Abstract
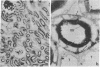
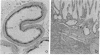
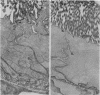
Immunocytochemistry with a specific antiserum and protein A-horseradish peroxidase permits visualization of the sites of gamma-glutamyltransferase [(5-glutamyl)-peptide:amino-acid 5-glutamyltransferase, EC 2.3.2.2] at both the light- and the electron-microscope levels. As seen by light microscopy, the enzyme is localized only in the proximal convolutions of the renal tubules. Electron microscopy reveals dense deposits of 3,3'-diaminobenzidine reaction product embedded in the glycocalyx along the entire luminal surface of the microvilli and in the basolateral membranes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. E., Bridges R. J., Meister A. Direct evidence for inter-organ transport of glutathione and that the non-filtration renal mechanism for glutathione utilization involves gamma-glutamyl transpeptidase. Biochem Biophys Res Commun. 1980 Sep 30;96(2):848–853. doi: 10.1016/0006-291x(80)91433-3. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Horiuchi S., Inoue M., Morino Y. Latent active site in rat-kidney gamma-glutamyl transpeptidase. The refolding process of the large subunit and characterization of the renatured enzyme. Eur J Biochem. 1980 Mar;105(1):93–102. doi: 10.1111/j.1432-1033.1980.tb04478.x. [DOI] [PubMed] [Google Scholar]
- Inoue M., Horiuchi S., Morino Y. Affinity labeling of rat kidney gamma-glutamyl transpeptidase by 6-diazo-5-oxo-D-norleucine. Eur J Biochem. 1979 Aug 15;99(1):169–177. doi: 10.1111/j.1432-1033.1979.tb13243.x. [DOI] [PubMed] [Google Scholar]
- Meister A., Griffith O. W., Novogrodsky A., Tate S. S. New aspects of glutathione metabolism and translocation in mammals. Ciba Found Symp. 1979;(72):135–161. doi: 10.1002/9780470720554.ch9. [DOI] [PubMed] [Google Scholar]
- Novikoff A. B., Novikoff P. M., Quintana N., Davis C. Diffusion artificats in 3,3'-diaminobenzidine cytochemistry. J Histochem Cytochem. 1972 Sep;20(9):745–749. doi: 10.1177/20.9.745. [DOI] [PubMed] [Google Scholar]
- Novikoff A. B., Novikoff P. M., Stockert R. J., Becker F. F., Yam A., Poruchynsky M. S., Levin W., Thomas P. E. Immunocytochemical localization of epoxide hydrase in hyperplastic nodules induced in rat liver by 2-acetylaminofluorene. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5207–5211. doi: 10.1073/pnas.76.10.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORLOWSKI M., MEISTER A. GAMMA-GLUTAMYL-P-NITROANILIDE: A NEW CONVENIENT SUBSTRATE FOR DETERMINATION AND STUDY OF L- AND D-GAMMA-GLUTAMYLTRANSPEPTIDASE ACTIVITIES. Biochim Biophys Acta. 1963 Aug 6;73:679–681. doi: 10.1016/0006-3002(63)90348-2. [DOI] [PubMed] [Google Scholar]
- Rutenburg A. M., Kim H., Fischbein J. W., Hanker J. S., Wasserkrug H. L., Seligman A. M. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem. 1969 Aug;17(8):517–526. doi: 10.1177/17.8.517. [DOI] [PubMed] [Google Scholar]
- Seligman A. M., Karnovsky M. J., Wasserkrug H. L., Hanker J. S. Nondroplet ultrastructural demonstration of cytochrome oxidase activity with a polymerizing osmiophilic reagent, diaminobenzidine (DAB). J Cell Biol. 1968 Jul;38(1):1–14. doi: 10.1083/jcb.38.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]