Abstract
The proper differentiation and threat of cancer rising from the application of induced pluripotent stem (iPS) cells are major bottlenecks in the field and are thought to be inherently linked to the pluripotent nature of iPS cells. To address this question, we have compared iPS cells to embryonic stem cells (ESCs), the gold standard of ground state pluripotency, in search for proteins that may improve pluripotency of iPS cells. We have found that when reprogramming somatic cells toward pluripotency, 1%–5% of proteins of 5 important cell functions are not set to the correct expression levels compared to ESCs, including mainly cell cycle proteins. We have shown that resetting cyclin A1 protein expression of early-passage iPS cells closer to the ground state pluripotent state of mouse ESCs improves the pluripotency and reduces the threat of cancer of iPS cells. This work is a proof of principle that reveals that setting expression of certain proteins correctly during reprogramming is essential for achieving ESC-state pluripotency. This finding would be of immediate help to those researchers in different fields of iPS cell work that specializes in cell cycle, apoptosis, cell adhesion, cell signaling, and cytoskeleton.
Introduction
Since the discovery of the method to reprogram fibroblasts into induced pluripotent stem (iPS) cells 5 years ago, it was difficult to make chimera mice, a measure of the pluripotent state of the iPS cells [1], which has improved dramatically in a short period of time [2–4]. The term “ground state” pluripotency of mouse embryonic stem cells (mESCs) was coined by Austin Smith's group in 2008 and refers to the true pluripotent state of mESCs before formation of the Epistem cells (EpiScs) [5,6]. Interestingly, insertion of KLF4, a reprogramming factor, has been found to revert EpiScs to ground state pluripotency, and Stat3 activation is a limiting step for reprogramming of mouse iPS (miPS) cells toward ground state pluripotency [7,8]. Moreover, we have found that modifying expression of Rem2 GTPase, a suppressor of the p53 pathway, can replace FGF2 signaling in human embryonic stem cells (hESCs) and can enhance the efficiency of reprogramming [9]. Taking this together, it suggests that iPS cells can be modified to more closely resemble the pluripotent state of ESCs, yet proteomic factors are yet to be fully described.
Here, we investigate if modification of cyclin A1 expression levels can modify the pluripotent state of iPS cells for a number of reasons. First, cyclin A1 has been found to be essential for mESC survival, but not mouse fibroblasts, suggesting a role in pluripotency [10], and second, our unbiased antibody array screen identified cyclin A1 as overexpressed in iPS cells compared to ESCs, the gold standard for ground state pluripotency, suggesting that during reprogramming, cyclin A1 protein expression levels are not set correctly.
Materials and Methods
Culture of ESCs
mESCs were made from the same black 6 mice that iPS cells were derived by following previously described methods [5,21]. mESCs and iPS cells were maintained in a G4 medium, which was changed every day. The medium formulation is as follows: DMEM (Gibco; # 21969-035), fetal bovine serum (Hyclone), MEM NEAA 100× (Gibco), penicillin (10,000 U/mL)/streptomycin (10,000 μg/mL) (100×) (Gibco; #15140-122), GlutaMAX 200 mM (Gibco; #35050-038), sodium pyruvate (Gibco; #11360), 2-Mercaptoethanol 50 mM (Gibco; #31350-010) (4°C), and LIF 1,000 U/mL (Chemicon; #ESG1107).
Human ESCs and iPS cells were derived and fully characterized as previously published and permission attained [22,23]. They were maintained on either human feeder layers or on matrigel-coated plates with the HUES medium, consisting of KO-DMEM (Invitrogen) supplemented with 10% KO-serum replacement (Invitrogen), 0.5% human albumin (Grifols), 2 mM Glutamax (Invitrogen), 50 μM 2-mercaptoethanol (Invitrogen), nonessential aminoacids (Cambrex), and 10 ng/mL bFGF (Peprotech). Cultures were maintained at 37°C, 5% CO2, with medium changes every other day. hESCs were routinely tested for normal karyotype. For hESC lines adapted to matrigel-coated plates, the HUES-conditioned media from irradiated mouse embryonic fibroblast (MEFs) were used instead. MEFs were cultured using 10% fetal calf serum (FCS) with the DMEM. hiPS cells were cultured the same as hESCs, and their generation has been previously described [24,25].
Generation of iPS cells
The generation of iPS cells using MEFs from black 6 mice has been described before [26]. For reprogramming experiments, about 50,000 or 100,000 cells were seeded per well of a 6-well plate and infected with retroviral supernatants of a FLAG-tagged polycistronic retroviral vector Oct3/4, Sox2, Klf4, and C-Myc (OSKM) in the presence of 1 mg/mL polybrene. Infection consisted of a 45-min spinfection at 750 g, washed with PBS and keratinocyte medium replaced. Two rounds of infections on consecutive days were performed. Two days after beginning the last round of infection, cells were trypsinized and seeded onto feeder layers of irradiated MEFs in the same culture medium. The medium was changed upon platting to G4 with LIF. Cultures were maintained at 37°C, 5% CO2, with medium changes every other day. iPS cells were picked, expanded, and characterized for morphology, gene expression, cell cycle, pluripotency, and teratomas [26].
Construct and virus production
cDNAs for Oct4 and Sox2 were amplified from mES RNA by RT-PCR. Klf-4 was amplified from IMAGE clone 5111134. c-Myc T58A mutant cDNA was amplified from DNA kindly provided by Dr. Luciano DiCroce. The amplified cDNAs were cloned into the EcoRI/ClaI sites of a modified pMSCVpuro vector, which allows the expression of N-terminal FLAG-tagged proteins. Retroviruses for the 4 factors were independently produced after transfecting the cell line Phoenix Amphotropic using the Fugene 6 reagent (Roche) according to the manufacturer's directions. After 24 h, the DMEM medium was replaced, cells were incubated at 32°C, and the viral supernatant was harvested after 24 and 48 h.
Lentiviral-based cyclin A1 shRNA constructs were purchased using a ps1HIV-H1 promoter backbone with a GFP tag. Lentiviruses were produced after transfecting the 293T cell line with packaging gag, pol and RRE, vesicular stomatitis virus G-protein, reverse element, and shRNA DNA using the Lipofectamine 2000 reagent according to the manufacturer's directions. After 24 h, the G4 medium was replaced, cells were incubated at 37°C, and the viral supernatant was harvested at 36 h. Two hundred thousand cells were infected with 1 mL of fresh viral supernatant.
Proteomic array
Cell-signaling antibody arrays were purchased from Sigma, and the manufacturer's instructions were followed. mESC lysates (derived from black 6 mice) were compared to miPS cell lysates (derived from the same strain of black 6 mice). Data extracted from arrays were analyzed by the Centre for Genomic Regulation (CRG) bio- informatics platform, and differences>1.5-fold are indicated in Fig. 1.
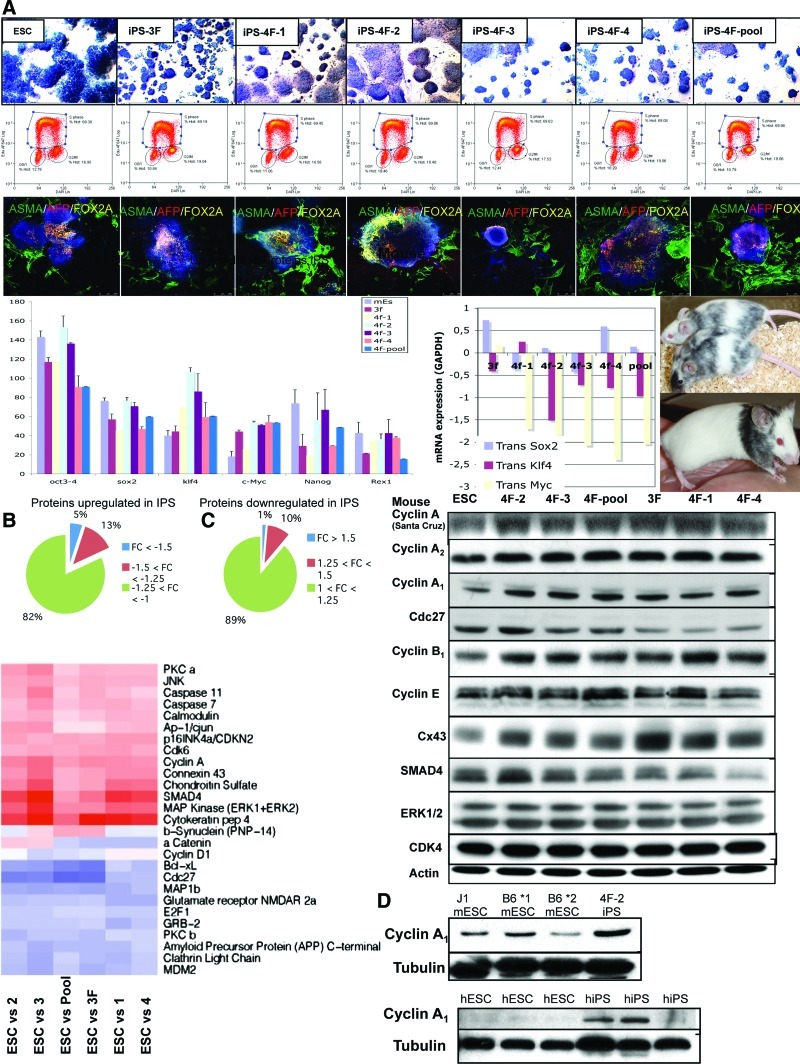
FIG. 1.
Characterization of mouse embryonic stem (ES) and induced pluripotent stem (iPS) cells. (A) Top 3 sets of panels: AP staining of C57/BL6 mouse ES cell (mESC) lines (2 lines tested, data not shown for both) and mouse iPS (miPS) cells (3F: 3 factors; 4F: 4 factors) made from MEFs of C57/BL6 mice (×100). 4F-pool is a pool of 4 iPS clones. Cell cycle analysis by fluorescence-activated cell sorting (FACS) for EDU (BRDU) in each clone demonstrates a high percentage of cells in the S phase in all cases. Note that iPS cells have a higher level of cells in the G2/M phase compared to mESCs. Confocal microscopy photos of EBs differentiating into ectoderm, endoderm, and endoderm demonstrating that mESCs can form all 3 germ layers, but some iPS clones formed low levels of ectoderm. See Supplementary Fig. S1 for further stainings. Lower panels: Graph of real-time polymerase chain reaction (RT-PCR) of endogenous and transgene pluripotency gene expression in ESCs and iPS cells. Transgene expression was almost silenced in iPS cell clones. Photos of chimera mice made from mESCs (top) and miPS cells (bottom). (B) Proteomic analyses using antibody arrays reveal differences between ES and iPS, implicating cyclin A1. Pie graph of differences in protein expression between mESCs and miPS cells and a heat map of antibody array showing changes in protein expression between ESCs and iPS cells. (C) Western blot validation of some of the proteins identified from the antibody array with the greatest changes. (D) Western blot in 3 mESC lines compared to the 1 miPS cell line (4F-2 from C above) for cyclin A1 expression levels to validate lower expression levels of cyclin A1 in mESC lines. Lower panel demonstrates western blot for 3 human ESC (hESC) lines and 3 human iPS (hiPS) cell lines, showing higher levels of cyclin A1 in the hiPS cell lines. AP, alkaline phosphatase; MEFs, mouse embryonic fibroblasts. Color images available online at www.liebertonline.com/scd
Immunocytochemistry, in vitro differentiation, and AP analysis
Cells were grown on plastic coverslide chambers and fixed with 4% paraformaldehyde (PFA). Differentiation toward endoderm, mesoderm, and neuroectoderm was carried out by plating embryoid body (EBs) on gelatin and the DMEM medium, with 20% FCS changed every second day for 2–3 weeks. Cells were then stained for appropriate markers described in the figures. Secondary antibodies used were all the Alexa Fluor Series from Invitrogen (all 1:500). Images were taken using a Leica SP5 confocal microscope. Direct AP activity was analyzed using an Alkaline Phosphatase Blue/Red Membrane Substrate solution kit according to the manufacturer's guidelines (Sigma). Briefly, cells were washed once in cold PBS, fixed with cold 4% PFA for 1–2 min, washed with PBS once, and stained with the AP mix until color develops (usually 5–10 min).
Flow cytometry analyses
All analyses were performed on MoFlo cell sorter (Dako Cytomation)-running Summit software. For measuring proliferation, we have used the commercial kit from Invitrogen the Click-iT EdU AlexaFluor647 Flow Cytometry Assay kit following the manufacturer's instructions. For the proliferation assay using the click-IT kit, instead of using the supplied DNA dyes, we used a homemade dye 4′,6-diamidino-2-phenylindole (DAPI)-staining solution (0.1M Tris Base, pH 7.4; 0.9% or 150 mM NaCl; 1 mM CaCl2; 0.5 mM MgCl2; 0.2% bovine serum albumin; 0.1% Nonidet P40; and 10 mg/mL DAPI) 0.5 mL/test (2 h/room temperature or overnight 4°C).
Western blot
Western blot analyses were performed, as previously described, using extracts of cells collected by centrifugation, washed twice in PBS and lyzed in 1× lysis buffer [50 nM Tris-HCl, 70 mM 2-mercaptoethanol, and 1% sodium dodecylsulfate (SDS)], and the concentration of total protein was measured by the Bradford assay. Lysates were then boiled for 5 min and subjected to 12% polyacrylamide SDS gel or 4%–12% SDS-resolving gel (Invitogen) electrophoresis. After electrophoresis, proteins were transferred to a nitrocellulose membrane using submerged transfer apparatus (BioRad), filled with 25 mM Tris Base, 200 mM glycine, and 20% methanol. After blocking with 5% nonfat dried milk in TBS-T [50 mM Tris- HCl (pH 8.0), 150 mM NaCl, and 0.1% Tween 20], the membrane was incubated with the primary antibodies diluted in TBS-T and washed extensively. Primary antibodies were Cyclin A (sc596), Cyclin A2 (Epitomics E399), Cyclin A1 (sc-15383), Cdc27 (sc13154), Actin, Cyclin B1, Cyclin E, p16INK4a, CDK4 and Cx43 (Santa Cruz), SMAD4 (Upstate), and ERK1/2 (Sigma M5670). The membrane was washed 3 times with TBS-T and then incubated with the appropriate horseradish peroxidase-linked secondary antibody (Amersham). The detection was performed with the Western Breeze Immunodetection Kit (Invitrogen).
Real-time PCR
Total mRNA was isolated using TRIZOL, and 1 μg was used to synthesize cDNA using the Invitrogen Cloned AMV First-Strand cDNA synthesis kit. One to 2 μL of the reaction was used to quantify gene expression by quantitative PCR for transgenes and endogenous pluripotent genes as previously described [27].
Soft-agar assay
Briefly, 6-well plates were coated with 1 mL of a 0.4% agar/G4 medium gel, between 25,000 and 2,500 mESCs or iPS cells plated in a 0.3% gel placed on top. LIF was maintained in serum (10% FCS) in the agar gels to control growth factor conditions. O.5mls of G4 media was changed every 3 days, and assays were left for 2–3 weeks, stained, and the number of colonies was counted and expressed as a percentage of the total number of cells plated.
Chimera and teratoma assay
Chimeras were made as previously described [2–4,28]. Briefly, 11–12 black 6 iPS cells were collected and microinjected into blastocytes from albino mice that had a part of its inner cell mass removed. Chimera blastocytes were then injected back into female black 6 mice and the F1 litter analyzed. Teratomas were performed by injecting 2×105 iPS cells subcutaneously in both flanks of 8-week-old SCID mice. After 3–5 weeks, tumors were removed, weighed, and processed for histology. Apoptosis was assessed in sections of teratomas using the tunnel assay.
Results and Discussion
Proteomic analysis reveals differences between ESCs and iPS, implicating cyclin A1
Here, we have compared the proteomic expression differences between mESCs and iPS cells (both derived from C57/BL6 mice) to determine proteins important in fine tunning pluripotency of iPS cells (using mESCs as the gold standard). All mESC and iPS cell lines derived were from the same strain of C57/BL6 (black 6) mice to reduce the clonal variation, following established retroviral methods with either 3 factors (3F; Oct4/Sox2/Klf4) or 4 factors (4F; Oct4/Sox2/Klf4/c-Myc), and assayed at the same passage number [1]. We analyzed 3 mES lines and 6 miPS lines, one of which was a pool of 4 iPS clones (4F-pool). We also verified the mouse work in 3 hESC lines, 1 fully reprogrammed human iPS (hiPS) cell line, and 2 partially reprogrammed hiPS cell lines, making a total of 6 ESC lines and 9 iPS cell lines studied. Characterization of mESC and miPS cell lines demonstrated that they were able to differentiate into the 3 germ layers, able to form chimera mice, stained positive for alkaline phosphatase (AP), and expressed the main pluripotency markers, with no major changes in the cell cycle profile (Fig. 1 and Supplementary Fig S1; Supplementary Data are available online at www.liebertpub.com/scd). Real-time polymerase chain reaction (RT-PCR) for pluripotency gene expression revealed similar expression levels between iPS cells and mESCs, and that the retroviral transgene expression was silenced (Fig. 1).
To assess differences in protein expression, we used antibody arrays of the best-described proteins known to be involved in 5 main cell functions: (i) cell cycle, (ii) apoptosis, (iii) cell adhesion, (iv) cell signaling, and (v) cytoskeleton. We found that about 82% to 89% of proteins were similarly expressed in iPS cells compared to mESCs, and that there were between 1% and 5% differences (>1.5-fold) in protein expression, with more differences being upregulated proteins in iPS cells (Fig. 1). Interestingly, proteins involved in the cell cycle control were the more frequently associated with changes between mESCs and iPS cells, followed by proteins controlling cell signaling and apoptosis (Fig. 1).
We found a number of protein targets already known for having a critical role in ESC pluripotency such as ERK, SMAD4, and cyclin A, further validating this approach [10,11] (Fig. 1). Overall, the array data were in good agreement with the validation by western blot methodology, suggesting that the array had performed well (Fig. 1).
We observed that the cdc27 protein levels were similar between 4F-2 and 4F-3 iPS and mESCs, and lower in iPS clones 4F-1 and 4F-4, compared to mESCs (Fig. 1C). We found that cyclin A1, normally exclusively expressed in germ cells only, was upregulated in iPS cells compared to mESCs, more so than cyclin A2 (Fig. 1C). Further, 3 mESC lines tested demonstrated a lower level of expression of cyclin A1 protein in mESC lines (Fig. 1D). Further validation in hESCs and hiPS cell lines by western blot demonstrated that cyclin A1 is upregulated in 2 of the 3 iPS cell lines and slightly increased in 1 hiPS cell compared to hESC lines (Fig. 1D).
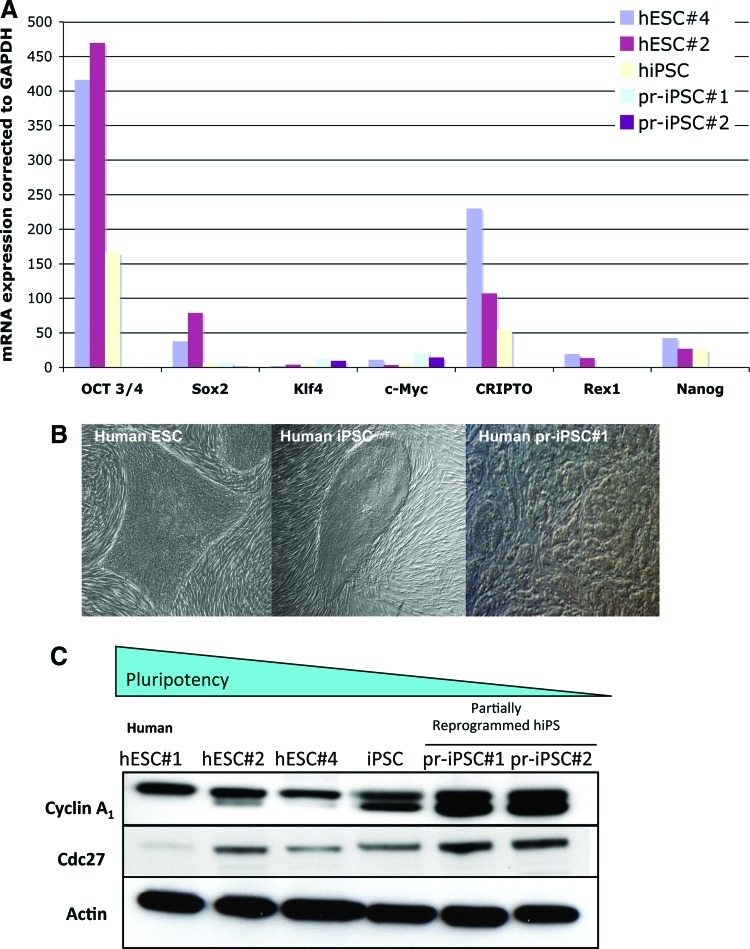
Given this, we validated expression levels of cdc27 and cyclin A1 in hESCs compared to hiPS and partially reprogrammed hiPS cells that were made at the same time and assessed at the same passage, to determine expression levels associated with lower levels of pluripotency (partially reprogrammed hiPS). Partially reprogrammed hiPS cells were determined based on morphology and expression of a battery of pluripotency genes (Fig. 2). We found that cyclin A1 protein levels were low in hESCs and increased in the partially reprogrammed hiPS cells, which are less pluripotent (Fig. 2). Cdc27 was expressed slightly higher in the 2 partially reprogrammed hiPS cell clones in contrast to the miPS cells (Fig. 2). Because a recent in vivo study functionally demonstrated that cyclin A is specific for mESC survival [10], suggesting a role for the cell cycle kinase in maintaining pluripotency and that we had validated high levels of cyclin A1 in partially reprogrammed hiPS, we focused on cyclin A1.
FIG. 2.
Validation in human ESCs and iPS cells. (A) RT-PCR of pluripotency genes in 2 hESC lines, hiPS cells and partially reprogrammed iPS cells (#1 and #2). (B) Photos of hESCs, hiPS, and partially reprogrammed iPS clones revealing morphological differences with the pluripotent state. (C) Validation by the western blot methodology of cyclin A1 and cdc27 in hESCs, hiPS clones and partially reprogrammed hiPS clones. Note that with reduced pluripotent potential in the partially reprogrammed iPS cells (#1 and #2), cyclin A1 protein levels increase compared to hESCs. hESCs have low levels of cyclin A1 protein expression and higher pluripotent potential. Color images available online at www.liebertonline.com/scd
Modification of cyclin A1 protein levels resets the pluripotent state of iPS cells
Next, we asked the question if modification of the cyclin A1 expression levels could modify the pluripotency of iPS cells by knocking down cyclin A1 levels in iPS clones using short hairpin RNA (shRNA) tagged with GFP (Supplementary Fig. S2). We have used 3 methods of analysis of pluripotency, (i) RT-PCR of pluripotency gene expression, (ii) in vitro differentiation to the 3 germ layers, and (iii) a gold standard functional test of pluripotency, making chimera mice. This approach is both an intensive and extensive analysis. For treatment of cyclin A1 protein levels in ESCs and iPS cells in Fig. 3, we chose to perform an intensive analysis with the 3 tests of pluripotency described above. Therefore, we tested 3 iPS cell lines for RT-PCR of pluripotency gene expression. For chimera mice assays, we chose 3 pluripotent cell lines with ∼300 embryo transfers for each of the conditions described in the figures (with and without cyclin A1 shRNA treatment).
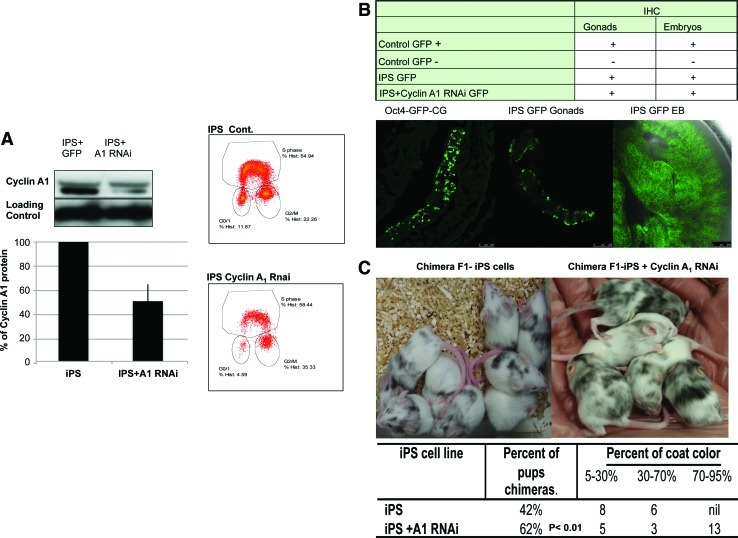
FIG. 3.
Modification of cyclin A1 protein levels resets the pluripotent state of iPS cells. (A) Western Blot analysis of cyclin A1 protein levels in iPS clones with stable lentiviral shRNA for cyclin A1, demonstrating 50% knockdown of protein expression. Graph showing quantification of cyclin A1 protein levels after shRNA treatment. An FAC analysis of a cell cycle profile of miPS cell clones compared to treated with stable lentiviral shRNA for cyclin A1. (B) Table summarizing presence of green fluorescent protein (GFP) in gonads or the embryo body of chimera mice made from GFP iPS cells or GFP iPS cells treated with RNAi for cyclin A1. Below are photographs of gonads (with control gonads, CG, of a GFP-OCT4 mouse) and the embryo body at d12.5 showing GFP-cyclin A1RNAi signal. (C) A functional pluripotency assay was performed by making chimera mice; the table summarizes the percentage of chimeras and the percent contribution to coat color of chimera mice for iPS cells with and without cyclin A1 knockdown. A total of 331 embryo transfers were made for iPS cell lines, and 300 embryo transfers were performed for iPS cell lines treated with cyclin A1 knockdown. Photographs showing example of chimera litter for each condition summarized in the table. Color images available online at www.liebertonline.com/scd
The cell cycle analysis revealed that knockdown of cyclin A1 caused an increase in the G2/M phase with a small decrease in the S phase (Fig. 3). Initial analysis of 6 main pluripotency gene expression levels did not reveal significant differences, although a small increase was seen in Nanog expression with knockdown of cyclin A1 (Supplementary Fig. S2). With in vitro differentiation, a difference in the ability to form the 3 germ layers was observed, suggesting an effect during differentiation (data not shown). To functionally test this in vivo, we assessed the ability of these cells to form chimeric mice. We found that knockdown of cyclin A1 protein levels led to an increase in the chimera rate from 42% to 62%, suggesting a better contribution to embryo development. Moreover, the percentage contribution to coat color increased significantly, with 62% of pups with >70% black coat color, demonstrating that reduced cyclin A1 levels in miPS gave better ability of iPS cells to contribute to the development of the embryo (Fig. 3). We also assessed GFP-positive cell contribution to embryo bodies at d13.5 and found with iPS GFP cell lines that they contributed to the embryo body and gonads, confirming that the miPS cells contributed to chimera formation (Fig. 3). Taken together, these data suggest that modification of cyclin A1 protein levels closer to that of mESCs (lower levels) can refine the pluripotent state of iPS cells. Interestingly, knockdown of cyclin A1 in mESCs resulted in no chimeric mice born, with embryos dying at a very early stage of embryo development (data not shown), supporting previous work that cyclin A is essential for mESC survival, although that work did not distinguish between cyclin A1 and A2 [10]. We advance that work to show that cyclin A1 is essential for mouse embryo development and survival. Moreover, we show from the chimera assays that only some miPS cell lines treated with shRNA cyclin A1 are able to proceed to the F1 generation, suggesting further that lower levels of cyclin A1 protein expression are essential for germ-line transmission (Supplementary Fig. S1).
Reducing cyclin A1 levels in high-expressing iPS clones reduces tumorigenicity
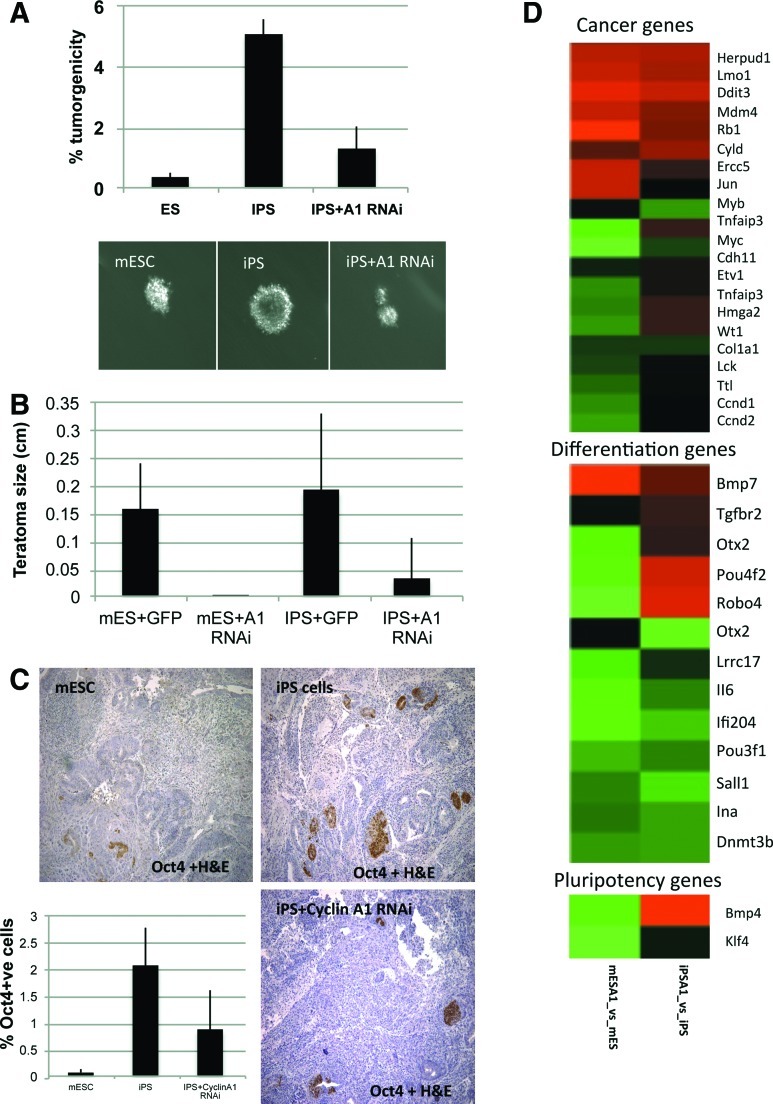
It is thought that the oncogenic threat of iPS cells is due to poor differentiation, determined by the pluripotent state achieved during reprogramming. Given that we could modify the pluripotent state of iPS by reducing cyclin A1 protein levels in iPS cells, we next asked if this could affect the tumorigenicity of iPS cells. We used an in vitro soft-agar assay to test anchorage-independent growth, a hallmark of malignancy. Small colonies were able to grow slowly at an incidence of 0.4% for ESCs and 5% for iPS cells. By reducing cyclin A1 protein levels using stable shRNA in iPS cells, we observed a 4-fold reduction in the tumorigenicity of miPS cells (Fig. 2). We tested tumorigenicity from another approach using a teratoma assay in vivo. We found that reduction of cyclin A1 protein levels could also reduce the size of teratomas formed by iPS significantly (Fig. 2).
To understand better the mechanism of how reduced cyclin A1 levels could be contributing to increased pluripotency and reduced tumorigenicity of iPS cells, the microarray analysis of the pluripotent cell lines with cyclin A1 knockdown reveals upregulation of cancer suppressor genes such as Rb1 (as well as Vhl and Btg1—data not shown) with downregulation of oncogenes Myc, Myb, and ccnd1 (Fig. 2). Moreover, we observed a decrease in important differentiation genes such as Dnmt3b, Pou3f1, and Sall1 in all 3 cell lines with an increase in the critical pluripotency gene Bmp4 with cyclin A1 reduction in the iPS cell lines. Taken together, these data suggest that cyclin A1 regulates indirectly or directly a set of genes important for tumor formation or suppression and as well for genes regulating ground state pluripotency.
To further explain the mechanism of how knockdown of cyclin A1 may be reducing tumorigenicity of iPS cells, we measured apoptosis (tunnel) in mouse teratomas by IHC (Fig. 4). We found that with iPS cells, a significant increase in apoptosis occurs with cyclin A1 knockdown (Supplementary Fig. S2). These results highlight that an increase in apoptosis as a result of cyclin A1 knockdown could explain the reduction of tumor growth in vivo. It has been previously shown that high c-Myc levels associated with lower cyclin A1 levels under growth factor-reduced conditions cause apoptosis [12,13].
FIG. 4.
Reduction of cyclin A1 protein levels reduces tumorgenicity. (A) Soft-agar tumorgenicity assay. Reduction of cyclin A1 protein levels reduces tumorgenicity of iPS cells. Quantification and photos of the soft-agar assay, demonstrating that iPS cells treated with stable lentiviral shRNA for cyclin A1 have reduced number of colonies forming in anchorage-independent conditions compared to controls. Note that the size of the mESC colony in the photos was the smallest assessed in the quantification. Three iPS clones were tested. This suggests that reduction of cyclin A1 in iPS reduces tumorigenicity. (B) In vivo tumorgenicity assay. Graph showing differences in the size of teratomas formed by iPS cells treated with stable lentiviral shRNA for cyclin A1 or controls compared to mESCs. (C) Photographs of teratomas formed by mESCs or iPS cells treated with stable lentiviral shRNA for cyclin A1 or controls stained for Oct4 expression with DAB compared to mESCs. Graph demonstrates quantification of the percent of Oct4-positive cells in teratomas. (D) Heat maps from Agilent gene expression arrays for 3 gene groups for mESCs and miPS cells with and without cyclin A1 RNAi treatment demonstrating a downregulation of important oncogenes (Myb, Myc, and cyclin D1) and an upregulation of important tumor suppressor genes (Rb) and pluripotency factors (BMP4), explaining the reduced tumorigenicity and increased pluripotency of iPS cells with reduced cyclin A1. Color images available online at www.liebertonline.com/scd
Given we had seen a reduction in tumorigenicity (both in vitro and in vivo measures) and better pluripotency of iPS with reduction of cyclin A1 protein levels in iPS cells, we thought that it could be a result of better differentiation of the cells. For iPS cells, we found that the number of Oct4-positive cells still present after 3 weeks of differentiation in vivo was reduced with knockdown of cyclin A1 protein levels (Fig. 2). These data suggest that the better differentiation of iPS cells with cyclin A1 knockdown may also contribute to the reduction of tumorigenicity of the iPS cells.
In conclusion, the data demonstrate that during reprogramming, achieving the correct expression levels of 1%–5% of proteins is essential to achieve closer to ground state pluripotency. We have shown that modifying cyclin A1 protein levels in iPS cells can increase pluripotency and reduce tumorigenicity of iPS cells. Recently, it has been shown that a number of cell cycle genes such as p27KIP, Cyclin D1, and p53 function play an important role in maintaining and attaining pluripotency of hESCs and iPS cells; however, a direct link of the cell cycle to the pluripotency program is yet to be established [9,14–20]. We expect that not all iPS cell lines would have overexpressed cyclin A1, and for those iPS clones, that would be good news. However, this work goes further; it is a proof of principle that setting certain protein expression correctly is essential for reaching ground state pluripotency equal to ESCs. This finding is of immediate help to those researchers in different fields of iPS that specialize in cell cycle, apoptosis, cell adhesion, cell signaling, and cytoskeleton.
Supplementary Material
Acknowledgments
The authors are indebted to the Center for Regenerative Medicine of Barcelona (CMRB) for allowing access to technical facilities and, especially, to José Miguel Andrés Vaquero, Mercé Martí, and Laura Batlle for assistance with flow cytometry, bioimaging, and generation of mouse chimeras (and mESC lines), respectively, and Sergi Mendez for assistance in making miPS cells. M.J.E. (RYC-2010-06512) and A.C. (RYC-2008-02772) are supported in part by the Programa Ramon y Cajal from the MICINN, supported by a grant from MICINN BFU2011–26596 to M.J.E. Additional support was provided by grants from the Fondo de Investigaciones Sanitarias (P1071209, PI061897, and CP05/00294), MICINN (BFU2009-13277, BFU2010-21823, PLE2009-0144, and ACI2010-1117), and Fondazione Guido Berlucchi 2010 to A.C.
Author Disclosure Statement
The authors declare that they have no competing financial interests.
References
- 1.Takahashi K. Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Okita K. Ichisaka T. Yamanaka S. Generation of germline- competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 3.Boland MJ. Hazen JL. Nazor KL. Rodriguez AR. Gifford W. Martin G. Kupriyanov S. Baldwin KK. Adult mice generated from induced pluripotent stem cells. Nature. 2009;461:91–94. doi: 10.1038/nature08310. [DOI] [PubMed] [Google Scholar]
- 4.Zhao XY. Li W. Lv Z. Liu L. Tong M. Hai T. Hao J. Guo CL. Ma QW, et al. iPS cells produce viable mice through tetraploid complementation. Nature. 2009;461:86–90. doi: 10.1038/nature08267. [DOI] [PubMed] [Google Scholar]
- 5.Ying QL. Wray J. Nichols J. Batlle-Morera L. Doble B. Woodgett J. Cohen P. Smith A. The ground state of embryonic stem cell self- renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall J. Guo G. Wray J. Eyres I. Nichols J. Grotewold L. Morfopoulou S. Humphreys P. Mansfield W, et al. Oct4 and LIF/Stat3 additively induce Kruppel factors to sustain embryonic stem cell self-renewal. Cell Stem Cell. 2009;5:597–609. doi: 10.1016/j.stem.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Yang J. van Oosten AL. Theunissen TW. Guo G. Silva JC. Smith A. Stat3 activation is limiting for reprogramming to ground state pluripotency. Cell Stem Cell. 7:319–328. doi: 10.1016/j.stem.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo G. Yang J. Nichols J. Hall JS. Eyres I. Mansfield W. Smith A. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development. 2009;136:1063–1069. doi: 10.1242/dev.030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edel MJ. Menchon C. Menendez S. Consiglio A. Raya A. Izpisua Belmonte JC. Rem2 GTPase maintains survival of human embryonic stem cells as well as enhancing reprogramming by regulating p53 and cyclin D1. Genes Dev. 2010;24:561–573. doi: 10.1101/gad.1876710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalaszczynska I. Geng Y. Iino T. Mizuno S. Choi Y. Kondratiuk I. Silver DP. Wolgemuth DJ. Akashi K. Sicinski P. Cyclin A is redundant in fibroblasts but essential in hematopoietic and embryonic stem cells. Cell. 2009;138:352–365. doi: 10.1016/j.cell.2009.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ying QL. Nichols J. Chambers I. Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self- renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 12.Barrett JF. Lewis BC. Hoang AT. Alvarez RJ., Jr. Dang CV. Cyclin A links c-Myc to adhesion-independent cell proliferation. J Biol Chem. 1995;270:15923–15925. doi: 10.1074/jbc.270.27.15923. [DOI] [PubMed] [Google Scholar]
- 13.Hoang AT. Lutterbach B. Lewis BC. Yano T. Chou TY. Barrett JF. Raffeld M. Hann SR. Dang CV. A link between increased transforming activity of lymphoma-derived MYC mutant alleles, their defective regulation by p107, and altered phosphorylation of the c-Myc transactivation domain. Mol Cell Biol. 1995;15:4031–4042. doi: 10.1128/mcb.15.8.4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y. Yin X. Qin H. Zhu F. Liu H. Yang W. Zhang Q. Xiang C. Hou P, et al. Two supporting factors greatly improve the efficiency of human iPSC generation. Cell Stem Cell. 2008;3:475–479. doi: 10.1016/j.stem.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Li H. Collado M. Villasante A. Strati K. Ortega S. Canamero M. Blasco MA. Serrano M. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature. 2009;460:1136–1139. doi: 10.1038/nature08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawamura T. Suzuki J. Wang YV. Menendez S. Morera LB. Raya A. Wahl GM. Belmonte JC. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature. 2009;460:1140–1144. doi: 10.1038/nature08311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong H. Takahashi K. Ichisaka T. Aoi T. Kanagawa O. Nakagawa M. Okita K. Yamanaka S. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460:1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Utikal J. Polo JM. Stadtfeld M. Maherali N. Kulalert W. Walsh RM. Khalil A. Rheinwald JG. Hochedlinger K. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature. 2009;460:1145–1148. doi: 10.1038/nature08285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menchon C. Edel MJ. Izpisua Belmonte JC. The cell cycle inhibitor p27 (Kip1) controls self-renewal and pluripotency of human embryonic stem cells by regulating the cell cycle, Brachyury and Twist. Cell Cycle. 2011;10:1435–1447. doi: 10.4161/cc.10.9.15421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edel MJ. Belmonte JC. The cell cycle and pluripotency: is there a direct link? Cell Cycle. 2010;9:2694–2695. doi: 10.4161/cc.9.14.12456. [DOI] [PubMed] [Google Scholar]
- 21.Batlle-Morera L. Smith A. Nichols J. Parameters influencing derivation of embryonic stem cells from murine embryos. Genesis. 2008;46:758–767. doi: 10.1002/dvg.20442. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Danes A. Richaud-Patin Y. Carballo-Carbajal I. Jimenez-Delgado S. Caig C. Mora S. Di Guglielmo C. Ezquerra M. Patel B, et al. Disease-specific phenotypes in dopamine neurons from human iPS-based models of genetic and sporadic Parkinson's disease. EMBO Mol Med. 2012;4:380–395. doi: 10.1002/emmm.201200215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raya A. Rodriguez-Piza I. Aran B. Consiglio A. Barri PN. Veiga A. Izpisua Belmonte JC. Generation of cardiomyocytes from new human embryonic stem cell lines derived from poor-quality blastocysts. Cold Spring Harb Symp Quant Biol. 2008;73:127–135. doi: 10.1101/sqb.2008.73.038. [DOI] [PubMed] [Google Scholar]
- 24.Raya A. Rodriguez-Piza I. Navarro S. Richaud-Patin Y. Guenechea G. Sanchez-Danes A. Consiglio A. Bueren J. Izpisua Belmonte JC. A protocol describing the genetic correction of somatic human cells and subsequent generation of iPS cells. Nat Protoc. 2010;5:647–660. doi: 10.1038/nprot.2010.9. [DOI] [PubMed] [Google Scholar]
- 25.Raya A. Rodriguez-Piza I. Guenechea G. Vassena R. Navarro S. Barrero MJ. Consiglio A. Castella M. Rio P, et al. Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells. Nature. 2009;460:53–59. doi: 10.1038/nature08129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez F. Barragan Monasterio M. Tiscornia G. Montserrat Pulido N. Vassena R. Batlle Morera L. Rodriguez Piza I. Izpisua Belmonte JC. Generation of mouse-induced pluripotent stem cells by transient expression of a single nonviral polycistronic vector. Proc Natl Acad Sci U S A. 2009;106:8918–8922. doi: 10.1073/pnas.0901471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aasen T. Raya A. Barrero MJ. Garreta E. Consiglio A. Gonzalez F. Vassena R. Bilic J. Pekarik V, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26:1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 28.Kang L. Wang J. Zhang Y. Kou Z. Gao S. iPS cells can support full-term development of tetraploid blastocyst-complemented embryos. Cell Stem Cell. 2009;5:135–138. doi: 10.1016/j.stem.2009.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.