Abstract
Human amniotic mesenchymal stem cells (hAMSCs) demonstrated partially pluripotent characteristics with a strong expression of Oct4 and Nanog genes and immunomodulatory properties characterized by the absence of HLA-DR and the presence of HLA-G and CD59. The hAMSCs were reprogrammed into induced pluripotent stem cells (iPSCs) that generate a promising source of universal cardiac cells. The hAMSC-derived iPSCs (MiPSCs) successfully underwent robust cardiac differentiation to generate cardiomyocytes. This study investigated 3 key properties of the hAMSCs and MiPSCs: (1) the reprogramming efficiency of the partially pluripotent hAMSCs to generate MiPSCs; (2) immunomodulatory properties of the hAMSCs and MiPSCs; and (3) the cardiac differentiation potential of the MiPSCs. The characteristic iPSC colony formation was observed within 10 days after the transduction of the hAMSCs with a single integration polycistronic vector containing 4 Yamanaka factors. Immunohistology and reverse transcription–polymerase chain reaction assays revealed that the MiPSCs expressed stem cell surface markers and pluripotency-specific genes. Furthermore, the hAMSCs and MiPSCs demonstrated immunomodulatory properties enabling successful engraftment in the SVJ mice. Finally, the cardiac differentiation of MiPSCs exhibited robust spontaneous contractility, characteristic calcium transience across the membrane, a high expression of cardiac genes and mature cardiac phenotypes, and a contractile force comparable to cardiomyocytes. Our results demonstrated that the hAMSCs are reprogrammed with a high efficiency into MiPSCs, which possess pluripotent, immunomodulatory, and precardiac properties. The MiPSC-derived cardiac cells express a c-kit cell surface marker, which may be employed to purify the cardiac cell population and enable allogeneic cardiac stem cell therapy.
Introduction
The generation of induced pluripotent stem cells (iPSCs) from differentiated adult cells has vast therapeutic implications in regenerative medicine. Many strategies have been developed for iPSC generation, including genomic integration, synthetic mRNA, small molecules, and protein-based reprogramming [1–4]. However, the identification of an optimal cell population, which can be readily induced into the pluripotent state, may be equally important. More noteworthy is that the current iPSC reprogramming strategy is an inefficient and slow process, which may limit their immediate usage in biological and translational research [5]. Differentiated cells are known to demonstrate lower reprogramming efficiency, and different somatic cells are found to possess differential reprogramming ability [6]. In human fibroblasts, only around 0.01% of the cells transduced with the 4 Yamanaka's factors (Sox2, Klf4, Oct4, cMyc; SKOM) form AP+ (alkaline phosphatase) iPSC colonies [7–9]. The robust and rapid generation of iPSCs has raised an important challenge in the field of stem cell research and regenerative medicine. In this study, we report a unique population of the human amniotic mesenchymal stem cells (hAMSCs) with a high reprogramming efficiency to generate iPSCs.
Placental tissue is readily available, easily procured without invasive procedures, and does not elicit ethical debate. Two regions of the amniotic membrane of the placenta contain the partially pluripotent epiblast population of the human amniotic epithelial cells and extraembryonic mesoderm population of hAMSCs [10]. These cells have been described as differentiating predominantly along the mesodermal lineage and as demonstrating precardiac commitment [11–13]. Furthermore, recent reports indicate partial pluripotency of the hAMSCs with a high expression of pluripotency-specific genes, Nanog and Oct4 [14]. In addition, the hAMSCs demonstrate the immunomodulatory properties that are known to suppress host immune responses. Interestingly, amniotic cells have never shown signs of aging and tumorigenecity even after propagation for more than 2 years in culture [15].
The hAMSCs were transduced via polycistronic lentivirus containing 4 transcription factors: Oct4, Sox2, c-Myc, and Klf4. The hypothesis that the robustly generated hAMSC-derived iPSCs (MiPSCs) will exhibit immunomodulatory and cardiac differentiation properties was tested. The findings from this study demonstrated that the hAMSCs generate a robust population of iPSCs (MiPSCs) characterized by stem cell surface markers, pluripotency genes, and immunomodulatory properties. More significantly, the MiPSCs readily demonstrated spontaneous contractility on day 12 of the cardiac differentiation protocol with mature cardiac phenotypes. This study suggests that these characteristics of MiPSCs may enable a source of universal cardiac cells.
Materials and Methods
hAMSC isolation from the human placenta
Human placentas were obtained from healthy subjects at the Stanford University Medical Center, Stanford, CA. All donors provided written informed consent before collection. Under stringent sterile conditions, the harvested placentas were placed in HBSS media (Invitrogen). The human amniotic membrane was carefully separated from the chorion, and the membrane was immediately washed 3 to 5 times with 0.9% NaCl solution to remove blood and mucus. The membrane was cut into 2×2 cm pieces and transferred into an enzymatic digestion buffer containing trypsin-EDTA (Invitrogen) in phosphate-buffered saline and incubated at 37°C for 10, 20, and 30 min. The digested tissue was centrifuged, and the supernatant was discarded. Then, the tissue was subjected to a second enzymatic digestion in 50 mL HBSS (Invitrogen) containing 50 mg type I collagenase (Invitrogen), 0.01% papain (Sigma), and 10% fetal bovine serum (FBS) for 2 h at 37°C. After digestion, the suspension was filtered through a sterile 70 mm filter (BD Biosciences), and the cells were collected by centrifugation at 200 g for 5 min. The collected cells were designated as hAMSCs. The sorted cells were cultured in the Dulbecco's modified Eagle medium (DMEM) supplemented with 110 mg/L sodium pyruvate, 4 mM l-glutamine, 10% FBS, 1% Pen-Strep, and 10 ng/mL EGF (R&D Systems) at 37°C, 5% CO2.The c-kit (+) sub-population of hAMSCs was fluorescent activated cell sort (FACS) sorted by excluding the HLA-DR, CD34, and CD45 (immunity and hematopoietic markers; Biolegend) cells and by including the SSEA3, SSEA4, TRA1-60, TRA1-81, thy-1, and c-kit (stemness and cardiac markers; Biolegend) cells.
Virus production and iPSCs generation
The plasmid of pHAGE2-EF1α-OKSM vectors (courtesy of Mostoslavsky, G, Ph.D., Boston University) was employed. To generate the virus, 293T cells were transfected at 90% confluence using lipofectamine (Invitrogen). For a 10 cm plate, 1 mL OPTIMEN (Invitrogen), 36 μL lipofectamine, and 24 μg DNA mixture (20:1:1:1:2; pHAGE2: tat: rev: gag/pol: vsv-g) were used. The virus was harvested over 3 days and concentrated by spinning for 1.5 h at 16,500 RPM at 4°C. Approximately, 100,000 hAMSCs were seeded using a 6-well plate and infected with 150 μL of the concentrated virus in the presence of polybrene (10 mg/mL; Sigma). The medium was replaced after 24 h with DMEM supplemented with 20% FBS (Invitrogen) and changed every 2 days. On day 6 postinfection, the cells were trypsinized (trypLE; Invitrogen), passaged at a 1:6 ratio, and cultured onto one 6-well plate preseeded with irradiated mouse embryonic fibroblasts (MEF-irr; GlobalStem) on a feeder layer of 0.2% gelatin (Sigma). The cells were grown until spontaneous colony formation using human embryonic stem cell (hESC) culture media containing knockout DMEM (Invitrogen) with 20% knockout serum (Invitrogen), 1 mM of l-glutamine (Invitrogen), 0.1 mM mercaptoethanol (Millipore), 1% nonessential amino-acid solution (Invitrogen), and 10 ng/mL of bFGF (R&D Systems).
Alkaline phosphatase and immunofluorescent staining
Alkaline phosphatase staining was performed using the Leukocyte Alkaline Phosphatase kit (Sigma). For immunofluorescent staining, cells were fixed with phosphate-buffered saline (PBS) containing 4% paraformaldehyde (Sigma) for 10 min at room temperature. After washing with PBS, the cells were permeated using 0.25% Triton X-100 (Sigma) for 10 min, then blocked with 10% goat serum (Sigma) for 1 h at room temperature. Finally, the primary antibodies were incubated for 45 min at room temperature. The antibodies used in this study included SSEA3, SSEA4, Tra-1–60, Tra-1-81, CD117, CD90, CD34, CD45, HLA-DR, HLA-G (Biolegend), and CD59 (Millipore). The cell nucleus was stained with 1 μg/mL Hoechst 33342 (Invitrogen). In order to immunostain the contractile cardiomyocytes, cardiac troponin T (cTNT; Abcam), Connexin43 (Sigma), and α-sarcomeric actin (Abcam) were utilized. To quantify the c-kit (+) MiPSCs, a flow cytometry assay was carried out using a BD LSR analyzer (BD Bioscience). The antibody used in this study was anti-CD117 (Biolegend). The data were analyzed by Flowjo (Tree Star).
Teratoma formation from MiPSCs
About 2×106 MiPSCs were suspended in 100 μL PBS with 20% Matrigel (BD Bioscience) and transplanted into the hind limbs of immunodeficient (SCID) mice. Five weeks after the injection, tumor formation was observed. Hematoxylin and eosin (H&E) histological stain was performed to determine the tissue formation from 3 germ lines.
Cell transplantation and survival in SVJ mice
About 2×106reporter gene (luciferase) transduced mouse ESCs (mESCs) (E14 cell line), MiPSCs, and hAMSCs were suspended in 100 μL PBS with 20% Matrigel (BD Bioscience) that were transplanted into the hind limbs of SVJ mice. Under general anesthesia, concurrent BLI was performed using the charged-coupled device camera (IVIS spectrum; Caliper) after an IP injection of D-luciferin at 375 mg/kg body weight. Bioluminescence images were acquired for 30 min at a 3 min interval using Living image 2.5 (Caliper).
Reverse transcription–polymerase chain reaction
Total RNA was extracted using Trizol reagent (Invitrogen) according to the manufacturer's recommendations. Two micrograms of total RNA was transcribed into cDNA using Superscript first-strand synthesis system (Invitrogen). The PCR products were size fractionated by 2% agarose gel electrophoresis (Invitrogen). For real-time PCR, genes were amplified using iQ SYBR Green Supermix (Applied Biosystems) and StepOne Plus Real-Time PCR Detection System (Applied Biosystems). All genes were amplified for 40 cycles. Specific gene expression was first normalized to GAPDH and then compared with the control groups. Primer sequences are shown in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/scd).
Cardiomyocytes differentiation of MiPSCs
MiPSCs were differentiated toward cardiac lineage following a modified protocol reported by Burridge et al. [5]. Briefly, MiPSCs were dissociated using TryLE Express (Invitrogen), and maintained/adapted onto a 1:400 geltrex (Invitrogen)-coated culture surface. Single cells of MiPSCs were passaged strictly every 3 days. The cells from passage 10 on geltrex were used for cardiac differentiation. On the day of study, MiPSCs were allowed to aggregate to form embryoid bodies (EBs) in a V-shaped bottom 96-well plate with 25 ng/mL bone morphogenetic protein 4 (BMP4; R&D Systems) and 5 ng/mL bFGF in a RPMI-1640-based serum-free induction media for 2 days, followed by another 2 days of culture in 10% FBS in ultra-low cluster plates. On day 4, EBs were adhered on a Matrigel (BD Bioscience)-coated plate and fed with 1% FBS in RPMI-1640-based media. The EBs were then maintained in this medium, which was changed every 3 days. On observation of spontaneous contractility of the EBs, beating cells were characterized by flow cytometry, immunostain, L-type calcium2+ current imaging, and atomic force microscopy (AFM) to measure the force exerted with each beat.
Flow cytometry for beating MiPSCs
Beating MiPSCs were mechanically detached from the culture surface and dissociated using TryLE Express (Invitrogen) for 5 min. The cells were fixed and permeabilized with PharMingen Perm/Fix solution for 30 min at 4°C, and incubated with primary antibody against human cTNT (1:200; Abcam) overnight at 4°C. The next day, the cells were stained with fit-C conjugated secondary antibody (1:500; Abcam) for 30 min at room temperature. The expression of markers was determined by FACS Calibur (BD Bioscience) and FlowJo software (Tree Star) to quantify the percentage of cTNT+ cells. The cells were stained with mouse IgM isotype antibodies (Biolegend) that were used as the control group.
Live-cell calcium imaging for the contractile MiPSCs
The contractile EBs were mechanically detached from the tissue culture surface and dissociated with TrypLE Express for 5 min. Single cells were allowed to attach onto gelatinized glass coverslips (Lab-Tek; Thermo Scientific/Nunc) for 5 days before imaging. On the day of imaging, the cells were stained with 5 μM Fluo-4 AM and 0.02% Pluronic F-127 (Invitrogen) for 15 min, and a fluorescence signal from intracellular Ca2+ was monitored using fast-line scanning (1.92 ms/line) on a confocal microscope (LSM 510 Meta; Carl Zeiss) with a ×63 lens (NA=1.4) [16]. Images were further analyzed using ImageJ software.
AFM for beating MiPSCs
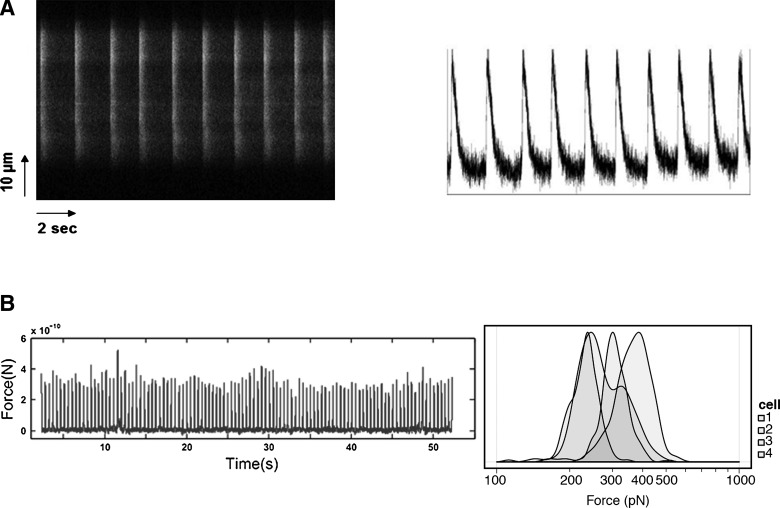
Contractile MiPSC-EBs were mechanically detached from the tissue culture surface and dissociated with TrypLE Express for 5 min. Single MiPSC were allowed to attach onto glass bottom plates for 3 days before the imaging. The culture media of MiPSCs were changed to a media containing 89% Tyrode's buffer, 10% fetal bovine serum (FBS), and 1% antibiotic-antimycotic (Cellgro) before the experiment. Cells were maintained at 36°C during the measurement. The beating of cells was measured by AFM (MFP-3D Bio) using a silicon nitride cantilever (spring constants ∼0.1 N/m; PPP-ContAu, NanoSensors). Cells were gently contacted by the cantilever tip with 400 pN of force. The cantilever tip remained in the position without Z-piezo feedback, and the deflection data were collected at a sample rate of 1 kHz for 2 min. The deflection trajectory was converted to force trajectory by multiplying by the spring constant and analyzed by using a Matlab (Mathworks) program. In the force trajectory, the amplitude of beating peaks provided the measurement of cell beating force. The full width at half maximum of beating peaks measured the beat duration. The reciprocal of the peak-to-peak interval measured the beating frequency.
Leukocyte-mediated cytotoxicity assays
In order to examine the immune characteristics of MiPSCs, a leukocyte-mediated cytotoxicity assay was performed. One week after an intramuscular injection of MiPSCs, hESCs (H7 line; Wicell), MEFs (derived from syngeneic SVJ strain), mouse iPSCs (derived from syngeneic SVJ strain), and hAMSCs into 2 SVJ mice per cell type, the mice were scarified for splenocyte isolation. The spleen was placed into a 70Um cell strainer (BD Biosciences) that was preplaced in a Petri dish with cold 20 mL RPMI-1640 (Invitrogen). Using the plunger end of the syringe, the spleen was forced through the cell strainer into the Petri dish. The cell strainer was rinsed with 5 mL RPMI-1640 and discarded. The suspended cells were transferred to a 15 mL conical tube and centrifuged at 1,200 rpm for 5 min at lower temperature (4°C–8°C). The supernatant was discarded and resuspended in a pellet in 1 mL ACK lysis buffer (Invitrogen). The cells were incubated at room temperature for 5–10 min, and 9 mL of RPMI-1640 were added and spun as earlier. The cells were resuspended in 5 mL RPMI-1640 with 10% FBS and 1% penicillin and streptomycin (PS). The cells were transferred into a 25 cm2 flask, incubated at 37°C, 5% CO2, and 95% humidity for at least 2 h to remove adherent cells. After 2 h, the splenocytes were cocultured with MiPSCs, MEF, H7, mouse iPSCs, and c-kit (+) hAMSCs in 24-well plates. After 3 days, the cytotoxic effects were measured by glucose-6-phosphate dehydrogenase (G6PD) released from damaged cells using the Vybrant Cyto-toxicity Assay Kit (Invitrogen).
Statistical analysis
Data were presented as the mean±standard deviation. A 2-way analysis of variance was performed. The Tukey–Kramer post hoc test was used for all pair-wise comparisons, and statistical significance was set at P<0.05. All statistical analyses were performed using the JMP statistical software package (SAS Institute).
Results
Immunomodulatory properties of hAMSCs
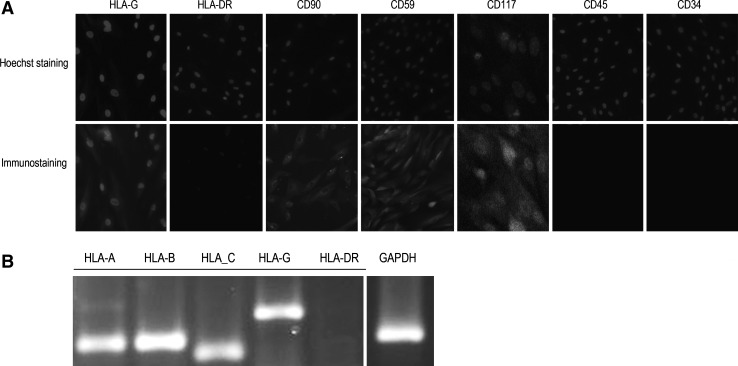
The hAMSCs were isolated from the amniotic membrane of the placenta. This population of hAMSCs demonstrated a high expression of CD59 by immunohistology, presence of HLA-G, and absence of HLA-DR by reverse transcription–polymerase chain reaction (RT-PCR) assays (Fig. 1A, B). Human HLA-G is overexpressed in the placenta to protect the fetal allograft during pregnancy [17]. In the human MSCs, HLA-G contributes to the immunomodulatory properties that allow MSCs to block alloimmune reactions [17,18]. Soluble HLA-G (sHLA-G) molecules produced by the placenta induce the apoptosis of activated CD8+ T-cells and inhibit CD4+ T-cell proliferation, which play a significant role in the immunosuppressive effects of the hAMSCs. CD59, a complement regulatory protein, prevents complement-mediated cell damage through the inhibition of the complement-mediated membrane attack complex [19].
FIG. 1.
Immunostain and reverse RT-PCR of the immunomodulatory properties of hAMSCs. (A) Immunostain demonstrates the specific immune characteristics of the hAMSCs: (+)CD59, (+)HLA-G, and (−)HLA-DR. Hoechst 33258 was used for nuclei staining. (B) Reverse RT-PCR analysis similarly confirms the (+)CD59, (+)HLA-G, and (−)HLA-DR profile of hAMSCs. hAMSCs, human amniotic mesenchymal stem cells; RT-PCR, reverse transcription–polymerase chain reaction.
Robust generation of iPSCs from hAMSCs (MiPSCs) and teratoma formation
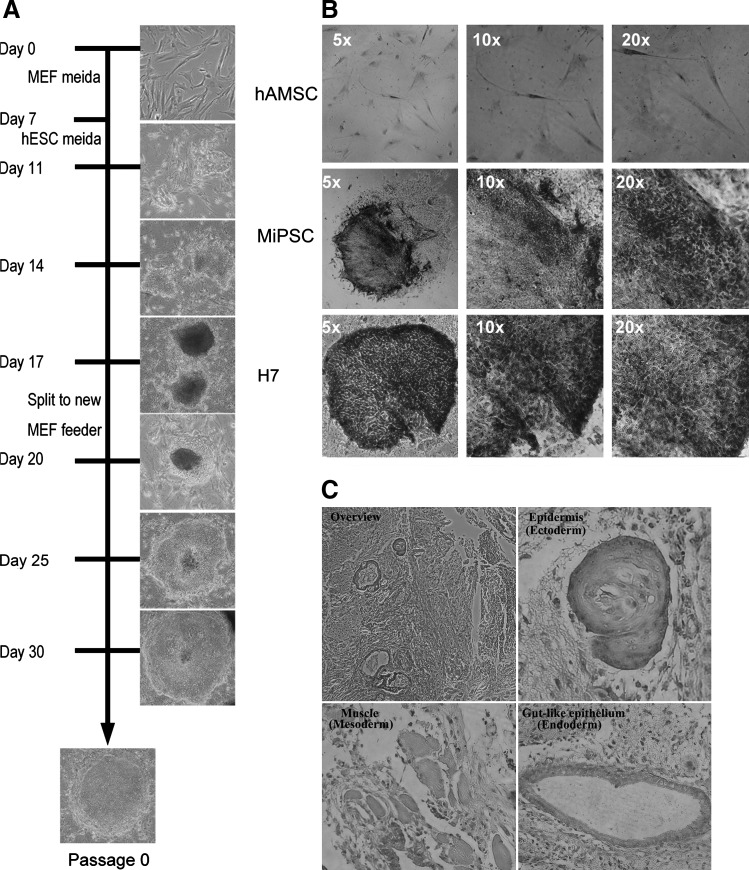
The characteristic colony formation resembling hESC colonies was observed around day 10 (Fig. 2A). The MiPSCs rapidly expanded on MEF feeder cells using hESC media. In this study, ∼200Oct4 positive colonies were found from 50,000 cells on day 18. However, from the fibroblast-derived iPSCs, 150 Oct4 positive colonies were found from 500,000 cells on day 34. These findings resulted in reprogramming the frequency of hAMSCs on day 18 to be 0.4% (200/50,000), while the fibroblasts on day 34 exhibited 0.03% (150/500,000) reprogramming frequency (Supplementary Fig. S1).These findings were confirmed by flow cytometry and real-time PCR, similarly demonstrating the high reprogramming efficacy of hAMSCs on day 14. Flow cytometry revealed ∼8-fold higher Oct4 positive cells, while RT-PCR demonstrated 20-fold higher Oct4 expression (Supplementary Figs. S2 and S3). Morphologically, the characteristic iPSC colonies were observed on day 20, which displayed a cobblestone appearance with prominent nucleoli and distinct individual cell border associated with high activity of alkaline phosphatases similar to the hESCs but not found in the hAMSCs (Fig. 2B). 2×106 MiPSCs were transplanted into the hind limbs of immunodeficient (SCID) mice. Five weeks after the injection, tumor formation was observed. H&E histological stain demonstrated evidence of 3 germ lines (Fig. 2C): epidermis (ectoderm), muscle (mesoderm), and gut-like epithelium (endoderm).
FIG. 2.
Generation of the MiPSCs from hAMSCs. (A) Timeline of the MiPSC generation from the hAMSCs demonstrates the first ESC-like colony found within the first 11 days after transduction. At day 20, the characteristic iPSC colonies were observed, which displayed a cobblestone appearance with prominent nucleoli and a distinct individual cell border. (B) The MiPSCs demonstrated high alkaline phosphatases activity similar to the H7 hESCs but that was not found in the hAMSCs. (C) Teratoma derived from the MiPSCs after transplantation into the hind limbs of SCID mouse was found 5 weeks after the injection. Hematoxylin and eosin stain confirmed that the tumor contained the tissue from 3 germ lines, including gut-like epithelial tissues (endoderm), muscle (mesoderm), and epidermis tissues (ectoderm). iPSC, induced pluripotent stem cells; MiPSC, hAMSC-derived iPSCs; hESCs, human embryonic stem cells.
MiPSCs displayed typical features of H7 hESCs
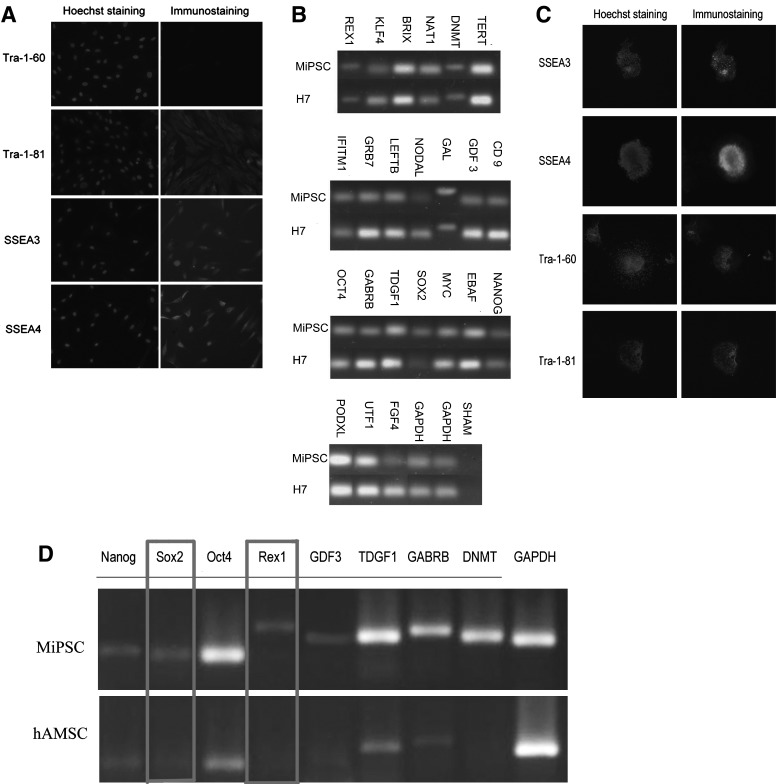
The hAMSCs demonstrate the partially pluripotent properties by the expression of SSEA3, SSEA4, and TRA-1-81(Fig. 3A). This unique characteristic of the hAMSCs has proved to be beneficial for iPSC generation. The RT-PCR data showed that MiPSCs expressed many hESC-pluripotency marker genes, including Oct4, Sox2, Nanog, growth, and differentiation factors 3 (GDF3), fibroblast growth factor 4 (FGF4), and Rex1 (Fig. 3B).Immunostaining results demonstrated that the MiPSCs expressed hESC-specific surface antigens, including SSEA3, SSEA4, Tra-1-60, and Tra-1-81(Fig. 3C). These findings were notable, as Sox2, Rex1, and Tra-1-60 were not seen in the native population of hAMSCs (Fig. 3D).
FIG. 3.
Stemness properties of the hAMSCs and MiPSCs. (A) Immunostaining of hAMSCs demonstrated(+)Tra-1-81, (+)SSEA3, (+)SSEA4, and (−)Tra-1-60 demonstrating partial pluripotency of the hAMSCs. (B) RT-PCR was performed to compare a total of 23 ESC marker expressions between the H7 hESCs and MiPSCs. (C) Immunostain of the MiPSCs was positive for SSEA3, SSEA4, Tra-1-60, and Tra-1-80. Note Tra-1-60 was negative for the hAMSCs. (D) Comparison of the stemness properties between the hAMSCs and MiPSCs demonstrated the absence of Sox2 and Rex1 in the hAMSCs.
MiPSCs retained the immunomodulatory properties of hAMSC
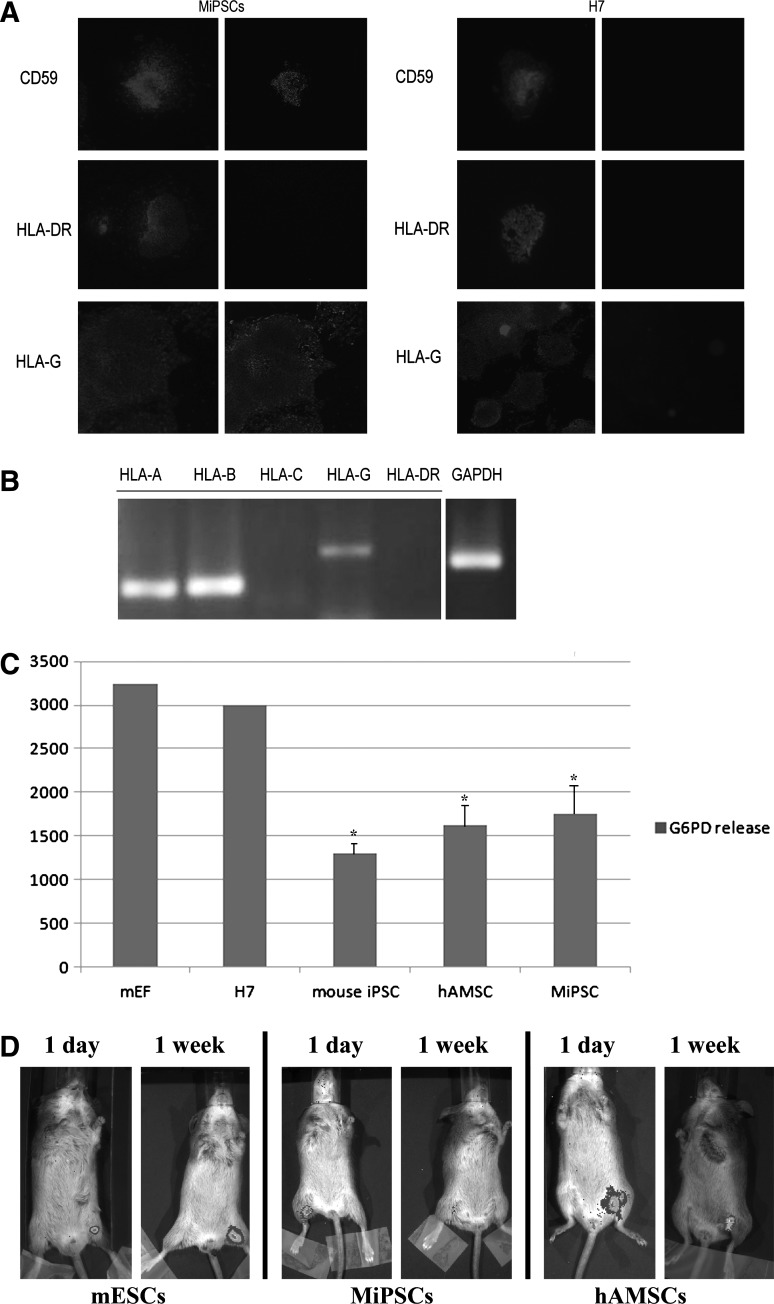
The MiPSCs expressed high levels of HLA-G and CD59, while the expression of HLA-DR [major histocompatibility complex (MHC) class II] and HLA-C (MHC class I) were not detected by immunohistology and RT-PCR assays (Fig. 4A, B). These findings suggested that the absence of MHC Class I and II in the MiPSCs will enable transplantation across the MHC barriers to overcome some of the major immunologic obstacles faced by these cells. Furthermore, the positive expression of CD59 and HLA-G and the absence of HLA-DR and HLA-Cin the MiPSCs indicate that the immunomodulatory property of the hAMSCs is retained during the reprogramming process. These characteristics were not observed in the H7 hESCs (Fig. 4A). Real-time PCR was performed to measure the expression of HLA-G in MiPSCs, hAMSCs, H7 hESCs, and iPSCs from fibroblasts. The results revealed that HLA-G and CD59 were only expressed in hAMSCs and MiPSCs. The markers were found in neither H7 hESCs nor iPSCs from fibroblasts (Supplementary Fig. S4).To examine the immune characteristics of MiPSCs, 2 immunocompetent SVJ mice underwent an intramuscular injection with each of the following cell types: MiPSCs, H7 hESCs, MEFs (nonsyngeneic), mouse iPSCs (syngeneic), and hAMSCs. One week after the intramuscular injection, the splenocytes of the recipient mice were removed and cocultured with each cell type. The leukocyte-mediated cytotoxicity was assessed by measuring the G6PD release in each coculture. The cytotoxicity in the splenocyte cocultures containing the H7 hESCs and MEFs significantly increased when compared with the MiPSCs and hAMSCs (P<0.05, n=5, Fig. 4C). Splenocytes consist of a variety of immune cell populations including T and B lymphocytes, dendritic cells, and macrophages, which have different immune functions. These data confirm the immunosuppressive effects from the absence of HLA-DR to attenuate the response of the T-helper cells to activate the killer T cells and macrophages and the presence of HLA-G and CD59 to induce the apoptosis of activated CD8+ T-cells, inhibit CD4+ T-cell proliferation, and attenuate complement-mediated cytotoxicity [18,20,21].These findings provide evidence that the transplanted MiPSCs may survive in vivo by inhibiting the host immune response.
FIG. 4.
Immune characteristics of the MiPSCs. (A) Immunostain confirms that both CD 59 and HLA-G were positive and HLA-DR was negative in the MiPSCs (left). However, this profile was not seen with the H7 hESCs (right). (B) RT-PCR analysis was performed to confirm the absence of HLA-DR and HLA-C in the MiPSCs. (C) Splenocytes cocultured with MEF, hESCs, MiPSCs, mouse iPSCs, and hAMSCs for 24 h. Significantly decreased cytotoxicity (P<0.05, n=5) was observed in the cocultures containing the MiPSCs, hAMSCs, and mouse iPSCs (syngeneic). (D) Mouse ESCs, MiPSCs, and hAMSCs were injected into the hind limbs of immunocompetent SVJ mice. The mESCs and hAMSCs demonstrated robust BLI survival signals, while the MiPSCs did not do so at week 1. MEF, mouse embryonic fibroblasts.
The in vivo study demonstrated robust BLI survival signal by the luciferase-transduced mESCs (syngeneic) and hAMSCs transplanted into the mouse hindlimb at week 1 (Fig. 4D). These results verified that HLA-G and CD59 played an important role in the in vivo survival of the hAMSCs. However, the luciferase-transduced MiPSCs only survived 5 days in the SVJ mouse, which is contrary to the in vitro data and robust survival of hAMSCs. There may be other nonimmunological reasons for their poor survival, which is currently under investigation.
C-kit (+) MiPSCs displayed robust cardiac differentiation
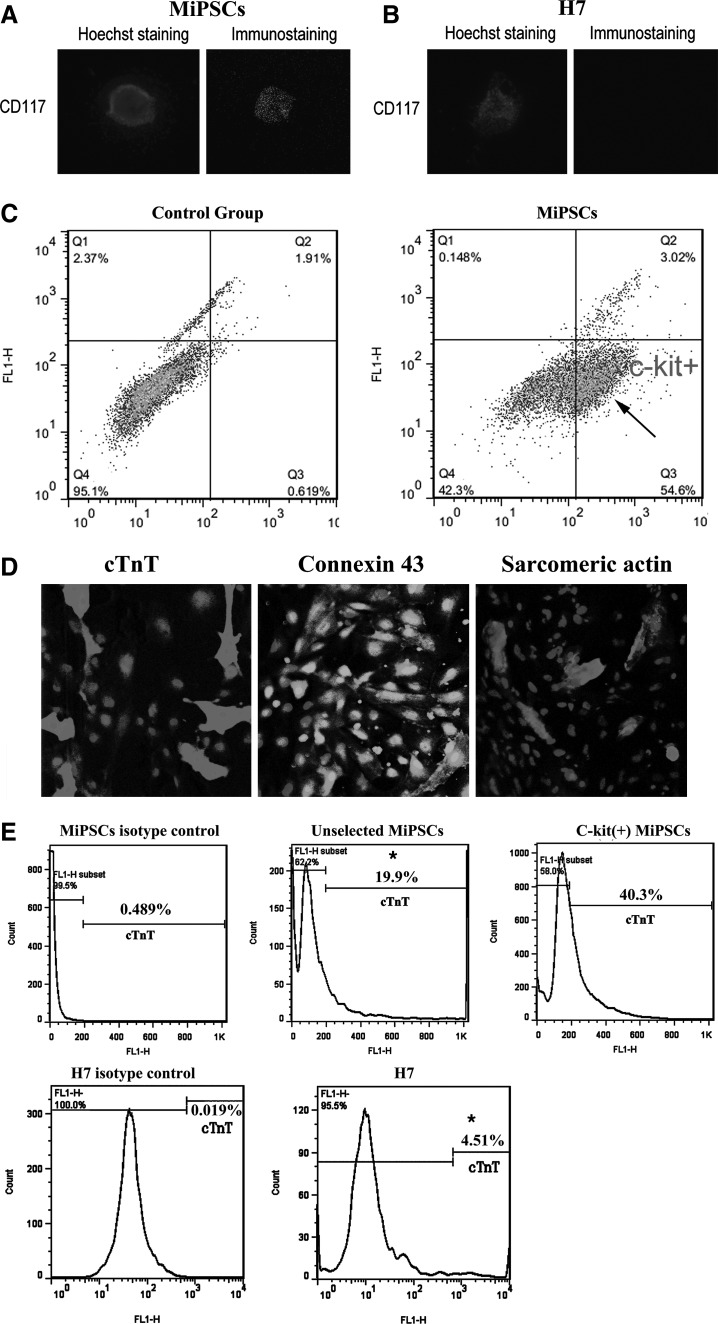
The c-kit (+) hAMSC sub-population represented a highly selective sub-population. They were sorted by FACS by excluding the HLA-DR, CD34, and CD45 (immunity and hematopoietic markers) cells and by including the SSEA3, SSEA4, TRA1-60, TRA1-81, thy-1, and c-kit (stemness and cardiac markers) cells. C-kit (CD117) is considered an important surface marker of precardiac mesodermal progenitor cells [22,23]. This marker was not found in the H7 hESCs but was expressed robustly in the human MiPSCs (Fig. 5A, B). Flow cytometry assay revealed that the proportion of c-kit positive MiPSCs (MiPSC+s) was ∼54.6% of the total MiPSCs. IgM isotype for the MiPSCs served as the control group, demonstrating negligible signals (Fig. 5C).
FIG. 5.
Cardiac differentiation profile of the MiPSCs. (A) C-kit (CD 117) surface marker was positive in MiPSCs. (B) C-kit (CD 117) surface marker was negative in H7 hESCs. (C) Flow cytometry for MiPSCs demonstrated significant c-kit (+) MiPSC sub-population (arrow). The proportion of c-kit (+) MiPSCs was ∼55%. The control group, IgM isotype stain, exhibited negligible c-kit expression. (D) The contractile areas of the differentiated MiPSCs were characterized by the colocalization of cTNT, Connexin 43, and alpha-sarcomeric actin. Hoechst 33258 was used for nuclei staining (blue). (E) Flow cytometry analysis revealed ∼40.3% cTNT+ cells derived from c-kit (+) MiPSCs, 19.9%* cTnT+ cells derived from unselected MiPSCs, and 4.5%* cTNT+ cells derived from H7 hESCs (*P<0.05, n=3). The cells derived from both MiPSCs and H7 cells were stained with mouse IgM isotype antibodies to be used as the control group, respectively. cTNT, cardiac troponin T.
In order to investigate the cardiac differentiation potential of MiPSC+s, the cells underwent cardiac differentiation. Contractile areas were observed at day 12 after induction with the modified cardiac differentiation protocol [5] (Supplementary Movies S1 and S2). By day 28, more than 95% of the EBs generated from the MiPSCs was found to be spontaneously beating. Immunohistology and flow cytometry of the beating cells showed the robust colocalization of cTnT, connexin 43, and α-sarcomeric actin in the contractile areas (Fig. 5D). Furthermore, ∼40% of the cells derived from the MiPSC+s were found to be (+)cTNT in comparison to the 19.9%* (+)cTnT cells from the unselected MiPSCs, and 4.51%* (+)cTnT cells from H7 cells (*P<0.05, n=3, Fig. 5E).The contractile MiPSC-derived cardiomyocytes exhibited the characteristic L-type Ca2+ current/transient peaks across the membrane with each depolarization, as indicated by the calcium-dependent intracellular fluorescence intensity detected by confocal microscopy (Fig. 6A). Finally, AFM analysis of the 4 representative contractile MiPSC-derived cardiomyocytes showed that the maximal contractile force of each beat was comparable to that of native cardiomyocytes (10-0.4∼10-0.6 nN) (Fig. 6B).In comparison to other published studies on the cardiac differentiation of iPSCs, MiPSC+s demonstrated a robust cardiac differentiation potential and generated an enriched pool of mature cardiac cells [1,8,24].
FIG. 6.
Electrophysiological characterization of the contractile MiPSC-derived cardiac cells. (A) Live-cell calcium imaging of the contractile MiPSC-derived cardiac cells exhibited a characteristic calcium transient with each depolarization. The left image represents a time-lapsed fluorescence image of a line scan of confocal stain, and the right image indicates the calcium peaks. The MiPSC-derived cardiac cells demonstrated mature cardiomyocyte characteristics. (B) The histogram of atomic force microscopy that evaluates the force exerted by each contraction of a representative MiPSC-derived cardiac cell (left image). The maximal contractile force measured for 4 representative beating cells was comparable to that of native cardiomyocytes (right image, 10-0.4∼10-0.6 nN).
Discussion
In this study, the high reprogramming efficiency of the hAMSCs to generate induced pluripotent stem cells (MiPSCs) was demonstrated. The MiPSCs retained the immunomodulatory and precardiac mesodermal properties of the hAMSCs. The partially pluripotent property of hAMSCs marked with an endogenous expression of Oct4 and Nanog conferred high reprogramming capability. The data revealed that the ESC-like colony morphology appeared within 10 days, which represents a rapid and expeditious reprogramming process [1,25]. Published reports suggest a 4-week duration for human iPSC generation from skin fibroblasts, which could be shortened to ∼14 to 20 days when enhanced with small molecules [25]. Some investigations have reported that the endogenous expression of reprogramming genes by adult somatic cells facilitates the generation of iPSCs [26,27]. However, an optimal adult somatic target cell population has not been identified. Although the 4Yamanaka factors were employed to transduce the hAMSCs into iPSCs, it is possible that 1 or more Yamanaka factors could be replaced by the endogenous pluripotency genes. Many studies have demonstrated that reduced number of Yamanaka factors such as Oct4 along with either c-Myc or Klf4 can reprogram adult mouse neural stem cells that endogenously expressed Sox2 [26]. The hAMSCs exhibit a high expression of Nanog and Oct4 with positive stem cell surface markers including SSEA3, SSEA4, Tra-1-81, and thy-1. However, the precise role of endogenous expression of Nanog and Oct4 in the rapid reprogramming process is not clear. The current study suggests that this unique population of hAMSCs represents a potentially promising source of cells for the robust and rapid generation of iPSCs that may overcome some critical issues in iPSC generation.
The MiPSCs maintain an immunomodulatory capability through the presence of CD59 and HLA-G and the absence of HLA-DR and HLA-C expression. The primary function of HLA-DR is to present peptide antigens, potentially foreign in origin, to the immune system for the purpose of eliciting T-helper cell responses that eventually lead to the production of antibodies against the same peptide antigen. On the other hand, HLA-G plays a role in immune tolerance during pregnancy for the placenta situated along the maternal-fetal border. The presence of sHLA-G has been associated with higher pregnancy rates [20]. The sHLA-G molecules induce apoptosis of the activated T-cells and inhibit CD4+ T-cell proliferation, effectively inhibiting alloimmune reactions [18,21]. In addition, CD59, a complement regulatory protein, prevents complement-mediated cell damage through inhibition of the complement membrane attack complex [19].
Immunologic reactions are considered a critical issue in stem cell-based therapy when using nonmatched stem cells. To address this issue, autologous iPSCs derived from the patient's somatic cells are considered a potentially landmark solution. Even in a situation where an immediate treatment is unnecessary, a therapeutic strategy to wait for ex vivo reprogramming and expansion with the subsequent re-transplantation of autologous stem cells may not be financially and clinically feasible [28]. Recently, Zhao et al. reported that the cells differentiated from iPSCs can induce T-cell-dependent immune responses in syngeneic recipients [29]. This represents a major challenge for iPSC transplantation therapy. Rather, an off-the-shelf supply of universal iPSC-derived cells may be more preferable. The data from this study suggest that the MiPSC-derived cells could make allogeneic iPSC transplantation therapy possible.
Finally, the MiPSCs retain the c-kit (CD117) cell surface marker (MiPSCs+). As described in many studies, c-kit is an important surface marker of cardiac progenitor cells in adult stem cells and has not been found in ESCs [30]. In 2009, Okabe and colleagues reprogrammed c-kit (+) hematopoietic cells from mouse to pluripotent stem cells by the direct viral transfer of iPSCs factors, but the c-kit expression did not persist in the iPSCs [31]. In our current study, the MiPSC+s derived from the hAMSCs demonstrated enhanced cardiac differentiation efficiency through a high percentage of spontaneous contractility in a short period of time. Furthermore, these MiPSC+-derived cardiac cells exhibited consistent development into a mature cardiomyocyte phenotype. Cardiac differentiation from iPSCs holds great promise for regenerative medicine; however, low reprogramming efficiency and suboptimal differentiation efficiency limit cardiomyocyte generation. Various molecular strategies, small molecules, and peptides led to numerous cardiac differentiation protocols [24,32–34]. However, the efficacy of these approaches was modest. An alternative solution is to select a cell population that may be predisposed to high reprogramming and cardiac differentiation efficiency.
Our study demonstrated that the unique characteristics of MiPSCs+ may enable a robust, rapid, and safe method for generating universal, off-the-shelf supply of cardiac lineage committed cells. The findings validated a cell-specific approach to translate novel stem cell biology to induce pluripotency and cardiac differentiation.
Supplementary Material
Acknowledgments
This study is funded by NIH R01 HL097516 (PY). The authors thank Dr. Xiaoxing Xiong and Dr. Peng Wang for their technical assistance in the cell imaging.
Author Disclosure Statement
Boehringer-Ingelheim: research support; General Electric Healthcare: research support.
References
- 1.Takahashi K. Tanabe K. Ohnuki M. Narita M. Ichisaka T. Tomoda K. Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Warren L. Manos PD. Ahfeldt T. Loh YH. Li H. Lau F. Ebina W. Mandal PK. Smith ZD, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi Y. Desponts C. Do JT. Hahm HS. Scholer HR. Ding S. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell. 2008;3:568–574. doi: 10.1016/j.stem.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Kim D. Kim CH. Moon JI. Chung YG. Chang MY. Han BS. Ko S. Yang E. Cha KY. Lanza R. Kim KS. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4:472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burridge PW. Thompson S. Millrod MA. Weinberg S. Yuan X. Peters A. Mahairaki V. Koliatsos VE. Tung L. Zambidis ET. A universal system for highly efficient cardiac differentiation of human induced pluripotent stem cells that eliminates interline variability. PLoS One. 2011;6:e18293. doi: 10.1371/journal.pone.0018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrne JA. Nguyen HN. Reijo Pera RA. Enhanced generation of induced pluripotent stem cells from a subpopulation of human fibroblasts. PLoS One. 2009;4:e7118. doi: 10.1371/journal.pone.0007118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esteban MA. Wang T. Qin B. Yang J. Qin D. Cai J. Li W. Weng Z. Chen J. Ni S. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell. 2010;6:71–79. doi: 10.1016/j.stem.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K. Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Yu J. Vodyanik MA. Smuga-Otto K. Antosiewicz-Bourget J. Frane JL. Tian S. Nie J. Jonsdottir GA. Ruotti V, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 10.Miki T. Strom SC. Amnion-derived pluripotent/multipotent stem cells. Stem Cell Rev Rep. 2006;2:133–141. doi: 10.1007/s12015-006-0020-0. [DOI] [PubMed] [Google Scholar]
- 11.Hoelters J. Ciccarella M. Drechsel M. Geissler C. Gulkan H. Bocker W. Schieker M. Jochum M. Neth P. Nonviral genetic modification mediates effective transgene expression and functional RNA interference in human mesenchymal stem cells. J Gene Med. 2005;7:718–728. doi: 10.1002/jgm.731. [DOI] [PubMed] [Google Scholar]
- 12.Khoo MLM. Shen B. Tao H. Ma DDF. Long-term serial passage and neuronal differentiation capability of human bone marrow mesenchymal stem cells. Stem Cells Dev. 2008;17:883–896. doi: 10.1089/scd.2007.0185. [DOI] [PubMed] [Google Scholar]
- 13.Hwang NS. Varghese S. Lee HJ. Zhang Z. Ye Z. Bae J. Cheng L. Elisseeff J. In vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cells. Proc Natl Acad Sci U S A. 2008;105:20641–20646. doi: 10.1073/pnas.0809680106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miki T. Lehmann T. Cai H. Stolz DB. Strom SC. Stem cell characteristics of amniotic epithelial cells. Stem Cells. 2005;23:1549–1559. doi: 10.1634/stemcells.2004-0357. [DOI] [PubMed] [Google Scholar]
- 15.Walther G. Gekas J. Bertrand OF. Amniotic stem cells for cellular cardiomyoplasty: promises and premises. Catheter Cardiovasc Interv. 2009;73:917–924. doi: 10.1002/ccd.22016. [DOI] [PubMed] [Google Scholar]
- 16.Yazawa M. Hsueh B. Jia X. Pasca AM. Bernstein JA. Hallmayer J. Dolmetsch RE. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature. 2011;471:230–234. doi: 10.1038/nature09855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouteiller P. Blaschitz A. The functionality of HLA-G is emerging. Immunol Rev. 1999;167:233–244. doi: 10.1111/j.1600-065x.1999.tb01396.x. [DOI] [PubMed] [Google Scholar]
- 18.Nasef A. Mathieu N. Chapel A. Frick J. Francois S. Mazurier C. Boutarfa A. Bouchet S. Gorin NC. Thierry D. Fouillard L. Immunosuppressive effects of mesenchymal stem cells: involvement of HLA-G. Transplantation. 2007;84:231–237. doi: 10.1097/01.tp.0000267918.07906.08. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y. Qiao F. Abagyan R. Hazard S. Tomlinson S. Defining the CD59-C9 binding interaction. J Biol Chem. 2006;281:27398–27404. doi: 10.1074/jbc.M603690200. [DOI] [PubMed] [Google Scholar]
- 20.Rebmann V. Switala M. Eue I. Grosse-Wilde H. Soluble HLA-G is an independent factor for the prediction of pregnancy outcome after ART: a German multi-centre study. Hum Reprod. 2010;25:1691–1698. doi: 10.1093/humrep/deq120. [DOI] [PubMed] [Google Scholar]
- 21.Le Bouteiller P. Blaschitz A. The functionality of HLA-G is emerging. Immunol Rev. 1999;167:233–244. doi: 10.1111/j.1600-065x.1999.tb01396.x. [DOI] [PubMed] [Google Scholar]
- 22.Orlic D. Kajstura J. Chimenti S. Jakoniuk I. Anderson SM. Li B. Pickel J. McKay R. Nadal-Ginard B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 23.Orlic D. Kajstura J. Chimenti S. Limana F. Jakoniuk I. Quaini F. Nadal-Ginard B. Bodine DM. Leri A. Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang L. Soonpaa MH. Adler ED. Roepke TK. Kattman SJ. Kennedy M. Henckaerts E. Bonham K. Abbott GW. Linden RM. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 25.Lin T. Ambasudhan R. Yuan X. Li W. Hilcove S. Abujarour R. Lin X. Hahm HS. Hao E. Hayek A. A chemical platform for improved induction of human iPSCs. Nat Methods. 2009;6:805–808. doi: 10.1038/nmeth.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JB. Zaehres H. Wu G. Gentile L. Ko K. Sebastiano V. Arauzo-Bravo MJ. Ruau D. Han DW. Zenke M. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature. 2008;454:646–650. doi: 10.1038/nature07061. [DOI] [PubMed] [Google Scholar]
- 27.Shi Y. Tae Do J. Desponts C. Hahm HS. Scholer HR. Ding S. A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;2:525–528. doi: 10.1016/j.stem.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Patel S. King C. Lim P. Habiba U. Dave M. Porecha R. Rameshwar P. Personalizing stem cell research and therapy: the arduous road ahead or missed opportunity? Curr Pharmacogenomics Person Med. 2010;8:25–36. doi: 10.2174/1875692111008010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao T. Zhang ZN. Rong Z. Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 30.Murry CE. Soonpaa MH. Reinecke H. Nakajima H. Nakajima HO. Rubart M. Pasumarthi KBS. Virag JI. Bartelmez SH. Poppa V. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 31.Okabe M. Otsu M. Ahn DH. Kobayashi T. Morita Y. Wakiyama Y. Onodera M. Eto K. Ema H. Nakauchi H. Definitive proof for direct reprogramming of hematopoietic cells to pluripotency. Blood. 2009;114:1764–1767. doi: 10.1182/blood-2009-02-203695. [DOI] [PubMed] [Google Scholar]
- 32.Wu X. Ding S. Ding Q. Gray NS. Schultz PG. Small molecules that induce cardiomyogenesis in embryonic stem cells. J Am Chem Soc. 2004;126:1590–1591. doi: 10.1021/ja038950i. [DOI] [PubMed] [Google Scholar]
- 33.Ieda M. Fu JD. Delgado-Olguin P. Vedantham V. Hayashi Y. Bruneau BG. Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kattman SJ. Witty AD. Gagliardi M. Dubois NC. Niapour M. Hotta A. Ellis J. Keller G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.