Abstract
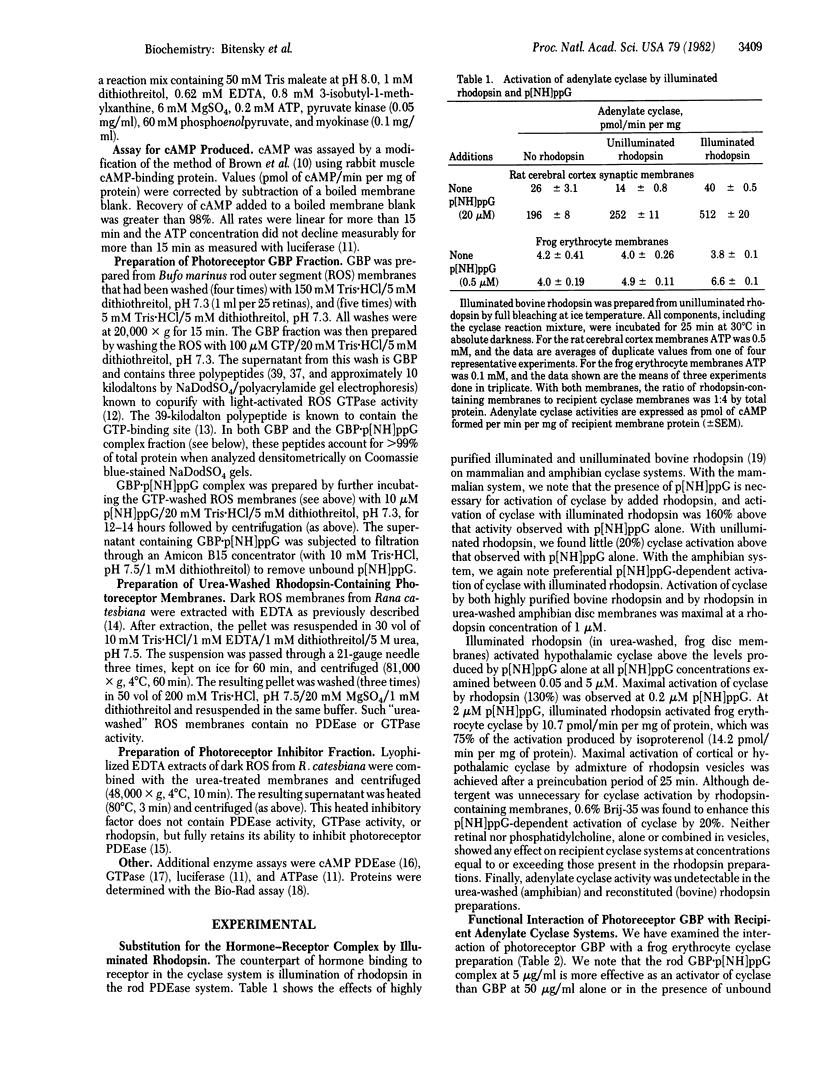
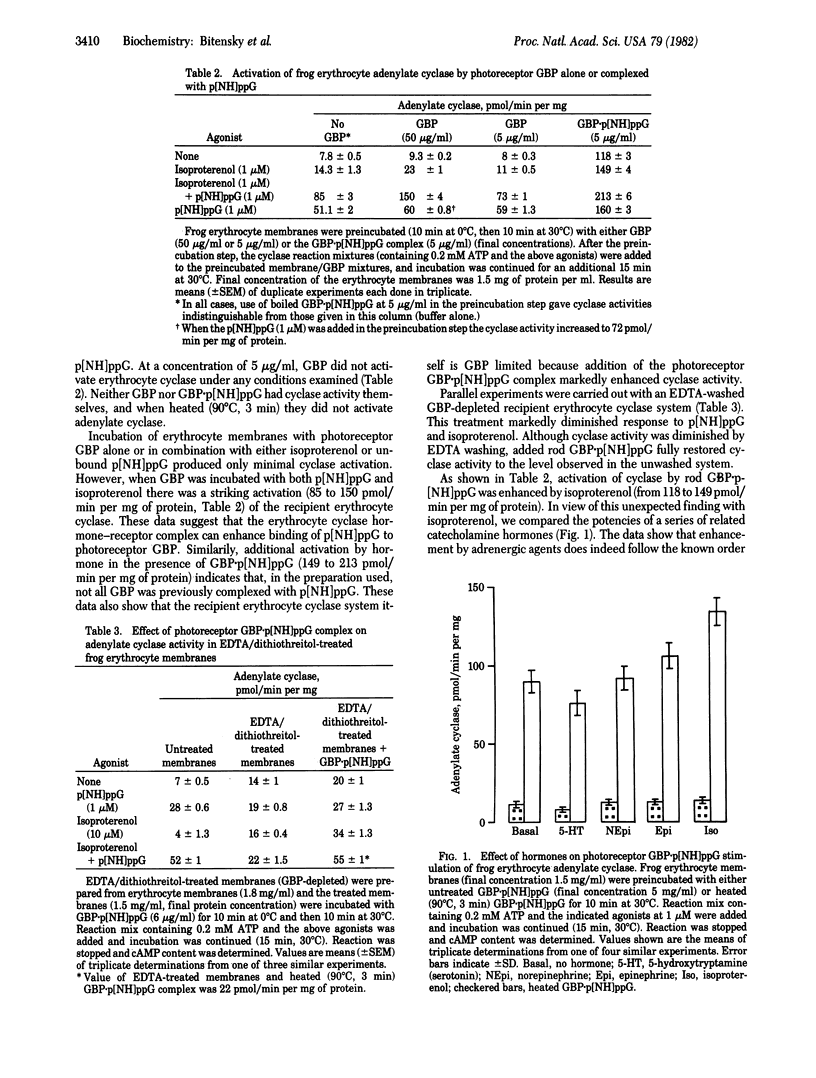
Previous studies have noted profound similarities between the regulation of light-activated 3',5'-cyclic nucleotide phosphodiesterase (3',5'-cyclic-nucleotide 5'-nucleotidohydrolase, EC 3.1.4.17) in retinal rods and hormone-activated adenylate cyclase [ATP pyrophosphate-lyase (cyclizing), EC 4.6.1.1] in a variety of tissues. We report here the functional exchange of components isolated from the photoreceptor system, which displayed predicted functional characteristics when incubated with recipient adenylate cyclase systems from rat cerebral cortical and hypothalamic synaptic membranes and frog erythrocyte ghosts. We demonstrate functional exchange of photoreceptor components at each of three loci: the hormone receptor, the GTP-binding protein (GBP), and the catalytic moiety of adenylate cyclase. Illuminated (but not unilluminated) rhodopsin was fund to mimic the hormone-receptor complex, causing GTP-dependent activation of adenylate cyclase. The photoreceptor GBP complexed with guanosine 5'-[beta, gamma)imidotriphosphate (p[NH]ppG) produced a marked activation of recipient adenylate cyclase systems. Much smaller activation was observed when GBP was not complexed with p[NH]ppG. A heat-stable photoreceptor phosphodiesterase inhibitor reduced both basal and Mn2+-activated adenylate cyclase activities and this inhibition was reversed by photoreceptor GBP.p[NH]ppG. These data demonstrate a remarkable functional compatibility between subunits of both systems and furthermore imply that specialized peptide domains responsible for protein-protein interactions are highly conserved.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bitensky M. W., Wheeler G. L., Aloni B., Vetury S., Matuo Y. Light- and GTP-activated photoreceptor phosphodiesterase: regulation by a light-activated GTPase and identification of rhodopsin as the phosphodiesterase binding site. Adv Cyclic Nucleotide Res. 1978;9:553–572. [PubMed] [Google Scholar]
- Bourne H. R., Kaslow D., Kaslow H. R., Salomon M. R., Licko V. Hormone-sensitive adenylate cyclase. Mutant phenotype with normally regulated beta-adrenergic receptors uncoupled with catalytic adenylate cyclase. Mol Pharmacol. 1981 Sep;20(2):435–441. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown B. L., Albano J. D., Ekins R. P., Sgherzi A. M. A simple and sensitive saturation assay method for the measurement of adenosine 3':5'-cyclic monophosphate. Biochem J. 1971 Feb;121(3):561–562. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. Split genes and RNA splicing. Science. 1979 Apr 20;204(4390):264–271. doi: 10.1126/science.373120. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Implications of RNA-RNA splicing in evolution of eukaryotic cells. Science. 1978 Dec 22;202(4374):1257–1260. doi: 10.1126/science.364651. [DOI] [PubMed] [Google Scholar]
- Downs R. W., Jr, Spiegel A. M., Singer M., Reen S., Aurbach G. D. Fluoride stimulation of adenylate cyclase is dependent on the guanine nucleotide regulatory protein. J Biol Chem. 1980 Feb 10;255(3):949–954. [PubMed] [Google Scholar]
- Dumler I. L., Etingof R. N. Protein inhibitor of cyclic adenosine 3':5'-monophosphate phosphodiesterase in retina. Biochim Biophys Acta. 1976 Apr 8;429(2):474–478. doi: 10.1016/0005-2744(76)90295-3. [DOI] [PubMed] [Google Scholar]
- Hong K., Hubbell W. L. Preparation and properties of phospholipid bilayers containing rhodopsin. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2617–2621. doi: 10.1073/pnas.69.9.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley J. B. Isolation and recombination of bovine rod outer segment cGMP phosphodiesterase and its regulators. Biochem Biophys Res Commun. 1980 Jan 29;92(2):505–510. doi: 10.1016/0006-291x(80)90362-9. [DOI] [PubMed] [Google Scholar]
- Kimmich G. A., Randles J., Brand J. S. Assay of picomole amounts of ATP, ADP, and AMP using the luciferase enzyme system. Anal Biochem. 1975 Nov;69(1):187–206. doi: 10.1016/0003-2697(75)90580-1. [DOI] [PubMed] [Google Scholar]
- Kühn H. Light- and GTP-regulated interaction of GTPase and other proteins with bovine photoreceptor membranes. Nature. 1980 Feb 7;283(5747):587–589. doi: 10.1038/283587a0. [DOI] [PubMed] [Google Scholar]
- Miki N., Keirns J. J., Marcus F. R., Freeman J., Bitensky M. W. Regulation of cyclic nucleotide concentrations in photoreceptors: an ATP-dependent stimulation of cyclic nucleotide phosphodiesterase by light. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3820–3824. doi: 10.1073/pnas.70.12.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee C., Caron M. G., Coverstone M., Lefkowitz R. J. Identification of adenylate cyclase-coupled beta-adrenergic receptors in frog erythrocytes with (minus)-[3-H] alprenolol. J Biol Chem. 1975 Jul 10;250(13):4869–4876. [PubMed] [Google Scholar]
- Neufeld A. H., Levy H. M. A second ouabain-sensitive sodium-dependent adenosine triphosphate in brain microsomes. J Biol Chem. 1969 Dec 10;244(23):6493–6497. [PubMed] [Google Scholar]
- Northup J. K., Sternweis P. C., Smigel M. D., Schleifer L. S., Ross E. M., Gilman A. G. Purification of the regulatory component of adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6516–6520. doi: 10.1073/pnas.77.11.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeuffer T. GTP-binding proteins in membranes and the control of adenylate cyclase activity. J Biol Chem. 1977 Oct 25;252(20):7224–7234. [PubMed] [Google Scholar]
- Rasenick M. M., Bitensky M. W. Partial purification and characterization of a macromolecule which enhances fluoride activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4628–4632. doi: 10.1073/pnas.77.8.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasenick M. M., Stein P. J., Bitensky M. W. The regulatory subunit of adenylate cyclase interacts with cytoskeletal components. Nature. 1981 Dec 10;294(5841):560–562. doi: 10.1038/294560a0. [DOI] [PubMed] [Google Scholar]
- Rodbell M., Lin M. C., Salomon Y., Londos C., Harwood J. P., Martin B. R., Rendell M., Berman M. Role of adenine and guanine nucleotides in the activity and response of adenylate cyclase systems to hormones: evidence for multisite transition states. Adv Cyclic Nucleotide Res. 1975;5:3–29. [PubMed] [Google Scholar]
- Rogers J., Early P., Carter C., Calame K., Bond M., Hood L., Wall R. Two mRNAs with different 3' ends encode membrane-bound and secreted forms of immunoglobulin mu chain. Cell. 1980 Jun;20(2):303–312. doi: 10.1016/0092-8674(80)90616-9. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Biochemical properties of hormone-sensitive adenylate cyclase. Annu Rev Biochem. 1980;49:533–564. doi: 10.1146/annurev.bi.49.070180.002533. [DOI] [PubMed] [Google Scholar]
- Shinozawa T., Sen I., Wheeler G., Bitensky M. Predictive value of the analogy between hormone-sensitive adenylate cyclase and light-sensitive photoreceptor cyclic GMP phosphodiesterase: a specific role for a light-sensitive GTPase as a component in the activation sequence. J Supramol Struct. 1979;10(2):185–190. doi: 10.1002/jss.400100208. [DOI] [PubMed] [Google Scholar]
- Uchida S., Wheeler G. L., Yamazaki A., Bitensky M. W. A GTP-protein activator of phosphodiesterase which forms in response to bleached rhodopsin. J Cyclic Nucleotide Res. 1981;7(2):95–104. [PubMed] [Google Scholar]
- Wheeler G. L., Bitensky M. W. A light-activated GTPase in vertebrate photoreceptors: regulation of light-activated cyclic GMP phosphodiesterase. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4238–4242. doi: 10.1073/pnas.74.10.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler M., Tishler M., Bitensky M. W. Agaridoxin: a fungal catecholamine which acts as an alpha 1 agonist of mammalian hypothalamic adenylate cyclase. Brain Res. 1982 Jan 14;231(2):387–398. doi: 10.1016/0006-8993(82)90375-4. [DOI] [PubMed] [Google Scholar]
- Yamazaki A., Bartucca F., Ting A., Bitensky M. W. Reciprocal effects of an inhibitory factor on catalytic activity and noncatalytic cGMP binding sites of rod phosphodiesterase. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3702–3706. doi: 10.1073/pnas.79.12.3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki A., Sen I., Bitensky M. W., Casnellie J. E., Greengard P. Cyclic GMP-specific, high affinity, noncatalytic binding sites on light-activated phosphodiesterase. J Biol Chem. 1980 Dec 10;255(23):11619–11624. [PubMed] [Google Scholar]


