Abstract
We have developed a cell-free system from Escherichia coli for studying illegitimate recombination between nonhomologous DNA molecules. The recombination is stimulated by oxolinic acid, an inhibitor of DNA gyrase. The stimulation is abolished by coumermycin A1 and is not found in extracts of nalidixic acid-resistant (gyrA) mutants. We therefore inferred that DNA gyrase directly participates in illegitimate recombination, at least in the presence of oxolinic acid [Ikeda, H., Moriya, K. & Matsumoto, T. (1981) Cold Spring Harbor Symp. Quant. Biol. 45, 399--408]. The structure of recombinant DNA molecules formed in the presence of oxolinic acid from a cross between phage lambda and plasmid pBR322 DNAs was analyzed by heteroduplex mapping. Among nine isolates tested, two recombinants were formed by the insertion of the plasmid into the lambda genome. The seven other recombinants had more complicated genome structures. Insertion of pBR322 was accompanied by a deletion on one of the genomes. In all cases, the end points of deletions coincided with one end of the pBR322 insertion. Recombination sites seemed to be distributed randomly on the lambda and pBR322 genomes. Analysis of nucleotide sequences of the recombination junctions proved that the crossover took place between nonhomologous DNA sequences. A model for DNA gyrase-mediated illegitimate recombination is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Daniels D. L., de Wet J. R., Blattner F. R. New map of bacteriophage lambda DNA. J Virol. 1980 Jan;33(1):390–400. doi: 10.1128/jvi.33.1.390-400.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons S. W., MacCosham V., Baldwin R. L. On the mechanism of production of tandem genetic duplications in phage lambda. J Mol Biol. 1975 Jun 15;95(1):83–89. doi: 10.1016/0022-2836(75)90337-x. [DOI] [PubMed] [Google Scholar]
- Franklin N. C. Extraordinary recombinational events in Escherichia coli. Their independence of the rec+ function. Genetics. 1967 Apr;55(4):699–707. doi: 10.1093/genetics/55.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Fisher L. M., Ohmori H., O'Dea M. H., Mizuuchi K. DNA gyrase: site-specific interactions and transient double-strand breakage of DNA. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):391–398. doi: 10.1101/sqb.1981.045.01.053. [DOI] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Itoh T., Tomizawa J. I. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4772–4776. doi: 10.1073/pnas.74.11.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingery R., Echols H. Integration, excision, and transducing particle genesis by bacteriophage lambda. Cold Spring Harb Symp Quant Biol. 1968;33:721–727. doi: 10.1101/sqb.1968.033.01.082. [DOI] [PubMed] [Google Scholar]
- Gottesman M. E., Yarmolinsky M. B. The integration and excision of the bacteriophage lambda genome. Cold Spring Harb Symp Quant Biol. 1968;33:735–747. doi: 10.1101/sqb.1968.033.01.084. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Matsumoto T. Transcription promotes recA-independent recombination mediated by DNA-dependent RNA polymerase in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4571–4575. doi: 10.1073/pnas.76.9.4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
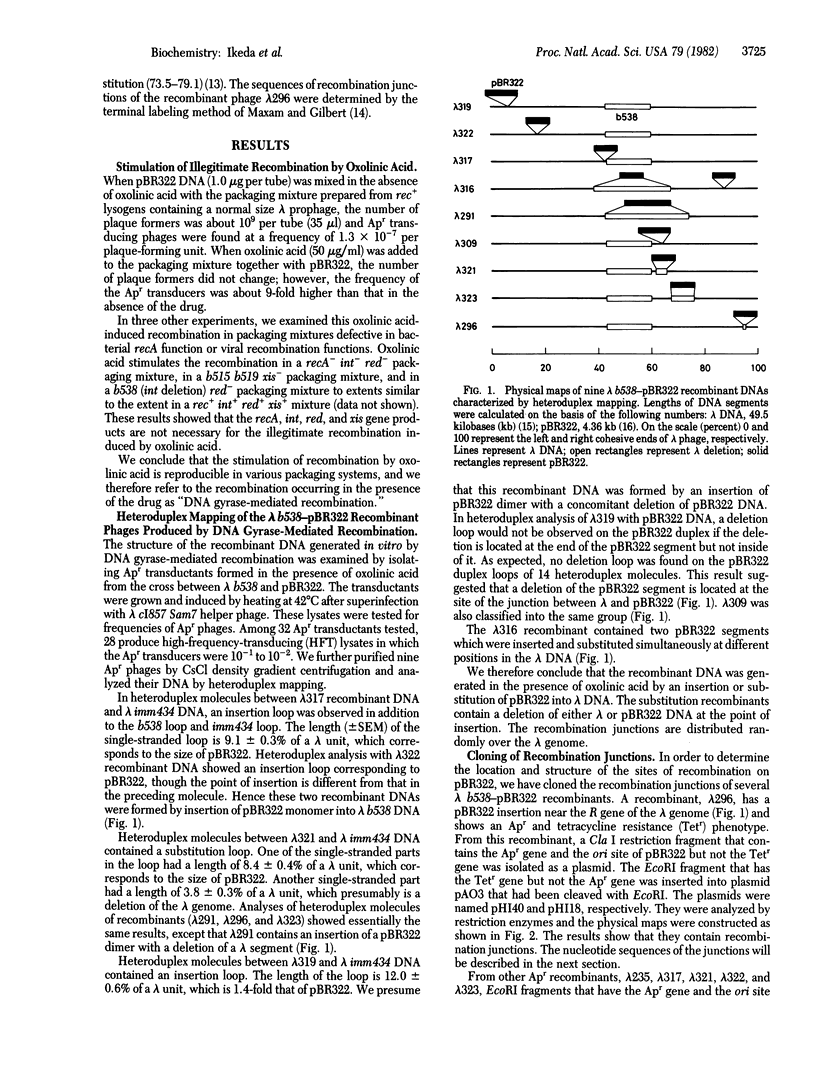
- Ikeda H., Moriya K., Matsumoto T. In vitro study of illegitimate recombination: involvement of DNA gyrase. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):399–408. doi: 10.1101/sqb.1981.045.01.054. [DOI] [PubMed] [Google Scholar]
- Inselburg J. Formation of deletion mutations in recombination-deficient mutants of Escherichia coli. J Bacteriol. 1967 Oct;94(4):1266–1267. doi: 10.1128/jb.94.4.1266-1267.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb A. J., Lustig A. The molecular weight of concanavalin A. Biochim Biophys Acta. 1968 Oct 21;168(2):366–367. doi: 10.1016/0005-2795(68)90161-x. [DOI] [PubMed] [Google Scholar]
- Klevan L., Wang J. C. Deoxyribonucleic acid gyrase-deoxyribonucleic acid complex containing 140 base pairs of deoxyribonucleic acid and an alpha 2 beta 2 protein core. Biochemistry. 1980 Nov 11;19(23):5229–5234. doi: 10.1021/bi00564a012. [DOI] [PubMed] [Google Scholar]
- Kobayashi I., Ikeda H. Formation of recombinant DNA of bacteriophage lambda by recA function of Escherichia coli without duplication, transcription, translation, and maturation. Mol Gen Genet. 1977 Jun 24;153(3):237–245. doi: 10.1007/BF00431589. [DOI] [PubMed] [Google Scholar]
- Kobayashi I., Ikeda H. On the role of recA gene product in genetic recombination: an analysis by in vitro packaging of recombinant DNA molecules formed in the absence of protein synthesis. Mol Gen Genet. 1978 Oct 25;166(1):25–29. doi: 10.1007/BF00379725. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison A., Cozzarelli N. R. Site-specific cleavage of DNA by E. coli DNA gyrase. Cell. 1979 May;17(1):175–184. doi: 10.1016/0092-8674(79)90305-2. [DOI] [PubMed] [Google Scholar]
- Oka A., Nomura N., Morita M., Sugisaki H., Sugimoto K., Takanami M. Nucleotide sequence of small ColE1 derivatives: structure of the regions essential for autonomous replication and colicin E1 immunity. Mol Gen Genet. 1979 May 4;172(2):151–159. doi: 10.1007/BF00268276. [DOI] [PubMed] [Google Scholar]
- Reeke G. N., Jr, Becker J. W., Edelman G. M. The covalent and three-dimensional structure of concanavalin A. IV. Atomic coordinates, hydrogen bonding, and quaternary structure. J Biol Chem. 1975 Feb 25;250(4):1525–1547. [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Szybalski E. H., Szybalski W. A comprehensive molecular map of bacteriophage lambda. Gene. 1979 Nov;7(3-4):217–270. doi: 10.1016/0378-1119(79)90047-7. [DOI] [PubMed] [Google Scholar]


