Abstract
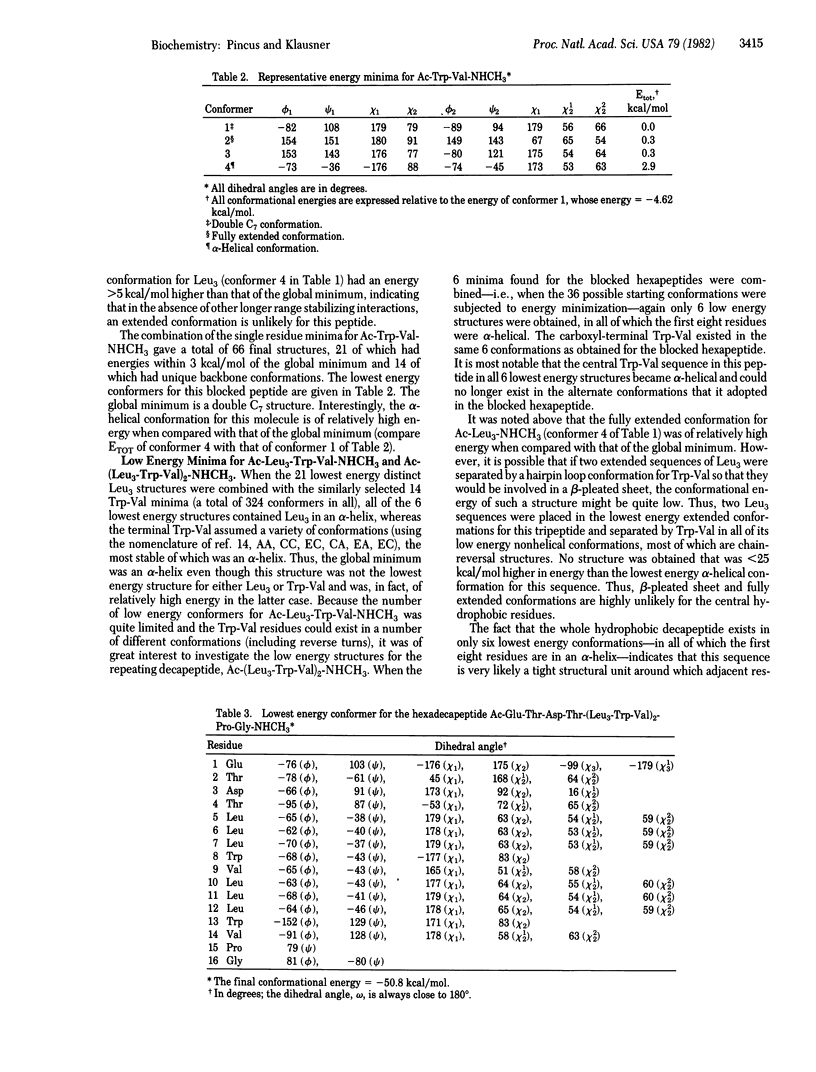
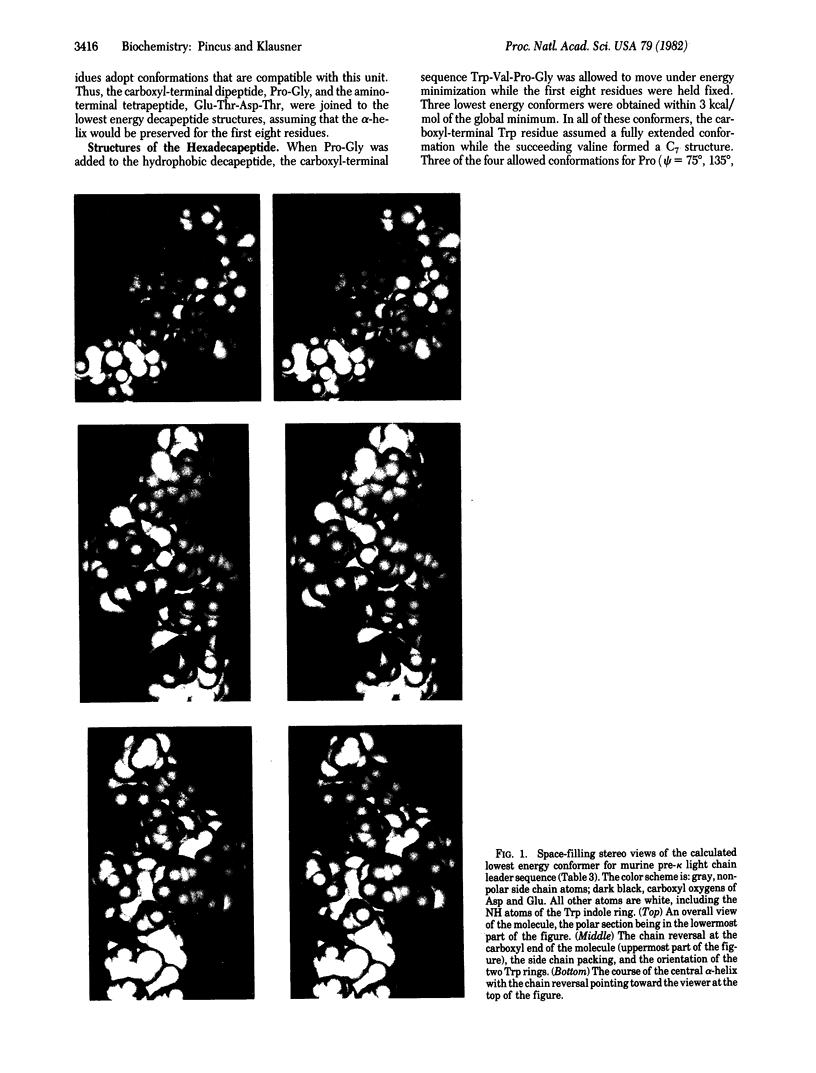
The three-dimensional structure of the signal sequence for murine kappa light chain has been calculated by using conformational energy calculations. These calculations, based on tested and reliable potential energy functions, employ a novel global search technique to identify the lowest energy structures for the hexadecapeptide signal sequence, Glu-Thr-Asp-Thr-(Leu3-Trp-Val)2-Pro-Gly.l It has been found that the core hydrophobic sequence, Leu3-Trp-Val-Leu3, adopts an alpha-helical conformation that is terminated by chain reversal conformations for the four residues, Trp-Val-Pro-Gly. The amino-terminal four residues adopt low energy conformations that are fully compatible with the succeeding alpha-helix. The immediately neighboring sequence., Asp-Thr, exists in a single lowest energy double-equatorial conformation, whereas the first two residues, Glu-Thr, can adopt a variety of low energy conformations. The calculations arrive at a highly structured and specific model for the conformation of a leader sequence, compatible with recent experimental data.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burstein Y., Schechter I. Primary structures of N-terminal extra peptide segments linked to the variable and constant regions of immunoglobulin light chain precursors: implications on the organization and controlled expression of immunoglobulin genes. Biochemistry. 1978 Jun 13;17(12):2392–2400. doi: 10.1021/bi00605a022. [DOI] [PubMed] [Google Scholar]
- Carne T., Scheele G. Amino acid sequences of transport peptides associated with canine exocrine pancreatic proteins. J Biol Chem. 1982 Apr 25;257(8):4133–4140. [PubMed] [Google Scholar]
- Dygert M., Gō N., Scheraga H. A. Use of a symmetry condition to compute the conformation of gramicidin S1. Macromolecules. 1975 Nov-Dec;8(6):750–761. doi: 10.1021/ma60048a016. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A. The spontaneous insertion of proteins into and across membranes: the helical hairpin hypothesis. Cell. 1981 Feb;23(2):411–422. doi: 10.1016/0092-8674(81)90136-7. [DOI] [PubMed] [Google Scholar]
- Garnier J., Gaye P., Mercier J. C., Robson B. Structural properties of signal peptides and their membrane insertion. Biochimie. 1980;62(4):231–239. doi: 10.1016/s0300-9084(80)80397-x. [DOI] [PubMed] [Google Scholar]
- Némethy G., Scheraga H. A. Protein folding. Q Rev Biophys. 1977 Aug;10(3):239–252. doi: 10.1017/s0033583500002936. [DOI] [PubMed] [Google Scholar]
- Rosenblatt M., Beaudette N. V., Fasman G. D. Conformational studies of the synthetic precursor-specific region of preproparathyroid hormone. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3983–3987. doi: 10.1073/pnas.77.7.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele G., Jacoby R., Carne T. Mechanism of compartmentation of secretory proteins: transport of exocrine pancreatic proteins across the microsomal membrane. J Cell Biol. 1980 Dec;87(3 Pt 1):611–628. doi: 10.1083/jcb.87.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman S. S., Pottle M. S., Némethy G., Scheraga H. A. Conformational analysis of the 20 naturally occurring amino acid residues using ECEPP. Macromolecules. 1977 Jan-Feb;10(1):1–9. doi: 10.1021/ma60055a001. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. S., Scheraga H. A. Influence of local interactions on protein structure. I. Conformational energy studies of N-acetyl-N'-methylamides of Pro-X and X-Pro dipeptides. Biopolymers. 1977 Apr;16(4):811–843. doi: 10.1002/bip.1977.360160408. [DOI] [PubMed] [Google Scholar]