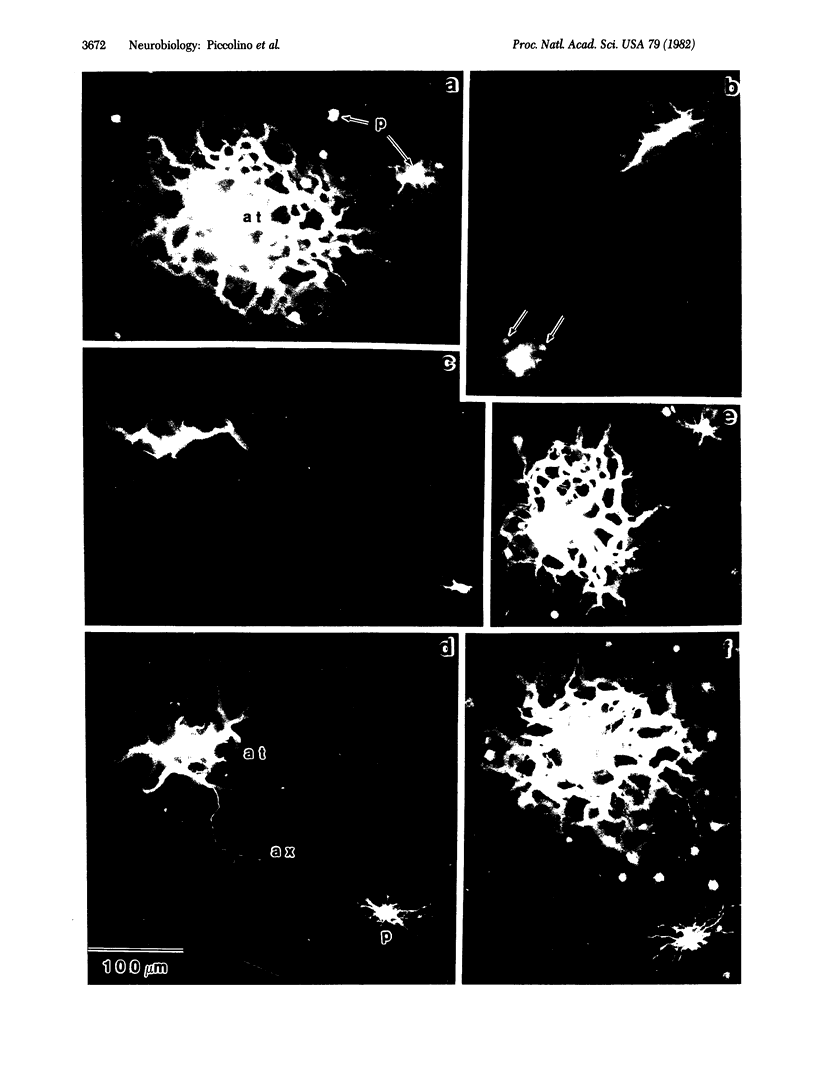
Abstract
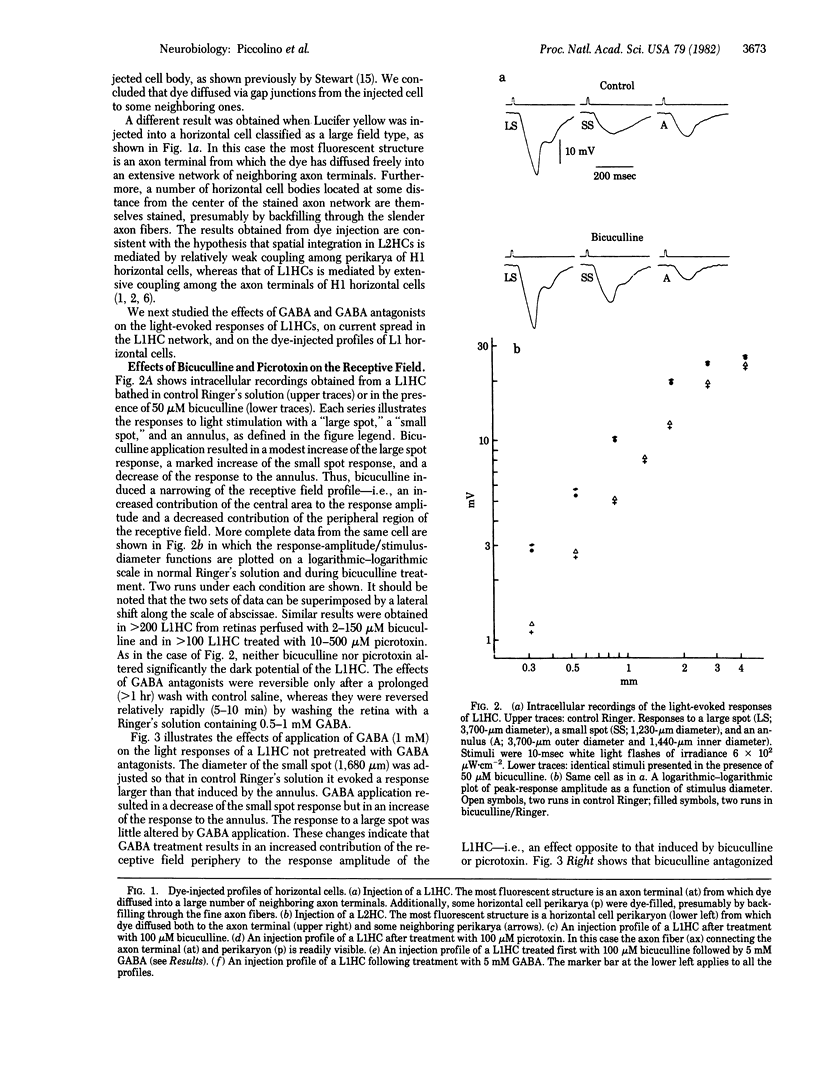
The antagonists of gamma-aminobutyric acid, bicuculline and picrotoxin, were found to narrow the receptive field profile of the large field horizontal cell (L1HC) in the turtle retina when added to the perfusion medium in micromolar concentrations. The coupling resistance between neighboring L1HCs was increased by bicuculline or picrotoxin. Under control conditions, the dye Lucifer yellow injected into one L1HC diffused into a large number of neighboring L1HCs; bicuculline or picrotoxin greatly restricted dye passage between these same cells. We conclude that antagonists of gamma-aminobutyric acid decrease the conductance of gap junctions between L1HCs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. V., Spira M. E., Spray D. C. Permeability of gap junctions between embryonic cells of Fundulus: a reevaluation. Dev Biol. 1978 Jul;65(1):114–125. doi: 10.1016/0012-1606(78)90184-7. [DOI] [PubMed] [Google Scholar]
- Byzov A. L. Vzaimodeistvie mezhdu gorizontal'nymi kletkami setchatki cherepakhi. Neirofiziologiia. 1975;7(3):279–286. [PubMed] [Google Scholar]
- Carew T. J., Kandel E. R. Two functional effects of decreased conductance EPSP's: synaptic augmentation and increased electrotonic coupling. Science. 1976 Apr 9;192(4235):150–153. doi: 10.1126/science.943847. [DOI] [PubMed] [Google Scholar]
- Cervetto L., MacNichol E. F., Jr Inactivation of horizontal cells in turtle retina by glutamate and aspartate. Science. 1972 Nov 17;178(4062):767–768. doi: 10.1126/science.178.4062.767. [DOI] [PubMed] [Google Scholar]
- Detwiler P. B., Hodgkin A. L. Electrical coupling between cones in turtle retina. J Physiol. 1979 Jun;291:75–100. doi: 10.1113/jphysiol.1979.sp012801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaume C., Spira M. E., Korn H. Uncoupling of invertebrate electrotonic synapses by carbon dioxide. Neurosci Lett. 1980 Apr;17(1-2):197–202. doi: 10.1016/0304-3940(80)90084-1. [DOI] [PubMed] [Google Scholar]
- Hollyfield J. G., Rayborn M. E., Sarthy P. V., Lam D. M. The emergence, localization and maturation of neurotransmitter systems during development of the retina in Xenopus laevis. I. Gamma aminobutyric acid. J Comp Neurol. 1979 Dec 15;188(4):587–598. doi: 10.1002/cne.901880406. [DOI] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Pancreatic acinar cells: acetylcholine-evoked electrical uncoupling and its ionic dependency. J Physiol. 1978 Jan;274:81–06. doi: 10.1113/jphysiol.1978.sp012135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A. Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J Physiol. 1970 May;207(3):623–633. doi: 10.1113/jphysiol.1970.sp009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam D. M., Lasater E. M., Naka K. I. gamma-Aminobutyric acid: a neurotransmitter candidate for cone horizontal cells of the catfish retina. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6310–6313. doi: 10.1073/pnas.75.12.6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam D. M., Su Y. Y., Swain L., Marc R. E., Brandon C., Wu J. Y. Immunocytochemical localisation of L-glutamic acid decarboxylase in the goldfish retina. Nature. 1979 Apr 5;278(5704):565–567. doi: 10.1038/278565a0. [DOI] [PubMed] [Google Scholar]
- Lamb T. D. Spatial properties of horizontal cell responses in the turtle retina. J Physiol. 1976 Dec;263(2):239–255. doi: 10.1113/jphysiol.1976.sp011630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeper H. F., Copenhagen D. R. Mixed rod-cone responses in horizontal cells of snapping turtle retina. Vision Res. 1979;19(4):407–412. doi: 10.1016/0042-6989(79)90105-6. [DOI] [PubMed] [Google Scholar]
- Leeper H. F. Horizontal cells of the turtle retina. II. Analysis of interconnections between photoreceptor cells and horizontal cells by light microscopy. J Comp Neurol. 1978 Dec 15;182(4 Pt 2):795–809. doi: 10.1002/cne.901820504. [DOI] [PubMed] [Google Scholar]
- Marc R. E., Stell W. K., Bok D., Lam D. M. GABA-ergic pathways in the goldfish retina. J Comp Neurol. 1978 Nov 15;182(2):221–244. doi: 10.1002/cne.901820204. [DOI] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. The generation and spread of S-potentials in fish (Cyprinidae). J Physiol. 1967 Sep;192(2):437–461. doi: 10.1113/jphysiol.1967.sp008308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J., Piccolino M., Gerschenfeld H. M. Involvement of small-field horizontal cells in feedback effects on green cones of turtle retina. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4616–4619. doi: 10.1073/pnas.78.7.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolino M., Gerschenfeld H. M. Characteristics and ionic processes involved in feedback spikes of turtle cones. Proc R Soc Lond B Biol Sci. 1980 Jan 17;206(1165):439–463. doi: 10.1098/rspb.1980.0007. [DOI] [PubMed] [Google Scholar]
- Rose B., Loewenstein W. R. Permeability of a cell junction and the local cytoplasmic free ionized calcium concentration: a study with aequorin. J Membr Biol. 1976 Aug 27;28(1):87–119. doi: 10.1007/BF01869692. [DOI] [PubMed] [Google Scholar]
- Simon E. J. Two types of luminosity horizontal cells in the retina of the turtle. J Physiol. 1973 Apr;230(1):199–211. doi: 10.1113/jphysiol.1973.sp010183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira M. E., Bennett M. V. Synaptic control of electrotonic coupling between neurons. Brain Res. 1972 Feb 25;37(2):294–300. doi: 10.1016/0006-8993(72)90674-9. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Harris A. L., Bennett M. V. Gap junctional conductance is a simple and sensitive function of intracellular pH. Science. 1981 Feb 13;211(4483):712–715. doi: 10.1126/science.6779379. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Harris A. L., Bennett M. V. Voltage dependence of junctional conductance in early amphibian embryos. Science. 1979 Apr 27;204(4391):432–434. doi: 10.1126/science.312530. [DOI] [PubMed] [Google Scholar]
- Stewart W. W. Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell. 1978 Jul;14(3):741–759. doi: 10.1016/0092-8674(78)90256-8. [DOI] [PubMed] [Google Scholar]
- Stewart W. W. Lucifer dyes--highly fluorescent dyes for biological tracing. Nature. 1981 Jul 2;292(5818):17–21. doi: 10.1038/292017a0. [DOI] [PubMed] [Google Scholar]
- Turin L., Warner A. E. Intracellular pH in early Xenopus embryos: its effect on current flow between blastomeres. J Physiol. 1980 Mar;300:489–504. doi: 10.1113/jphysiol.1980.sp013174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P., Burkhardt D. A., Nagy A. R. Synaptic connections linking cones and horizontal cells in the retina of the pikeperch (Stizostedion vitreum). J Comp Neurol. 1979 Aug 15;186(4):541–559. doi: 10.1002/cne.901860404. [DOI] [PubMed] [Google Scholar]
- Yamada E., Ishikawa T. The fine structure of the horizontal cells in some vertebrate retinae. Cold Spring Harb Symp Quant Biol. 1965;30:383–392. doi: 10.1101/sqb.1965.030.01.038. [DOI] [PubMed] [Google Scholar]
- Yazulla S., Brecha N. Binding and uptake of the GABA analogue, 3H-muscimol, in the retinas of goldfish and chicken. Invest Ophthalmol Vis Sci. 1980 Dec;19(12):1415–1426. [PubMed] [Google Scholar]
- Yazulla S., Brecha N. Localized binding of [3H]muscimol to synapses in chicken retina. Proc Natl Acad Sci U S A. 1981 Jan;78(1):643–647. doi: 10.1073/pnas.78.1.643. [DOI] [PMC free article] [PubMed] [Google Scholar]