Abstract
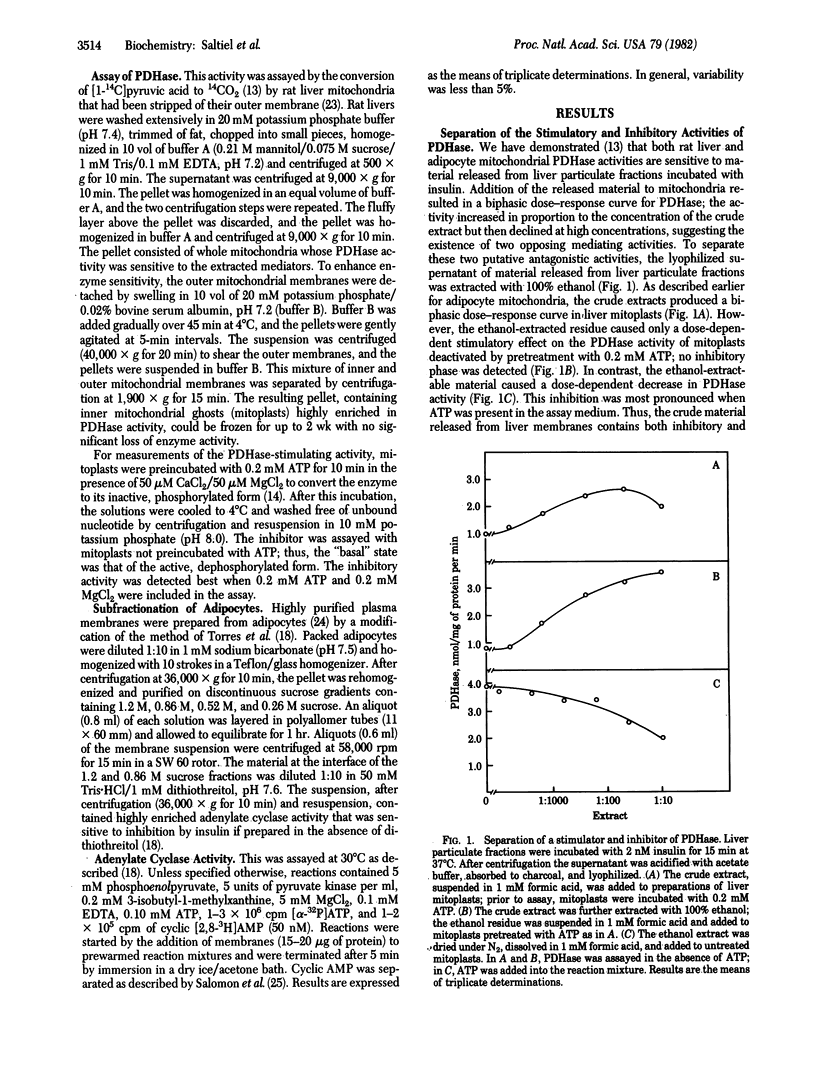
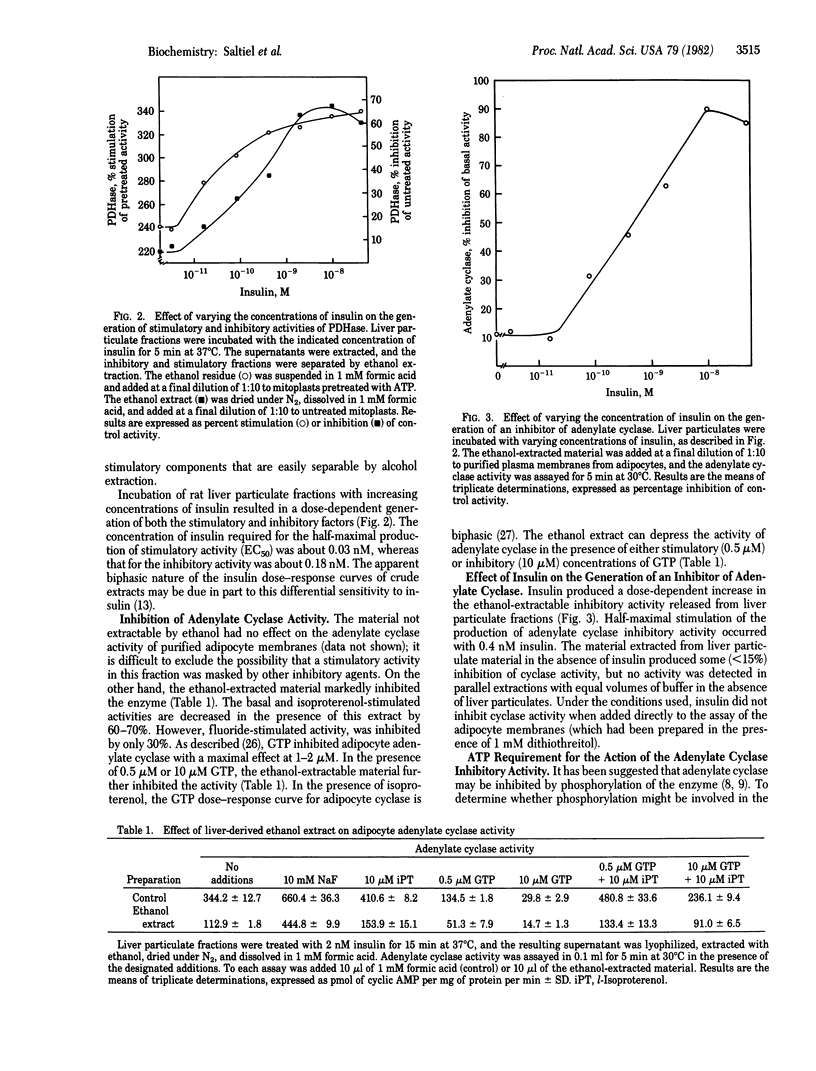
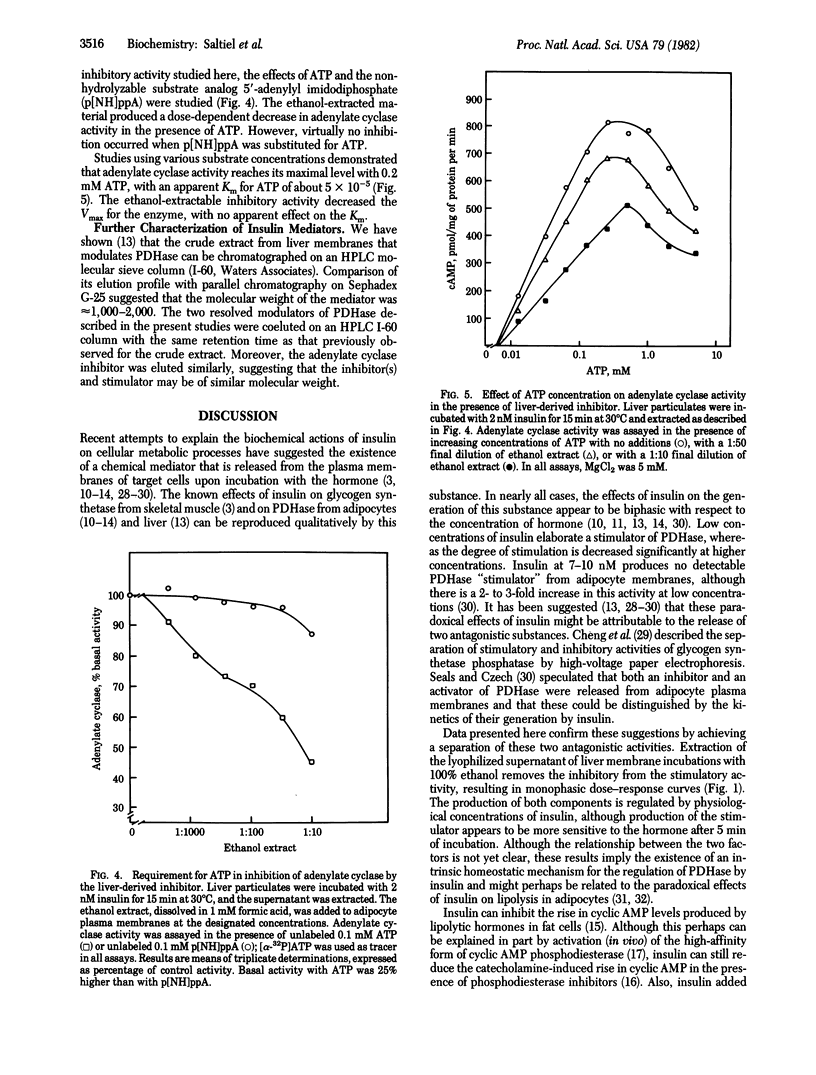
Recent evidence suggests that certain actions of insulin may be mediated by the selective generation of chemically undefined intracellular substances. Incubation of rat liver particulate fraction with low concentrations of insulin enhances the release into the supernatant of a substance that stimulates mitochondrial pyruvate dehydrogenase. Higher concentrations of insulin release less stimulating activity. It is possible to resolve activities that stimulate and inhibit pyruvate dehydrogenase by differential ethanol extraction of the supernatant solutions. The elaboration of both factors is dependent upon the presence of insulin in a dose-dependent manner. Moreover, fractions that contain the pyruvate dehydrogenase-inhibiting activity also inhibit adipocyte basal and hormonally stimulated adenylate cyclase. The production of this adenylate cyclase inhibitory activity is also stimulated by insulin. Cyclase inhibition is virtually abolished when the nonhydrolyzable ATP analog, 5'-adenylyl imidodiphosphate, is included in the assay. These results indicate that the bimodal effects of insulin on certain functions may be ascribed to the generation of at least two distinct chemical substances that show opposing activities, which may operate by regulating phosphorylation reactions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butcher R. W., Baird C. E., Sutherland E. W. Effects of lipolytic and antilipolytic substances on adenosine 3',5'-monophosphate levels in isolated fat cells. J Biol Chem. 1968 Apr 25;243(8):1705–1712. [PubMed] [Google Scholar]
- Butcher R. W., Sneyd J. G., Park C. R., Sutherland E. W., Jr Effect of insulin on adenosine 3',5'-monophosphate in the rat epididymal fat pad. J Biol Chem. 1966 Apr 10;241(7):1651–1653. [PubMed] [Google Scholar]
- Cheng K., Galasko G., Huang L., Kellogg J., Larner J. Studies on the insulin mediator. II. Separation of two antagonistic biologically active materials from fraction II. Diabetes. 1980 Aug;29(8):659–661. doi: 10.2337/diab.29.8.659. [DOI] [PubMed] [Google Scholar]
- Claus T. H., El-Maghrabi M. R., Pilkis S. J. Modulation of the phosphorylation state of rat liver pyruvate kinase by allosteric effectors and insulin. J Biol Chem. 1979 Aug 25;254(16):7855–7864. [PubMed] [Google Scholar]
- Constantopoulos A., Najjar V. A. The activation of adenylate cyclase. II. The postulated presence of (A) adenylate cyclase in a phospho (inhibited) form (B) a dephospho (activated) form with a cyclic adenylate stimulated membrane protein kinase. Biochem Biophys Res Commun. 1973 Aug 6;53(3):794–799. doi: 10.1016/0006-291x(73)90162-9. [DOI] [PubMed] [Google Scholar]
- Cooper D. M., Schlegel W., Lin M. C., Rodbell M. The fat cell adenylate cyclase system. Characterization and manipulation of its bimodal regulation by GTP. J Biol Chem. 1979 Sep 25;254(18):8927–8931. [PubMed] [Google Scholar]
- Coore H. G., Denton R. M., Martin B. R., Randle P. J. Regulation of adipose tissue pyruvate dehydrogenase by insulin and other hormones. Biochem J. 1971 Nov;125(1):115–127. doi: 10.1042/bj1250115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech M. P. Insulin action. Am J Med. 1981 Jan;70(1):142–150. doi: 10.1016/0002-9343(81)90421-6. [DOI] [PubMed] [Google Scholar]
- Harwood J. P., Löw H., Rodbell M. Stimulatory and inhibitory effects of guanyl nucleotides on fat cell adenylate cyclase. J Biol Chem. 1973 Sep 10;248(17):6239–6245. [PubMed] [Google Scholar]
- Hepp K. D., Renner R. Insulin action on the adenyl cyclase system: Antagonism to activation by lipolytic hormones. FEBS Lett. 1972 Feb 1;20(2):191–194. doi: 10.1016/0014-5793(72)80791-9. [DOI] [PubMed] [Google Scholar]
- Ho R. J., Sutherland E. W. Formation and release of a hormone antagonist by rat adipocytes. J Biol Chem. 1971 Nov 25;246(22):6822–6827. [PubMed] [Google Scholar]
- Illiano G., Cuatrecasas P. Modulation of adenylate cyclase activity in liver and fat cell membranes by insulin. Science. 1972 Feb 25;175(4024):906–908. doi: 10.1126/science.175.4024.906. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Cuatrecasas P. Insulin receptor: structure and function. Endocr Rev. 1981 Summer;2(3):251–263. doi: 10.1210/edrv-2-3-251. [DOI] [PubMed] [Google Scholar]
- Jarett L., Seals J. R. Pyruvate dehydrogenase activation in adipocyte mitochondria by an insulin-generated mediator from muscle. Science. 1979 Dec 21;206(4425):1407–1408. doi: 10.1126/science.505013. [DOI] [PubMed] [Google Scholar]
- Jiménez de Asúa L., Surian E. S., Flawia M. M., Torres H. N. Effect of insulin on the growth pattern and adenylate cyclase activity of BHK fibroblasts. Proc Natl Acad Sci U S A. 1973 May;70(5):1388–1392. doi: 10.1073/pnas.70.5.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiechle F. L., Jarett L., Kotagal N., Popp D. A. Partial purification from rat adipocyte plasma membranes of a chemical mediator which simulates the action of insulin on pyruvate dehydrogenase. J Biol Chem. 1981 Mar 25;256(6):2945–2951. [PubMed] [Google Scholar]
- Kono T., Barham F. W. Effects of insulin on the levels of adenosine 3':5'-monophosphate and lipolysis in isolated rat epididymal fat cells. J Biol Chem. 1973 Nov 10;248(21):7417–7426. [PubMed] [Google Scholar]
- Larner J., Galasko G., Cheng K., DePaoli-Roach A. A., Huang L., Daggy P., Kellogg J. Generation by insulin of a chemical mediator that controls protein phosphorylation and dephosphorylation. Science. 1979 Dec 21;206(4425):1408–1410. doi: 10.1126/science.228395. [DOI] [PubMed] [Google Scholar]
- Layne P., Constantopoulos A., Judge J. F., Rauner R., Najjar V. A. The occurrence of fluoride stimulated membrane phosphoprotein phosphatase. Biochem Biophys Res Commun. 1973 Aug 6;53(3):800–805. doi: 10.1016/0006-291x(73)90163-0. [DOI] [PubMed] [Google Scholar]
- Levey G. S., Lehotay D. C., Canterbury J. M., Bricker L. A., Meltz G. J. Isolation of a unique peptide inhibitor of hormone-responsive adenylate cyclase. J Biol Chem. 1975 Jul 25;250(14):5730–5733. [PubMed] [Google Scholar]
- Manganiello V., Vaughan M. An effect of insulin on cyclic adenosine 3':5'-monophosphate phosphodiesterase activity in fat cells. J Biol Chem. 1973 Oct 25;248(20):7164–7170. [PubMed] [Google Scholar]
- Marchmont R. J., Houslay M. D. A peripheral and an intrinsic enzyme constitute the cyclic AMP phosphodiesterase activity of rat liver plasma membranes. Biochem J. 1980 May 1;187(2):381–392. doi: 10.1042/bj1870381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons D. F., Williams G. R., Chance B. Characteristics of isolated and purified preparations of the outer and inner membranes of mitochondria. Ann N Y Acad Sci. 1966 Jul 14;137(2):643–666. doi: 10.1111/j.1749-6632.1966.tb50188.x. [DOI] [PubMed] [Google Scholar]
- Popp D. A., Kiechle F. L., Kotagal N., Jarett L. Insulin stimulation of pyruvate dehydrogenase in an isolated plasma membrane-mitochondrial mixture occurs by activation of pyruvate dehydrogenase phosphatase. J Biol Chem. 1980 Aug 25;255(16):7540–7543. [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Sahyoun N., Schmitges C. J., Siegel M. I., Cuatrecasas P. 2'-Deoxyadenosine-3'-monophosphate: a naturally occurring inhibitor of adenylate cyclase in amphibian and mammalian cells. Life Sci. 1976 Dec 15;19(12):1961–1969. doi: 10.1016/0024-3205(76)90132-6. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Saltiel A., Jacobs S., Siegel M., Cuatrecasas P. Insulin stimulates the release from liver plasma membranes of a chemical modulator of pyruvate dehydrogenase. Biochem Biophys Res Commun. 1981 Oct 15;102(3):1041–1047. doi: 10.1016/0006-291x(81)91643-0. [DOI] [PubMed] [Google Scholar]
- Schuurmans Stekhoven F., Bonting S. L. Transport adenosine triphosphatases: properties and functions. Physiol Rev. 1981 Jan;61(1):1–76. doi: 10.1152/physrev.1981.61.1.1. [DOI] [PubMed] [Google Scholar]
- Seals J. R., Czech M. P. Characterization of a pyruvate dehydrogenase activator released by adipocyte plasma membranes in response to insulin. J Biol Chem. 1981 Mar 25;256(6):2894–2899. [PubMed] [Google Scholar]
- Seals J. R., Czech M. P. Evidence that insulin activates an intrinsic plasma membrane protease in generating a secondary chemical mediator. J Biol Chem. 1980 Jul 25;255(14):6529–6531. [PubMed] [Google Scholar]
- Seals J. R., Jarett L. Activation of pyruvate dehydrogenase by direct addition of insulin to an isolated plasma membrane/mitochondria mixture: evidence for generated of insulin's second messenger in a subcellular system. Proc Natl Acad Sci U S A. 1980 Jan;77(1):77–81. doi: 10.1073/pnas.77.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon S. S., Brush J. S., Kitabchi A. E. Antilipolytic activity of insulin and proinsulin on ACTH and cyclic nucleotide-induced lipolysis in the isolated adipose cell of rat. Biochim Biophys Acta. 1970 Oct 6;218(1):167–169. doi: 10.1016/0005-2760(70)90104-9. [DOI] [PubMed] [Google Scholar]
- Torres H. N., Flawià M. M., Hernaez L., Cuatrecasas P. Effects of insulin on the adenylyl cyclase activity of isolated fat cell membranes. J Membr Biol. 1978 Sep 29;43(1):1–18. doi: 10.1007/BF01869039. [DOI] [PubMed] [Google Scholar]


