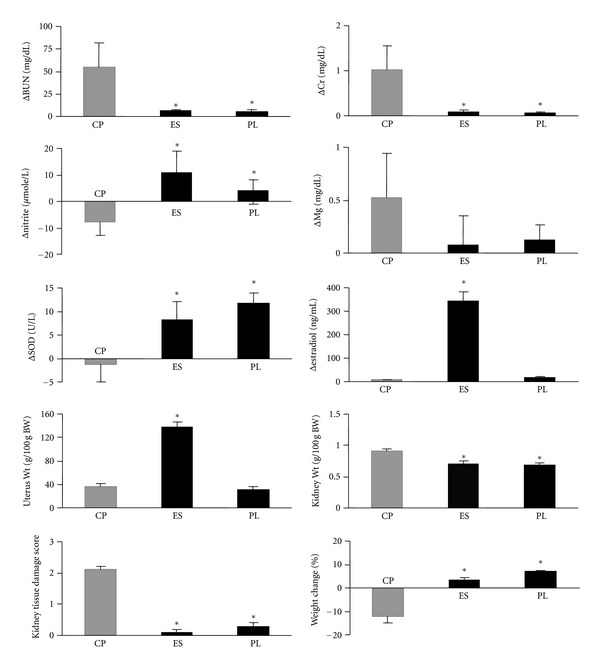
The serum difference levels (one week after CP administration − before CP administration; Δ) of BUN, Cr, Mg, SOD, estradiol, and nitrite; the kidney and the uterus weights; the KTDS in vehicle, estradiol, or CP (the positive control group) alone-treated ovariectomized groups. The star indicates significant difference (P < 0.05) when compared with the positive control groups. CP, ES, and PL on the horizontal axes stand for groups treated with CP (group 5), estradiol valerate (group 6), and vehicle (placebo, group 7), respectively.
The serum levels of BUN and Cr, and KTDS significantly increased in the positive control group (P < 0.05) when compared with negative control groups (groups 6 and 7). This finding confirms kidney toxicity induced by CP. No significant differences were observed in the serum Mg levels between positive and negative control groups; indicating that no Mg depletion occurred by CP during one-week treatment with CP. Administration of CP reduced the serum levels of nitrite and SOD, while increase of these parameters was observed in the negative control groups (P < 0.05). The serum level of estradiol and uterus weigh in group 6 increased significantly (P < 0.05) when compared with groups 5 and 7; indicating that estradiol administration increases the blood estrogen level.
3.2. Effect of Vitamin C, Vitamin E, or Losartan: Comparison between Positive Control (Group 5) and Case Groups (Groups 1, 2, 3, and 4)
The serum difference levels (one week after CP administration-before CP administration; Δ) of BUN, Cr, Mg, SOD, estradiol, and nitrite; the kidney and the uterus weights; KTDS in estradiol and CP (group 1), estradiol plus vitamin E and CP (group 2), estradiol plus vitamin C and CP (group 3), estradiol plus losartan and CP (group 4) treated ovariectomized groups in comparison with ovariectomized rats treated with CP alone (the positive control group; group 5) are demonstrated in Figure 2. At the presence of estradiol, vitamin C, vitamin E, or losartan not only did not decrease the serum levels of BUN and Cr, and kidney weight or KTDS in ovariectomized rats, but also significantly increased these parameters when compared with the positive control group (P < 0.05). No significant differences were observed in Δnitrite, ΔMg, and percentage change of weight between groups 1 to 5. The serum level of SOD in CP-treated rats was significantly increased by estradiol, estradiol plus vitamin C, and estradiol plus losartan (P < 0.05). Such finding was not observed for estradiol plus vitamin E.
Figure 2.
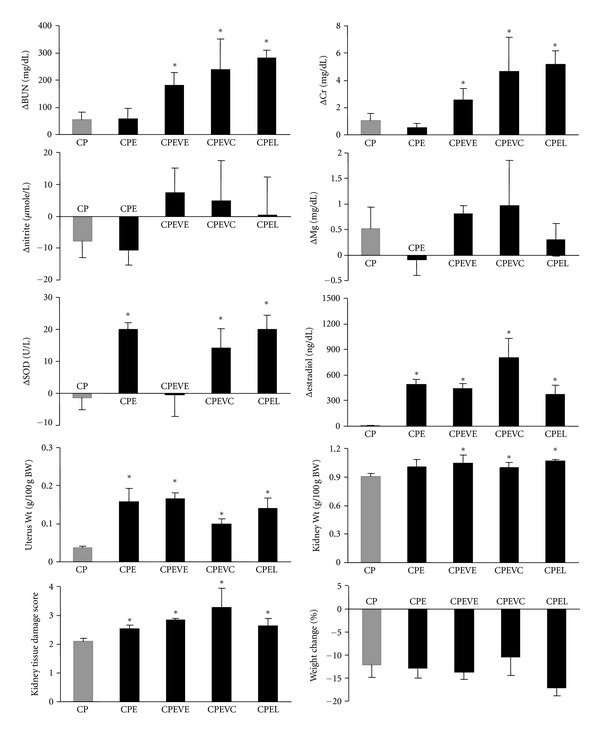
Serum difference levels (one week after CP administration − before CP administration; Δ) of BUN, Cr, Mg, SOD, estradiol, and nitrite; the kidney and the uterus weights; and KTDS in estradiol and CP, estradiol plus vitamin E and CP, estradiol plus vitamin C and CP, estradiol plus losartan and CP-treated ovariectomized groups (group 1–4) compared with ovariectomized rats treated with CP alone (positive control group). CP, CPE, CPEVE, CPEVC, and CPEL on the horizontal axes stand for groups treated with CP alone (group 5), estradiol and CP (group 1), estradiol plus vitamin E and CP (group 2), estradiol plus vitamin C and CP (group 3), and estradiol plus losartan and CP (group 4), respectively. The star indicates significant difference (P < 0.05) when compared with the positive control group.
According to these findings, the vitamins or losartan has no beneficial effects against CP-induced nephrotoxicity when estradiol is present. Samples of kidney tissue images from each experimental group are shown in Figure 3.
Figure 3.
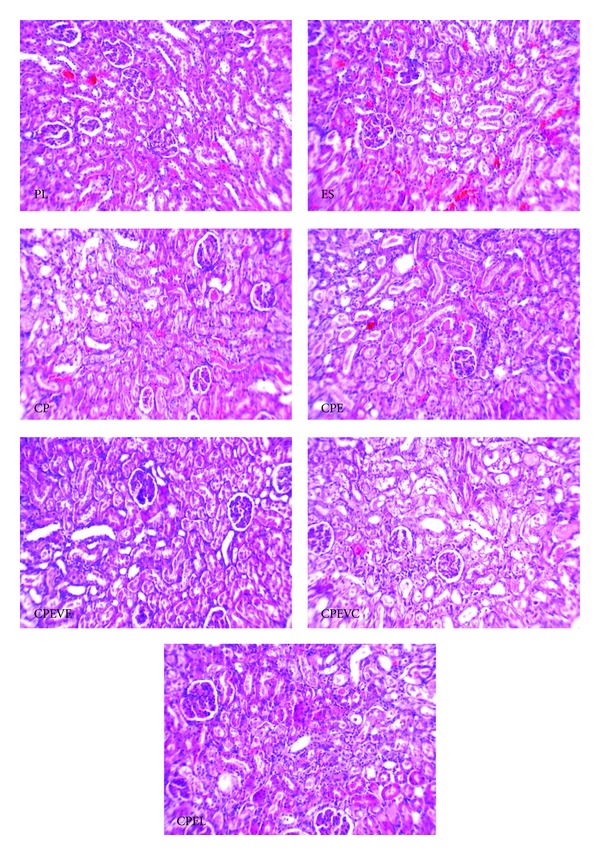
Images (magnification ×100) of kidney tissue. PL, ES, CP, CPE, CPEVE, CPEVC, and CPEL inside the images stand for groups treated with vehicle (placebo, group 7), estradiol valerate (group 6), CP alone (group 5), estradiol and CP (group 1), estradiol plus vitamin E and CP (group 2), estradiol plus vitamin C and CP (group 3), and estradiol plus losartan and CP (group 4), respectively. No tissue damages were seen in PL and ES, but higher and almost similar tissue damages were observed in other groups.
4. Discussion
Our findings indicate that coadministration of vitamin C, vitamin E, or losartan with estradiol in female rats has no protective effect on the onset or severity of nephrotoxicity induced by CP, although previous reports indicated nephroprotectant effects for vitamins and ATR1 blocker, losartan, against CP-induced nephrotoxicity [5, 6, 22, 23]. The incidence rates of both cardiovascular and renal diseases are gender related and are higher in males. In the cardiovascular system, protective role of estrogen before menopause is well known [44–46]. However, estrogen may enhance oxidative stress in the kidney [51], and the oxidative stress induced by estrogen will enhance CP-induced injury to the tubules. In this study, we demonstrated for the first time that estrogen did not reduce the severity of nephrotoxicity, and actually it abolished the regular protective effect of antioxidants such as vitamin C, vitamin E, and losartan against CP-induced nephrotoxicity [34, 54–56], despite that estrogen itself has an antioxidant effect too. Instead of physiological dose of estrogen, we used the pharmacological dose of estradiol to establish the effect of estradiol first, and the result of this study strongly suggests examining the dose response of estradiol for future studies.
4.1. CP, BUN, Cr, Kidney Weight, Body Weight, Estrogen, and KTDS
According to the serum levels of BUN and Cr, and pathology damage score, nephrotoxicity was verified in all CP-treated animals (Figure 2). CP also caused weight reduction similar to our previous study [34]. The uterus weight increased in all estradiol-treated groups due to the presence of estradiol. The kidney tissue weight increases in CP-induced nephrotoxicity [57], and our findings were similar in this study. The KTDS in CP plus estradiol-treated groups either with or without vitamins or losartan was significantly greater than that in CP alone treated group. This data reveals the more significant role of coadministration of estrogen that abolishes the protective effect of vitamins or losartan against CP-induced nephrotoxicity. There are two possible explanations for the observation. Estrogen and CP may have two different effects on endothelial nitric oxide (NO). The first increases NO, and the second decreases the level [58–62]. These two opposite effects may restore the serum level of NO. However, estrogen-induced NO may enhance the severity of nephrotoxicity [62]. The second explanation is related to formation of oxidative stress induced by ovariectomy [63] and by CP administration [3]. These oxidative stresses play an effective role to promote the kidney injury.
4.2. CP, Estrogen, and NO
CP alone causes dose-dependent declines in glomerular filtration rate (GFR) and renal blood flow due to renal vascular resistance changes [1, 4]. This phenomenon may limit CP transport to the kidney circulation. On the other hand, estrogen promotes formation of vasodilator biomarkers such as NO [58, 60, 61]. Our findings are in agreement with this item, as the serum level of NO metabolite (nitrite) elevated in the estrogen alone-treated group (Figure 1). NO involves in the process of CP-induced nephrotoxicity [64], and CP increases the level of inducible NO synthase [65] and decreases the level of endothelial NO synthase [59]. In addition, enhancement of NO level could increase the severity of toxicity [62]. It is also reported that inhibition of NO synthase aggravates CP-induced nephrotoxicity [57, 66]. Therefore, coadministration of CP and estrogen may promote the severity of toxicity due to estrogen-induced NO production. Another factor to consider is the availability of estrogen receptors in kidneys. Estradiol possibly sensitizes tubular cells to CP, similar to what happens in breast cancer cells [50], and promotes the tissue toxicity.
4.3. CP, Estrogen, and Oxidative Stress
It is well documented that abnormal production of reactive oxygen molecules, called oxidative stress, is involved in CP-induced nephrotoxicity, and CP produces renal oxidative stress, which disturbs renal function due to its toxicity [67]. Vitamins C and E reduce the level of SOD in CP-induced nephrotoxicity model [68]. Losartan also has antioxidant effect, and it may improve the renal function and decrease the severity of CP-induced nephrotoxicity [5, 6]. Estrogen also has antioxidant effect [54–56], and similar to vitamin E can modulate oxidative-stress-induced kidney toxicity [69, 70]. This is while other research indicated that estrogen enhances the oxidative stress in kidney [51]. In our study, the serum level of SOD was decreased by CP (Figure 1), and it was increased when estrogen alone or in combination of estrogen and vitamin C or losartan was administered. Opposite to these findings, Ulas and Cay [69] studied diabetic rat model, and no increment of SOD was detected in estradiol plus vitamin E treated rats. The level of serum SOD in ovariectomized rats treated either by estradiol or combination of estradiol and vitamin E was reported to be higher than nontreated ovariectomized animals [71], and this supplementation reduces lipid peroxidation [69]. But on the other hand, CP reduced the SOD level as an antioxidant defense system [72]. Considering our results, it seems that CP abolishes the antioxidant defense system induced by vitamin E and possibly estradiol due to possible pharmacokinetic or pharmacodynamic drug interactions.
4.4. CP, Estrogen, and Mg
The serum levels of Mg in CP-treated groups were not statistically different. CP-induced nephrotoxicity disturbs tubular reabsorption of Mg, and depletion of Mg enhances nephrotoxicity [73–76]. The CP-induced hypomagnesemia is not related to the total dose of CP [77], and in animal model, hypomagnesemia develops from the third week after CP administration [78]. Furthermore, an increase in the serum Mg level after supplemental treatment is not expected [79], and the serum level of Mg did not change in ovariectomized rats treated with estradiol and vitamin E [71]. Accordingly, it seems that one week was not enough to observe Mg depletion by CP in our model.
5. Conclusion
In the presence of estradiol in the particular pharmacological dose used in the study, vitamins C and E or losartan could not be nephroprotectant against CP-induced nephrotoxicity. The exact mechanisms need to be defined, but it seems that coadministration of the agents with CP in ovariectomized rats promotes the oxidative stress and potency of CP, which contribute to nephrotoxicity severity. This contribution abolishes protective effect of the vitamins and losartan.
Acknowledgments
This research was supported by Isfahan University of Medical Sciences (Grant no. 290223). The authors thank Dr. Alireza Rezaei for his assistance.
References
- 1.Winston JA, Safirstein R. Reduced renal blood flow in early cisplatin-induced acute renal failure in the rat. The American Journal of Physiology . 1985;249(4):F490–F496. doi: 10.1152/ajprenal.1985.249.4.F490. [DOI] [PubMed] [Google Scholar]
- 2.Ramesh G, Brian Reeves W. TNF-α mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. Journal of Clinical Investigation . 2002;110(6):835–842. doi: 10.1172/JCI15606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis CA, Nick HS, Agarwal A. Manganese superoxide dismutase attenuates cisplatin-induced renal injury: importance of superoxide. Journal of the American Society of Nephrology . 2001;12(12):2683–2690. doi: 10.1681/ASN.V12122683. [DOI] [PubMed] [Google Scholar]
- 4.Luke DR, Vadiei K, Lopez-Berestein G. Role of vascular congestion in cisplatin-induced acute renal failure in the rat. Nephrology Dialysis Transplantation . 1992;7(1):1–7. [PubMed] [Google Scholar]
- 5.Deegan PM, Nolan C, Ryan MP, Basinger MA, Jones MM, Hande KR. The role of the renin-angiotensin system in cisplatin nephrotoxicity. Renal Failure . 1995;17(6):665–674. doi: 10.3109/08860229509037634. [DOI] [PubMed] [Google Scholar]
- 6.Saleh S, Ain-Shoka AA, El-Demerdash E, Khalef MM. Protective effects of the angiotensin II receptor blocker losartan on cisplatin-induced kidney injury. Chemotherapy . 2009;55(6):399–406. doi: 10.1159/000262453. [DOI] [PubMed] [Google Scholar]
- 7.Naqshbandi A, Khan MW, Rizwan S, Rehman SU, Khan F. Studies on the protective effect of dietary fish oil on cisplatin induced nephrotoxicity in rats. Food and Chemical Toxicology . 2012;50(2):265–273. doi: 10.1016/j.fct.2011.10.039. [DOI] [PubMed] [Google Scholar]
- 8.Ashrafi F, Nematbakhsh M, Safari T, et al. A combination of vitamin C and losartan for cisplatin-induced nephrotoxicity in rats. Iranian Journal of Kidney Diseases . 2012;6(5):361–365. [PubMed] [Google Scholar]
- 9.Pérez-Rojas JM, Guerrero-Beltrán CE, Cruz C, Sánchez-González DJ, Martínez-Martínez CM, Pedraza-Chaverri J. Preventive effect of tert-butylhydroquinone on cisplatin-induced nephrotoxicity in rats. Food and Chemical Toxicology . 2011;49(10):2631–2637. doi: 10.1016/j.fct.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez-Gonzalez PD, Lopez-Hernandez FJ, Perez-Barriocanal F, Morales AI, Lopez-Novoa JM. Quercetin reduces cisplatin nephrotoxicity in rats without compromising its anti-tumour activity. Nephrology Dialysis Transplantation . 2011;26(11):3484–3495. doi: 10.1093/ndt/gfr195. [DOI] [PubMed] [Google Scholar]
- 11.Atasayar S, Gürer-Orhan H, Orhan H, Gürel B, Girgin G, Ozgunes H. Preventive effect of aminoguanidine compared to vitamin E and C on cisplatin-induced nephrotoxicity in rats. Experimental and Toxicologic Pathology . 2009;61(1):23–32. doi: 10.1016/j.etp.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 12.El-Sayed ESM, Abd-Ellah MF, Attia SM. Protective effect of captopril against cisplatin-induced nephrotoxicity in rats. Pakistan Journal of Pharmaceutical Sciences . 2008;21(3):255–261. [PubMed] [Google Scholar]
- 13.Liu XH, Li J, Li QX, Ai YX, Zhang L. Protective effects of ligustrazine on cisplatin-induced oxidative stress, apoptosis and nephrotoxicity in rats. Environmental Toxicology and Pharmacology . 2008;26(1):49–55. doi: 10.1016/j.etap.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Kim YH, Kim YW, Oh YJ, et al. Protective effect of the ethanol extract of the roots of Brassica rapa on cisplatin-induced nephrotoxicity in LLC-PK1 cells and rats. Biological and Pharmaceutical Bulletin . 2006;29(12):2436–2441. doi: 10.1248/bpb.29.2436. [DOI] [PubMed] [Google Scholar]
- 15.Mohan IK, Khan M, Shobha JC, et al. Protection against cisplatin-induced nephrotoxicity by Spirulina in rats. Cancer Chemotherapy and Pharmacology . 2006;58(6):802–808. doi: 10.1007/s00280-006-0231-8. [DOI] [PubMed] [Google Scholar]
- 16.Shimeda Y, Hirotani Y, Akimoto Y, et al. Protective effects of capsaicin against cisplatin-induced nephrotoxicity in rats. Biological and Pharmaceutical Bulletin . 2005;28(9):1635–1638. doi: 10.1248/bpb.28.1635. [DOI] [PubMed] [Google Scholar]
- 17.Karimi G, Ramezani M, Tahoonian Z. Cisplatin nephrotoxicity and protection by milk thistle extract in rats. Evidence-based Complementary and Alternative Medicine . 2005;2(3):383–386. doi: 10.1093/ecam/neh103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shirwaikar A, Malini S, Kumari SC. Protective effect of Pongamia pinnata flowers against cisplatin and gentamicin induced nephrotoxicity in rats. Indian Journal of Experimental Biology . 2003;41(1):58–62. [PubMed] [Google Scholar]
- 19.Mansour MA, Mostafa AM, Nagi MN, Khattab MM, Al-Shabanah OA. Protective effect of aminoguanidine against nephrotoxicity induced by cisplatin in normal rats. Comparative Biochemistry and Physiology C . 2002;132(2):123–128. doi: 10.1016/s1532-0456(02)00062-5. [DOI] [PubMed] [Google Scholar]
- 20.Saad SY, Al-Rikabi AC. Protection effects of taurine supplementation against cisplatin-induced nephrotoxicity in rats. Chemotherapy . 2002;48(1):42–48. doi: 10.1159/000048587. [DOI] [PubMed] [Google Scholar]
- 21.Somani SM, Husain K, Whitworth C, Trammell GL, Malafa M, Rybak LP. Dose-dependent protection by lipoic acid against cisplatin-induced nephrotoxicity in rats: antioxidant defense system. Pharmacology and Toxicology . 2000;86(5):234–241. doi: 10.1034/j.1600-0773.2000.d01-41.x. [DOI] [PubMed] [Google Scholar]
- 22.Greggi Antunes LM, D’arc J, Bianchi MDLP. Protective effects of vitamin C against cisplatin-induced nephrotoxicity and lipid peroxidation in adult rats: a dose-dependent study. Pharmacological Research . 2000;41(4):405–411. doi: 10.1006/phrs.1999.0600. [DOI] [PubMed] [Google Scholar]
- 23.Appenroth D, Fröb S, Kersten L, Splinter FK, Winnefeld K. Protective effects of vitamin E and C on cisplatin nephrotoxicity in developing rats. Archives of Toxicology . 1997;71(11):677–683. doi: 10.1007/s002040050444. [DOI] [PubMed] [Google Scholar]
- 24.Bräunlich H, Appenroth D, Fleck C. Protective effects of methimazole against cisplatin-induced nephrotoxicity in rats. Journal of Applied Toxicology . 1997;17(1):41–45. doi: 10.1002/(sici)1099-1263(199701)17:1<41::aid-jat388>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 25.Tokunaga J, Kobayashi M, Kitagawa A, Nakamura C, Arimori K, Nakano M. Protective effects of N-benzoyl amino acids on cisplatin nephrotoxicity in rats. Biological and Pharmaceutical Bulletin . 1996;19(11):1451–1456. doi: 10.1248/bpb.19.1451. [DOI] [PubMed] [Google Scholar]
- 26.Tokunaga J, Kobayashi M, Nakamura C, Kitagawa A, Arimori K, Nakano M. Protective effect of N-benzoyl-β-alanine against cisplatin nephrotoxicity in rats. Renal Failure . 1996;18(2):225–240. doi: 10.3109/08860229609052792. [DOI] [PubMed] [Google Scholar]
- 27.Appenroth D, Winnefeld K, Schroter H, Rost M. Beneficial effect of acetylcysteine on cisplatin nephrotoxicity in rats. Journal of Applied Toxicology . 1993;13(3):189–192. doi: 10.1002/jat.2550130309. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi T, Watanabe Y, Kumano K, et al. Protective effect of piperacillin against the nephrotoxicity of cisplatin in rats. Antimicrobial Agents and Chemotherapy . 1989;33(4):513–518. doi: 10.1128/aac.33.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rjiba-Touati K, Boussema IA, Belarbia A, Achour A, Bacha H. Protective effect of recombinant human erythropoietin against cisplatin-induced oxidative stress and nephrotoxicity in rat kidney. International Journal of Toxicology . 2011;30(5):510–517. doi: 10.1177/1091581810411931. [DOI] [PubMed] [Google Scholar]
- 30.Rjiba-Touati K, Ayed-Boussema I, Bouaziz C, et al. Protective effect of erythropoietin against cisplatin-induced nephrotoxicity in rats: antigenotoxic and antiapoptotic effect. Drug and Chemical Toxicology . 2012;35(1):89–95. doi: 10.3109/01480545.2011.589440. [DOI] [PubMed] [Google Scholar]
- 31.Lee KW, Jeong JY, Lim BJ, et al. Sildenafil attenuates renal injury in an experimental model of rat cisplatin-induced nephrotoxicity. Toxicology . 2009;257(3):137–143. doi: 10.1016/j.tox.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 32.Ulubaş B, Çimen MYB, Apa DD, Saritaş E, Muşlu N, Çimen ÖB. The protective effects of acetylsalicylic acid on free radical production in cisplatin induced nephrotoxicity: an experimental rat model. Drug and Chemical Toxicology . 2003;26(4):259–270. doi: 10.1081/dct-120024841. [DOI] [PubMed] [Google Scholar]
- 33.Sadzuka Y, Shimizu Y, Takino Y, Hirota S. Protection against cisplatin-induced nephrotoxicity in the rat by inducers and an inhibitor of glutathione S-transferase. Biochemical Pharmacology . 1994;48(3):453–459. doi: 10.1016/0006-2952(94)90274-7. [DOI] [PubMed] [Google Scholar]
- 34.Nematbakhsh M, Ashrafi F, Safari T, et al. Administration of vitamin E and losartan as prophylaxes in cisplatin-induced nephrotoxicity model in rats. Journal of Nephrology . 2012;25(3):410–417. doi: 10.5301/jn.5000018. [DOI] [PubMed] [Google Scholar]
- 35.Weijl NI, Elsendoorn TJ, Lentjes EGWM, et al. Supplementation with antioxidant micronutrients and chemotherapy-induced toxicity in cancer patients treated with cisplatin-based chemotherapy: a randomised, double-blind, placebo-controlled study. European Journal of Cancer . 2004;40(11):1713–1723. doi: 10.1016/j.ejca.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 36.Argyriou AA, Chroni E, Koutras A, et al. A randomized controlled trial evaluating the efficacy and safety of vitamin E supplementation for protection against cisplatin-induced peripheral neuropathy: final results. Supportive Care in Cancer . 2006;14(11):1134–1140. doi: 10.1007/s00520-006-0072-3. [DOI] [PubMed] [Google Scholar]
- 37.Pace A, Giannarelli D, Galiè E, et al. Vitamin e neuroprotection for cisplatin neuropathy: a randomized, placebo-controlled trial. Neurology . 2010;74(9):762–766. doi: 10.1212/WNL.0b013e3181d5279e. [DOI] [PubMed] [Google Scholar]
- 38.Gonçalves ARR, Fujihara CK, Mattar AL, et al. Renal expression of COX-2, ANG II, and AT1 receptor in remnant kidney: strong renoprotection by therapy with losartan and a nonsteroidal anti-inflammatory. American Journal of Physiology . 2004;286(5):F945–F954. doi: 10.1152/ajprenal.00238.2003. [DOI] [PubMed] [Google Scholar]
- 39.Vaziri ND, Bai Y, Ni Z, Quiroz Y, Pandian R, Rodriguez-Iturbe B. Intra-renal angiotensin II/AT1 receptor, oxidative stress, inflammation, and progressive injury in renal mass reduction. Journal of Pharmacology and Experimental Therapeutics . 2007;323(1):85–93. doi: 10.1124/jpet.107.123638. [DOI] [PubMed] [Google Scholar]
- 40.Ullman J, Eriksson S, Rundgren M. Losartan increases renal blood flow during isoflurane anesthesia in sheep. Acta Anaesthesiologica Scandinavica . 2001;45(9):1168–1175. doi: 10.1034/j.1399-6576.2001.450919.x. [DOI] [PubMed] [Google Scholar]
- 41.Dukacz SAW, Kline RL. Differing effects of enalapril and losartan on renal medullary blood flow and renal interstitial hydrostatic pressure in spontaneously hypertensive rats. Journal of Hypertension . 1999;17(9):1345–1352. doi: 10.1097/00004872-199917090-00016. [DOI] [PubMed] [Google Scholar]
- 42.Cho JJ, Cadet P, Salamon E, Mantione KJ, Stefano GB. The nongenomic protective effects of estrogen on the male cardiovascular system: clinical and therapeutic implications in aging men. Medical Science Monitor . 2003;9(3):RA63–RA68. [PubMed] [Google Scholar]
- 43.Chaudry IH, Choudhry MA. 17β-estradiol: a novel hormone for improving immune and cardiovascular responses following trauma-hemorrhage. Journal of Leukocyte Biology . 2008;83(3):518–522. doi: 10.1189/jlb.0607369. [DOI] [PubMed] [Google Scholar]
- 44.Dubey RK, Jackson EK. Cardiovascular protective effects of 17β-estradiol metabolites. Journal of Applied Physiology . 2001;91(4):1868–1883. doi: 10.1152/jappl.2001.91.4.1868. [DOI] [PubMed] [Google Scholar]
- 45.Mendelsohn ME. Protective effects of estrogen on the cardiovascular system. American Journal of Cardiology . 2002;89(12, supplement 1):12E–18E. doi: 10.1016/s0002-9149(02)02405-0. [DOI] [PubMed] [Google Scholar]
- 46.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. New England Journal of Medicine . 1999;340(23):1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 47.Nematbakhsh M, Ghadesi M, Hosseinbalam M, et al. Oestrogen promotes coronary angiogenesis even under normoxic conditions. Basic and Clinical Pharmacology and Toxicology . 2008;103(3):273–277. doi: 10.1111/j.1742-7843.2008.00286.x. [DOI] [PubMed] [Google Scholar]
- 48.Kang DH, Yu ES, Yoon KI, Johnson R. The impact of gender on progression of renal disease: potential role of estrogen-mediated vascular endothelial growth factor regulation and vascular protection. American Journal of Pathology . 2004;164(2):679–688. doi: 10.1016/S0002-9440(10)63155-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silbiger SR, Neugarten J. The role of gender in the progression of renal disease. Advances in Renal Replacement Therapy . 2003;10(1):3–14. doi: 10.1053/jarr.2003.50001. [DOI] [PubMed] [Google Scholar]
- 50.He Q, Liang CH, Lippard SJ. Steroid hormones induce HMG1 overexpression and sensitize breast cancer cells to cisplatin and carboplatin. Proceedings of the National Academy of Sciences of the United States of America . 2000;97(11):5768–5772. doi: 10.1073/pnas.100108697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beleh MA, Lin YC, Brueggemeier RW. Estrogen metabolism in microsomal, cell, and tissue preparations of kidney and liver from Syrian hamsters. Journal of Steroid Biochemistry and Molecular Biology . 1995;52(5):479–489. doi: 10.1016/0960-0760(95)00003-i. [DOI] [PubMed] [Google Scholar]
- 52.Nematbakhsh M, Talebi A, Nasri H, et al. Some evidence for sex-based differences in cisplatininduced nephrotoxicity in rats. Clinical and Experimental Medical Letters . 2012;53(1-2):29–32. [Google Scholar]
- 53.Haghighi M, Nematbakhsh M, Talebi A, et al. The role of angiotensin II receptor 1 (AT1) blockade in cisplatin-induced nephrotoxicity in rats: gender-related differences. Renal Failure . 2012;34(8):1046–1051. doi: 10.3109/0886022X.2012.700886. [DOI] [PubMed] [Google Scholar]
- 54.Song JY, Kim MJ, Jo HH, et al. Antioxidant effect of estrogen on bovine aortic endothelial cells. Journal of Steroid Biochemistry and Molecular Biology . 2009;117(1–3):74–80. doi: 10.1016/j.jsbmb.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 55.Mann V, Huber C, Kogianni G, Collins F, Noble B. The antioxidant effect of estrogen and Selective Estrogen Receptor Modulators in the inhibition of osteocyte apoptosis in vitro. Bone . 2007;40(3):674–684. doi: 10.1016/j.bone.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 56.Speir E, Yu ZX, Takeda K, Ferrans VJ, Cannon RO. Antioxidant effect of estrogen on cytomegalovirus-induced gene expression in coronary artery smooth muscle cells. Circulation . 2000;102(24):2990–2996. doi: 10.1161/01.cir.102.24.2990. [DOI] [PubMed] [Google Scholar]
- 57.Saad SY, Najjar TAO, Daba MH, Al-Rikabi AC. Inhibition of nitric oxide synthase aggravates cisplatin-induced nephrotoxicity: effect of 2-amino-4-methylpyridine. Chemotherapy . 2002;48(6):309–315. doi: 10.1159/000069714. [DOI] [PubMed] [Google Scholar]
- 58.Hamilton CA, Groves S, Carswell HVO, Brosnan MJ, Graham D, Dominiczak AF. Estrogen treatment enhances nitric oxide bioavailability in normotensive but not hypertensive rats. American Journal of Hypertension . 2006;19(8):859–866. doi: 10.1016/j.amjhyper.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 59.Leung EL, Fraser M, Fiscus RR, Tsang BK. Cisplatin alters nitric oxide synthase levels in human ovarian cancer cells: involvement in p53 regulation and cisplatin resistance. British Journal of Cancer . 2008;98(11):1803–1809. doi: 10.1038/sj.bjc.6604375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nematbakhsh M, Khazaei M. The effect of estrogen on serum nitric oxide concentrations in normotensive and DOCA Salt hypertensive ovariectomized rats. Clinica Chimica Acta . 2004;344(1-2):53–57. doi: 10.1016/j.cccn.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 61.Soylemez S, Gurdal H, Sepici A, Akar F. The effect of long-term resveratrol treatment on relaxation to estrogen in aortae from male and female rats: role of nitric oxide and superoxide. Vascular Pharmacology . 2008;49(2-3):97–105. doi: 10.1016/j.vph.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 62.Wink DA, Cook JA, Christodoulou D, et al. Nitric oxide and some nitric oxide donor compounds enhance the cytotoxicity of cisplatin. Nitric Oxide . 1997;1(1):88–94. doi: 10.1006/niox.1996.0108. [DOI] [PubMed] [Google Scholar]
- 63.Baxi DB, Singh PK, Vachhrajani KD, Ramachandran AV. Melatonin supplementation in rat ameliorates ovariectomy-induced oxidative stress. doi: 10.3109/13697137.2012.682108. Climacteric. In press. [DOI] [PubMed] [Google Scholar]
- 64.Srivastava RC, Farookh A, Ahmad N, Misra M, Hasan SK, Husain MM. Evidence for the involvement of nitric oxide in cisplatin-induced toxicity in rats. BioMetals . 1996;9(2):139–142. doi: 10.1007/BF00144618. [DOI] [PubMed] [Google Scholar]
- 65.Watanabe KI, Hess A, Bloch W, Michel O. Expression of inducible nitric oxide synthase (iNOS/NOS II) in the vestibule of guinea pigs after the application of cisplatin. Anti-Cancer Drugs . 2000;11(1):29–32. doi: 10.1097/00001813-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 66.Watanabe KI, Hess A, Michel O, Yagi T. Nitric oxide synthase inhibitor reduces the apoptotic change in the cisplatin-treated cochlea of guinea pigs. Anti-Cancer Drugs . 2000;11(9):731–735. doi: 10.1097/00001813-200010000-00010. [DOI] [PubMed] [Google Scholar]
- 67.Santos NAG, Catão CS, Martins NM, Curti C, Bianchi MLP, Santos AC. Cisplatin-induced nephrotoxicity is associated with oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Archives of Toxicology . 2007;81(7):495–504. doi: 10.1007/s00204-006-0173-2. [DOI] [PubMed] [Google Scholar]
- 68.Ajith TA, Usha S, Nivitha V. Ascorbic acid and α-tocopherol protect anticancer drug cisplatin induced nephrotoxicity in mice: a comparative study. Clinica Chimica Acta . 2007;375(1-2):82–86. doi: 10.1016/j.cca.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 69.Ulas M, Cay M. 17β-Estradiol and vitamin E modulates oxidative stress-induced kidney toxicity in diabetic ovariectomized rat. Biological Trace Element Research . 2011;144(1–3):821–831. doi: 10.1007/s12011-011-9025-x. [DOI] [PubMed] [Google Scholar]
- 70.Moorthy K, Sharma D, Basir SF, Baquer NZ. Administration of estradiol and progesterone modulate the activities of antioxidant enzyme and aminotransferases in naturally menopausal rats. Experimental Gerontology . 2005;40(4):295–302. doi: 10.1016/j.exger.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 71.Ulas M, Cay M. Effects of 17β-estradiol and vitamin E treatments on blood trace element and antioxidant enzyme levels in ovariectomized rats. Biological Trace Element Research . 2011;139(3):347–355. doi: 10.1007/s12011-010-8669-2. [DOI] [PubMed] [Google Scholar]
- 72.Maliakel DM, Kagiya TV, Nair CKK. Prevention of cisplatin-induced nephrotoxicity by glucosides of ascorbic acid and α-tocopherol. Experimental and Toxicologic Pathology . 2008;60(6):521–527. doi: 10.1016/j.etp.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 73.Goren MP. Cisplatin nephrotoxicity affects magnesium and calcium metabolism. Medical and Pediatric Oncology . 2003;41(3):186–189. doi: 10.1002/mpo.10335. [DOI] [PubMed] [Google Scholar]
- 74.Hodgkinson E, Neville-Webbe HL, Coleman RE. Magnesium depletion in patients receiving cisplatin-based chemotherapy. Clinical Oncology . 2006;18(9):710–718. doi: 10.1016/j.clon.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 75.Lajer H, Kristensen M, Hansen HH, Christensen S, Jonassen T, Daugaard G. Magnesium and potassium homeostasis during cisplatin treatment. Cancer Chemotherapy and Pharmacology . 2005;55(3):231–236. doi: 10.1007/s00280-004-0899-6. [DOI] [PubMed] [Google Scholar]
- 76.Lajer H, Kristensen M, Hansen HH, et al. Magnesium depletion enhances cisplatin-induced nephrotoxicity. Cancer Chemotherapy and Pharmacology . 2005;56(5):535–542. doi: 10.1007/s00280-005-1010-7. [DOI] [PubMed] [Google Scholar]
- 77.Ariceta G, Rodriguez-Soriano J, Vallo A, Navajas A. Acute and chronic effects of cisplatin therapy on renal magnesium homeostasis. Medical and Pediatric Oncology . 1997;28(1):35–40. doi: 10.1002/(sici)1096-911x(199701)28:1<35::aid-mpo7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 78.Mavichak V, Wong NLM, Quamme GA. Studies on the pathogenesis of cisplatin-induced hypomagnesemia in rats. Kidney International . 1985;28(6):914–921. doi: 10.1038/ki.1985.217. [DOI] [PubMed] [Google Scholar]
- 79.Virág V, May Z, Kocsis I, Blázovics A, Szentmihályi K. Effects of magnesium supplementation on the calcium and magnesium levels, redox homeostasis in normolipidemic and alimentary induced hyperlipidemic rats. Orvosi Hetilap . 2011;152(27):1075–1081. doi: 10.1556/OH.2011.29152. [DOI] [PubMed] [Google Scholar]


