Abstract
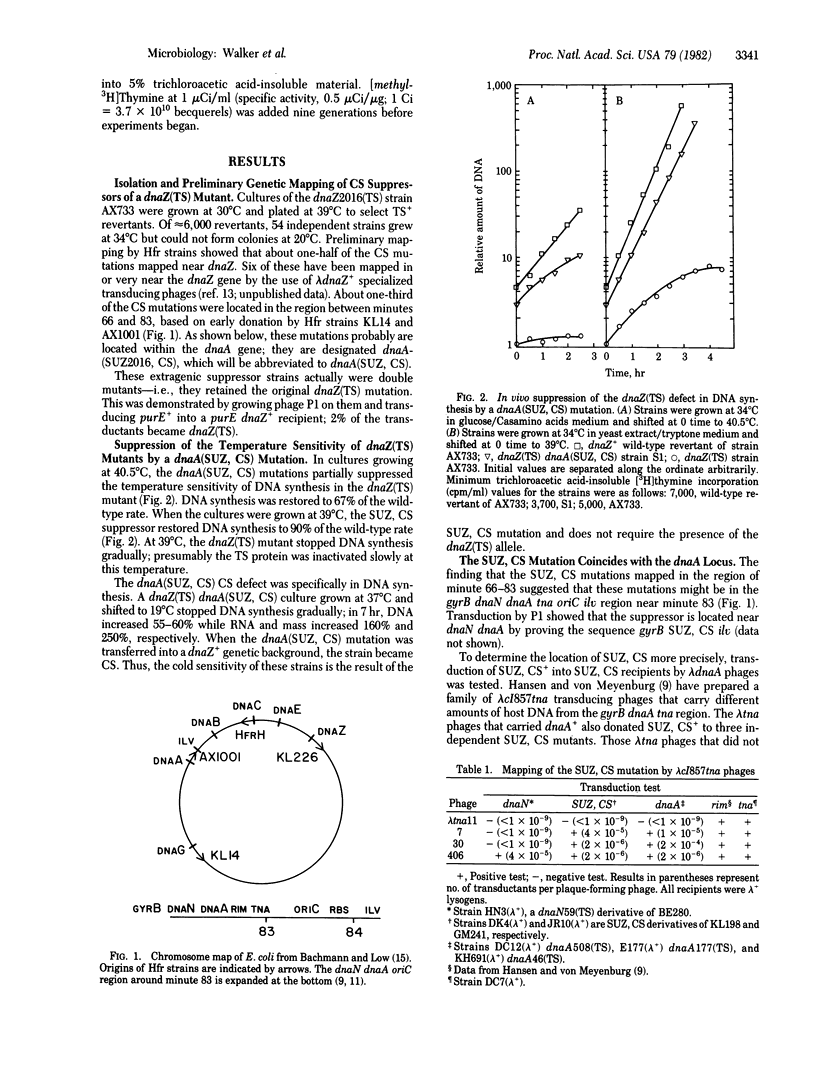
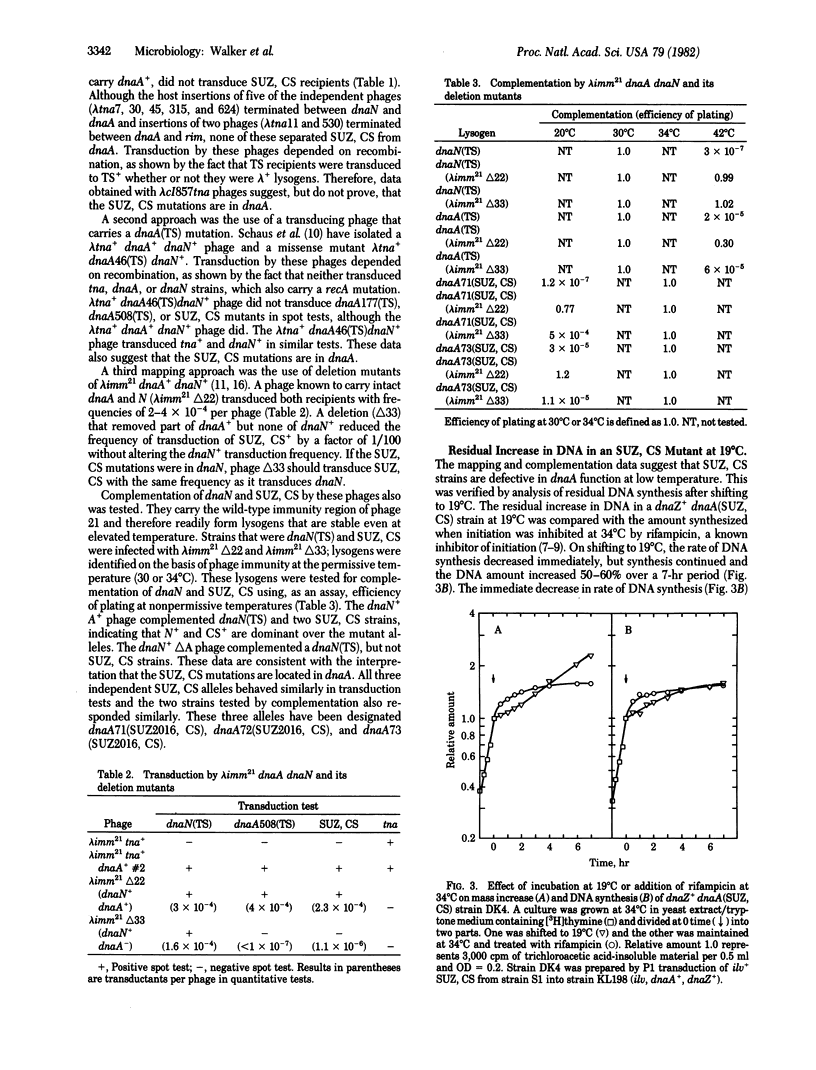
To define in vivo interactions of Escherichia coli DNA replication components, extragenic suppressors of a dnaZ(TS) mutant were isolated. A temperature-sensitive dnaZ mutant, which is defective in polymerization, was placed at 39 degrees C to select temperature-insensitive revertants. Some of these revertants also were cold sensitive, a phenotypic property that facilitated study of the suppressor. Mapping of the cold sensitivity indicated that some of the suppressor mutations are intragenic but others are located within the initiation gene, dnaA. The dnaA mutations that suppress the dnaZ(TS) defect are designated dnaA(SUZ, CS). The dnaA(SUZ, CS) strains have a defect in DNA synthesis at low temperature that is typical of an initiation defect. These data suggest that the dnaA product, an initiation factor, interacts in vivo with the dnaZ protein, a polymerization factor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdasarian M. M., Izakowska M., Bagdasarian M. Suppression of the DnaA phenotype by mutations in the rpoB cistron of ribonucleic acid polymerase in Salmonella typhimurium and Escherichia coli. J Bacteriol. 1977 May;130(2):577–582. doi: 10.1128/jb.130.2.577-582.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breschkin A. M., Mosig G. Multiple interactions of a DNA-binding protein in vivo. II. Effects of host mutations on DNA replication of phage T4 gene 32 mutants. J Mol Biol. 1977 May 15;112(2):295–308. doi: 10.1016/s0022-2836(77)80145-9. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. RNA polymerase. Annu Rev Biochem. 1971;40:711–740. doi: 10.1146/annurev.bi.40.070171.003431. [DOI] [PubMed] [Google Scholar]
- Chu H., Malone M. M., Haldenwang W. G., Walker J. R. Physiological effects of growth of an Escherichia coli temperature-sensitive dnaZ mutant at nonpermissive temperatures. J Bacteriol. 1977 Oct;132(1):151–158. doi: 10.1128/jb.132.1.151-158.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip C. C., Allen J. S., Gustafson R. A., Allen R. G., Walker J. R. Bacterial cell division regulation: characterization of the dnaH locus of Escherichia coli. J Bacteriol. 1974 Aug;119(2):443–449. doi: 10.1128/jb.119.2.443-449.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanegan J. B., Greenberg G. R. Regulation of deoxyribonucleotide biosynthesis during in vivo bacteriophage T4 DNA replication. Intrinsic control of synthesis of thymine and 5-hydroxymethylcytosine deoxyribonucleotides at precise ratio found in DNA. J Biol Chem. 1977 May 10;252(9):3019–3027. [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldenwang W. G., Walker J. R. Inhibition of bacteriophage M13 and phix174 duplex DNA replication and single-strand synthesis in temperature-sensitive dnaZ mutants of Escherichia coli. J Virol. 1977 Apr;22(1):23–30. doi: 10.1128/jvi.22.1.23-30.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldenwang W. G., Walker J. R. Parental RF formation on phages phiX174 and M13 requires the dnaZ gene product of Escherichia coli. Biochem Biophys Res Commun. 1976 Jun 7;70(3):932–938. doi: 10.1016/0006-291x(76)90681-1. [DOI] [PubMed] [Google Scholar]
- Hansen F. G., von Meyenburg K. Characterization of the dnaA, gyrB and other genes in the dnaA region of the Escherichia coli chromosome on specialized transducing phages lambda tna. Mol Gen Genet. 1979 Sep;175(2):135–144. doi: 10.1007/BF00425529. [DOI] [PubMed] [Google Scholar]
- Heil A., Zillig W. Reconstitution of bacterial DNA-dependent RNA-polymerase from isolated subunits as a tool for the elucidation of the role of the subunits in transcription. FEBS Lett. 1970 Dec;11(3):165–168. doi: 10.1016/0014-5793(70)80519-1. [DOI] [PubMed] [Google Scholar]
- Jarvik J., Botstein D. Conditional-lethal mutations that suppress genetic defects in morphogenesis by altering structural proteins. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2738–2742. doi: 10.1073/pnas.72.7.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung F. C., Glaser D. A. dnaA acts before dnaC in the initiation of DNA replication. J Bacteriol. 1978 Feb;133(2):755–762. doi: 10.1128/jb.133.2.755-762.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lark K. G. Evidence for the direct involvement of RNA in the initiation of DNA replication in Escherichia coli 15T. J Mol Biol. 1972 Feb 28;64(1):47–60. doi: 10.1016/0022-2836(72)90320-8. [DOI] [PubMed] [Google Scholar]
- Laurent S. J. Initiation of deoxyribonucleic acid replication in a temperature-sensitive mutant of B. subtilis: evidence for a transcriptional step. J Bacteriol. 1973 Oct;116(1):141–145. doi: 10.1128/jb.116.1.141-145.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry C., Kornberg A. DNA polymerase III holoenzyme of Escherichia coli. Purification and resolution into subunits. J Biol Chem. 1977 Sep 25;252(18):6478–6484. [PubMed] [Google Scholar]
- Messer W. Initiation of deoxyribonucleic acid replication in Escherichia coli B-r: chronology of events and transcriptional control of initiation. J Bacteriol. 1972 Oct;112(1):7–12. doi: 10.1128/jb.112.1.7-12.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prem veer Reddy G., Pardee A. B. Multienzyme complex for metabolic channeling in mammalian DNA replication. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3312–3316. doi: 10.1073/pnas.77.6.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy G. P., Mathews C. K. Functional compartmentation of DNA precursors in T4 phage-infected bacteria. J Biol Chem. 1978 May 25;253(10):3461–3467. [PubMed] [Google Scholar]
- Sakakibara Y., Mizukami T. A temperature-sensitive Escherichia coli mutant defective in DNA replication: dnaN, a new gene adjacent to the dnaA gene. Mol Gen Genet. 1980;178(3):541–553. doi: 10.1007/BF00337859. [DOI] [PubMed] [Google Scholar]
- Sakakibara Y., Tsukano H., Sako T. Organization and transcription of the dnaA and dnaN genes of Escherichia coli. Gene. 1981 Jan-Feb;13(1):47–55. doi: 10.1016/0378-1119(81)90042-1. [DOI] [PubMed] [Google Scholar]
- Sako T., Sakakibara Y. Coordinate expression of Escherichia coli dnaA and dnaN genes. Mol Gen Genet. 1980;179(3):521–526. doi: 10.1007/BF00271741. [DOI] [PubMed] [Google Scholar]
- Schaus N., O'Day K., Peters W., Wright A. Isolation and characterization of amber mutations in gene dnaA of escherichia coli K-12. J Bacteriol. 1981 Feb;145(2):904–913. doi: 10.1128/jb.145.2.904-913.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Weisberg R. A., Gottesman M. E. Prophage lambda at unusual chromosomal locations. II. Mutations induced by bacteriophage lambda in Escherichia coli K12. J Mol Biol. 1973 Oct 25;80(2):297–314. doi: 10.1016/0022-2836(73)90174-5. [DOI] [PubMed] [Google Scholar]
- Walker J. R., Henson J. M., Lee C. S. Isolation and characterization of plaque-forming lambdadnaZ+ transducing bacteriophages. J Bacteriol. 1977 Apr;130(1):354–365. doi: 10.1128/jb.130.1.354-365.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S., Hurwitz J. Involvement of escherichia coli dnaZ gene product in DNA elongation in vitro. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1053–1057. doi: 10.1073/pnas.73.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S. Mechanism of DNA elongation catalyzed by Escherichia coli DNA polymerase III, dnaZ protein, and DNA elongation factors I and III. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3511–3515. doi: 10.1073/pnas.73.10.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyskind J. W., Deen L. T., Smith D. W. Temporal sequence of events during the initiation process in Escherichia coli deoxyribonucleic acid replication: roles of the dnaA and dnaC gene products and ribonucleic acid polymerase. J Bacteriol. 1977 Mar;129(3):1466–1475. doi: 10.1128/jb.129.3.1466-1475.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


