Abstract
Purpose.
The complement system is closely linked to the pathogenesis of AMD. Several complement genes are expressed in RPE, and complement proteins accumulate in drusen. Further, a common variant of complement factor H (CFH) confers increased risk of developing AMD. Because the mechanisms by which changes in the function of CFH influence development of AMD are unclear, we examined ocular complement expression as a consequence of age in control and CFH null mutant mice.
Methods.
Gene expression in neuroretinas and RPE/choroid from young and aged WT and Cfh−/− C57BL/6J mice was analyzed by microarrays. Expression of a wide range of complement genes was compared with expression in liver.
Results.
An age-associated increased expression of complement, particularly C1q, C3, and factor B, in the RPE/choroid coincided with increased expression of the negative regulators Cfh and Cd59a in the neuroretina. Young mice deficient in CFH expressed Cd59a similar to WT, but failed to upregulate Cd59a expression with age. Hepatic expression of Cd59a increased with age regardless of Cfh genotype.
Conclusions.
While the connection between CFH deficiency and failure to upregulate CD59a remains unknown, these results suggest that expression of CD59 is tissue-specific and that neuroretinal regulation depends on CFH. This could contribute to the visual functional deficits and morphological changes in the Cfh−/− mouse retina that occur with age.
Here we characterize the complement transcriptome in neuroretina and RPE/choroid of young and aged mice. Further, we identify loss of neuroretinal upregulation of the membrane attack complex inhibitor Cd59a as the main consequence of complement factor H deficiency.
Introduction
AMD is the commonest cause of blindness in the Western world affecting more than 10% of persons above 60 years of age. Although the cellular pathogenesis is not fully understood, disease progression involves degeneration of the RPE. The RPE has several crucial functions. These include supporting the photoreceptors by supplying nutrients, removing shed outer segments, and formation of the outer blood-retinal barrier (BRB). One of the early clinical hallmarks of AMD is the funduscopic appearance of drusen, formed by accumulating deposits of lipids and proteins external to the RPE, outside the BRB.1–4
Age is the largest risk factor for AMD. Of the known single nucleotide polymorphisms, the Y402H in complement factor H (CFH) confers the highest risk. CFH is the principal fluid-phase inhibitor of the alternative pathway of the complement cascade. The level of complement activation correlates with CFH haplotype, and AMD patients have elevated plasma concentrations of activation split-products.5–8 Further, several complement components and regulators, including C3, C5, complement factor B (CFB) and CFH, have been identified in drusen of AMD patients.3,4,9
Most complement proteins are primarily synthesized in the liver, but numerous complement genes associated with the alternative and classical pathways are also constitutively expressed in the neuroretina and RPE. As such, the posterior eye expresses its own complement regulatory system, which may function independently in the ocular environment.10–14 For unknown reasons, ocular complement activation increases with age.11,13 This may have implications for drusen formation and AMD-pathogenesis. However, the mechanism by which dysregulation of complement via altered regulatory function of CFH leads to retinal injury is not known in detail. Therefore, we analyzed complement gene expression in the neuroretinas and RPE/choroid of young and old WT and Cfh−/− mice.
Materials and Methods
Animals
We used young (7–8 weeks of age) and aged (16–17 months of age) WT and Cfh−/− C57BL/6J mice, which do not harbor the rd8 mutation in the Crb gene.15 Cfh−/− mice were provided by Matthew Pickering.16 All experiments adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Harvest of Tissue and Isolation of RNA
Immediately following cervical dislocation, eyes were removed and hemi-dissected posterior to the ora serrata. The neuroretinas (NR) from both eyes were transferred to an RNA stabilization reagent solution (RNALater; Qiagen, Valencia, CA) for 24 hours, removed, and saved at −80°C. The RPE/choroid remaining in the two eyecups was gently scraped with a 25-gauge needle and transferred to an RNA extraction solution (TRIzol; Invitrogen, Grand Island, NY). Small pieces of liver were transferred to an RNA stabilization reagent solution (Qiagen) for 24 hours, removed, and saved at −80°C. No tissues from different mice were pooled. RNA from both RPE/choroid and NR was isolated using a standard RNA extraction solution (Invitrogen) protocol followed by a cleanup on mini spin columns (RNeasy; Qiagen). Traces of genomic DNA were degraded by treatment with amplification grade DNase I. RNA from livers was isolated using RNA isolation columns (Nucleospin RNA II; Macherey-Nagel, Bethlehem, PA).
Microarray
RNA quality and quantity were assessed using a bioanalyzer (Agilent, Horsholm, Denmark). RPE/choroid RNA was linearly amplified using an amplification kit (Ovation WGA; NuGEN Technologies, San Carlos, CA). RPE/choroid and NR-RNA underwent sense target labeling prior to hybridization to miniature arrays (Mouse Gene 1.0 ST Array GeneChips; Affymetrix, Santa Clara, CA). These microarrays (Affymetrix) were washed and stained in a fluidics station (Affymetrix) prior to scanning. All microarray data are MIAME compliant, and raw data have been deposited in the Gene Expression Omnibus repository (accession number GSE38671). The Cfh probeset on the miniature arrays (Affymetrix) contains thirty-two 25-mer probes covering 15 of the 22 exons (Integrated Genome Browser 6.6, www.bioviz.org, in the public domain). Therefore, it will also bind to the disrupted Cfh transcript in Cfh−/−, which explains the detection of Cfh expression in Cfh−/− mice.
qRT-PCR
Ocular RNAs (RPE/choroid, 300 ng; NR, 600 ng) were converted to cDNA using a transcription kit (Qiagen Quantitect RT; Qiagen). RNAs from livers (1 μg) were converted to cDNA using a first strand cDNA synthesis kit (RevertAid; Fermentas, Copenhagen, Denmark). QPCR master mix (Brilliant SYBR; Stratagene, Horsholm, Denmark) was used for qRT-PCR with the primers specified in the Table. For each run, a melting curve was generated to ascertain specificity. Threshold cycle (Ct) values were averaged from duplicates differing by less than one cycle. Fold changes between groups of animals were calculated from a beta actin-normalized amount of transcript as 2(Ct, beta actin−Ct, target gene)
Table. .
qRT-PCR Primer Sequences
|
Gene |
Accession |
Forward (5′-3′) |
Reverse (5′-3′) |
| Actb | NM_007393.3 | TCCAAGTATCCATGAAATAAGTGG | GCAGTACATAATTTACACAGAAGC |
| C3 | NM_009778.2 | AGACACAAAGGACCTGGAACTGCT | AGGCAGTCTTCTTCGGTGTGTGAA |
| C1qc | NM_007574.2 | GTTCAACAGCAAGCAGGTCA | ACCAGAGAAGACGCTGTTGG |
| Cfh | NM_009888.3 | TCCTTTAGGCTGGCAGTTGGATCT | TCATTGATCCACCCATCTGCACCA |
| Cfb | NM_008198.2 | TGATGTGGCCCTAGTCAAGCTCAA | GAGCAACTGTTCCTTGTGCTGCTT |
| Cd59a | NM_001111060.1 | GAGCATGAGCACAGTCACTGGCG | GAACACAGCCAGAAGCAGCAGGAG |
| Serping1 | NM_009776.3 | TAGAGCCTTCTCAGATCCCGA | ACTCGTTGGCTACTTTACCCA |
Statistics
Microarray data were normalized using robust multiarray averaging (RMA). Data were checked for outliers using principal component analysis. ANOVA analysis of data generated a standard P value. P values were adjusted using the Benjamini & Hochberg method and the false discovery rate was set at ≤0.05 to create gene lists that showed significant differentially expressed genes and exons between the different groups of mice (Partek Genomics Suite; Partek, St. Louis, MO). The unpaired t-test with Welch's correction for unequal variances was used for analysis of the qRT-PCR using graphing software (GraphPad Prism v. 4.03 for Windows; GraphPad Software, La Jolla, CA). P values ≤0.05 were considered statistically significant.
Results
Complement Transcriptome in Neuroretina and RPE/Choroid
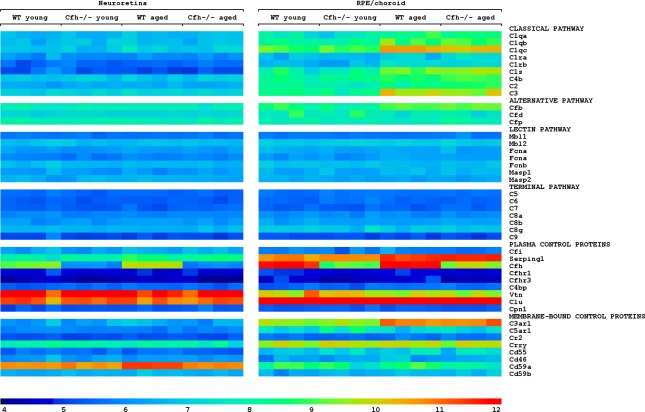
To characterize the full expression of complement-associated genes in the back of the eye, we isolated RNA from neuroretina (NR) and RPE/choroid from individual mice and analyzed it using genome-wide Affymetrix microarrays. In RPE/choroid, we did not detect any significant differences in the expression of complement-associated genes between WT and Cfh−/− mice. With age, however, components of the classical and the alternative pathways including C1q, C1r, C1s, C3, and Cfb were upregulated in both normal and mutant strains. Genes associated with either the lectin or the terminal pathways were not expressed at detectable levels in the RPE/choroid of young or old mice. The genes encoding the C1-inhibitor, Serping1, and the C3a receptor, C3aR1, were both expressed at high levels and also increased with age. Regardless of age, genes encoding the negative regulators, vitronectin (Vtn), Crry and in particular clusterin (Clu) were expressed at high levels, while Cd46 and Cd55 were expressed at low levels (Fig. 1).
Figure 1. .
The complement transcriptome in neuroretina and RPE/choroid. Heat map showing that aging resulted in the upregulation of several genes associated with activation of the classical and alternative pathways in RPE/choroid together with upregulation of negative regulators of both early and late pathways in RPE/choroid and neuroretina. Old animals deficient in Cfh, however, failed to upregulate Cd59a. To visualize differential regulation, normalized expression values are shown in log2 scale with arbitrary units. Values ranged from 3.5 to 12.5 and 97.0% are within the chosen scale. See Supplementary Table S1 for numeric values (see Supplementary Material and Supplementary Table S1, http://www.iovs.org/content/53/10/6324/suppl/DC1).
In NR, we primarily detected expression of genes encoding negative regulators. Thus, Vtn, Clu, and Cd59a were expressed at high levels. Notably, while Vtn and Clu remained unchanged with age, the expression of Cd59a increased with age, but only in WT mice. Thus, besides the deficient expression of Cfh, lack of neuroretinal upregulation of Cd59a with age represented the only significant difference between WT and Cfh−/− mice. Further, in WT mice, the expression of Cfh was highest in RPE/choroid, while the expression of Cd59a was highest in NR (Fig. 1). The numerical values for all the complement genes can be found in Supplementary Table S1 (see Supplementary Material and Supplementary Table S1, http://www.iovs.org/content/53/10/6324/suppl/DC1) and a full list of all differentially regulated genes identified in the microarray can be found in Supplementary Tables S2–S5 (see Supplementary Material and Supplementary Tables S2–S5, http://www.iovs.org/content/53/10/6324/suppl/DC1).
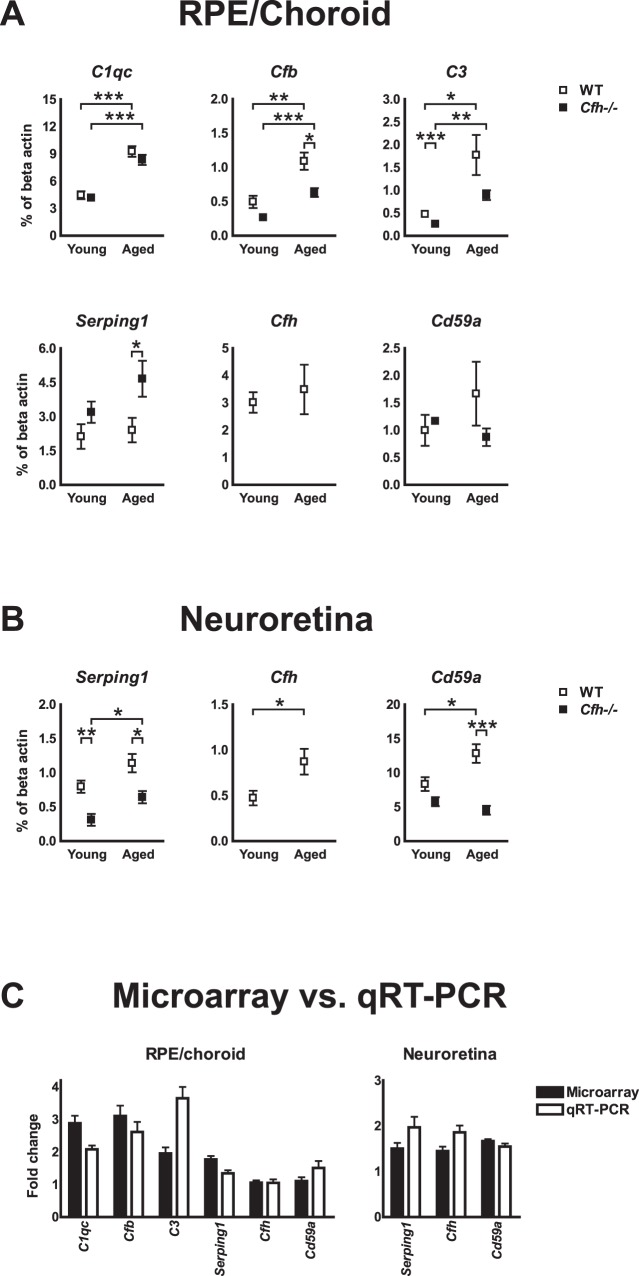
qRT-PCR of selected complement genes confirmed the lack of neuroretinal upregulation of Cd59a with age and showed good agreement with results obtained from microarrays. However, a lower expression of Cfb and C3 and a higher expression of Serping1 were noted in RPE/choroid of Cfh−/− mice (Fig. 2).
Figure 2. .
Validation of deficient neuroretinal upregulation of Cd59a with age. qRT-PCR analysis of selected genes that were differentially regulated in microarrays. (A) RPE/choroid. (B) Neuroretina. Average expression normalized to beta actin; 5–6 mice in each group. *P < 0.05. **P < 0.01. ***P < 0.001. (C) Comparison of age-dependent fold change from microarray and qRT-PCR. Data pooled from WT and Cfh−/− mice except values for Cfh and Cd59a, where only data from WT mice are shown. All error bars represent SEM.
Systemic Expression of Complement Genes
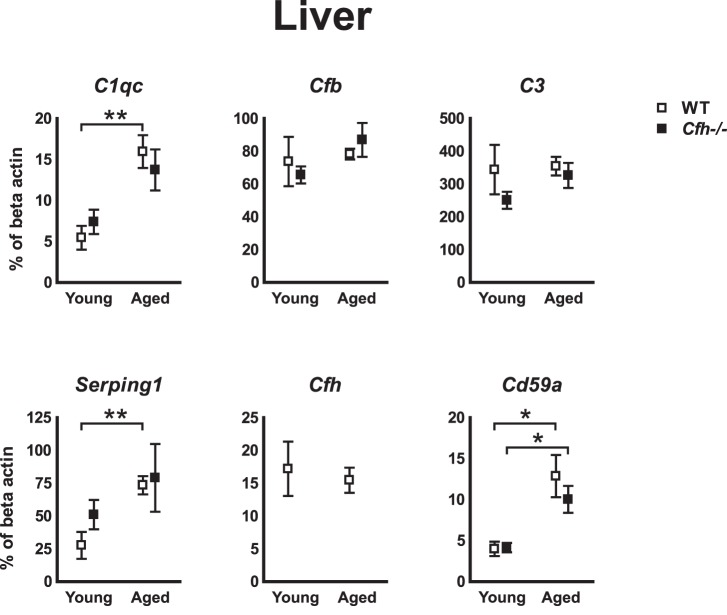
To test whether the observed differential expression of complement genes, in particular Cd59a, was specific to ocular tissue, we analyzed the expression of selected genes in the liver using qRT-PCR. Because most complement genes are expressed in the liver, we analyzed the same set of genes that were tested in RPE/choroid. Indeed, Cd59a increased with age in the liver from both WT and Cfh−/− mice. Further, an indication of increased activity in the classical pathway with age was noted. As such, with age C1qc and Serping1 increased in WT mice and showed a similar tendency in Cfh−/− mice. The increased expression of Cfb and C3 with age observed in the RPE/choroid was not recapitulated in the liver (Fig. 3).
Figure 3. .
Hepatic expression of Cd59a increases with age independently of Cfh. qRT-PCR analyze of selected complement genes in the liver revealed increased expression of Cd59a with age in both WT and Cfh−/− mice. The classical components C1qc and Serping1 also increased with age (only tendency in Cfh−/− mice), while there was no differential expression of Cfb or C3; 5 to 6 mice in each group. Error bars represent SEM. *P < 0.05. **P < 0.01.
Spatial and Temporal Expression of Cd59a
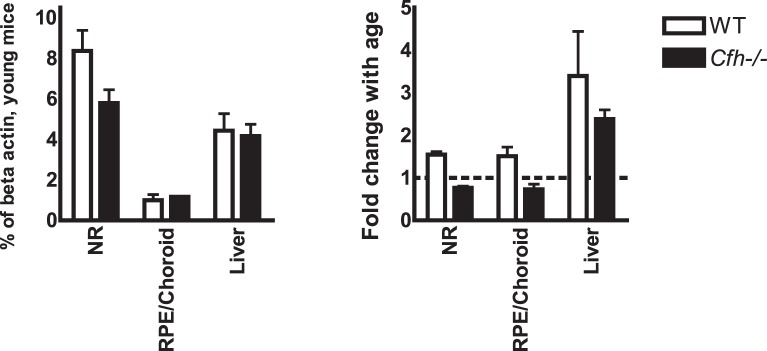
Taking the qRT-PCR data on Cd59a together, a pattern of tissue-specific expression and regulation emerges. Thus, the rank order of Cd59a expression in young mice (neuroretina > liver > RPE/choroid) showed the highest expression in neuroretinas and was independent of Cfh genotype. With age, however, loss of Cfh resulted in loss of ocular upregulation of Cd59a, whereas the liver displayed increased expression regardless of Cfh genotype (Fig. 4).
Figure 4. .
Only ocular tissue from Cfh−/− mice displays deficient up-regulation of Cd59a with age. Comparison of qRT-PCR data from different tissues, revealed a tissue-specific basal expression and regulation of Cd59a. The expression in neuroretinas of young mice was highest, whereas the upregulation with age was more marked in the liver. The dotted line represents cutoff between positive and negative fold change.
Discussion
To examine the role of complement factor H in the ocular complement regulatory system, we analyzed the expression of complement-related genes in neuroretinas and RPE/choroid of young and old WT and Cfh−/− mice. With age, several complement genes were upregulated in both the neuroretina and RPE/choroid of WT mice.
The profile of murine ocular complement gene expression has been analyzed previously, and although there is some disagreement with regards to the neuroretinal up-regulation of complement factors,11,13 it is generally agreed that RPE/choroid displays increased expression of certain complement factors with age.11,12 From our results, however, it is evident that this increase coincides with a neuroretinal upregulation of complement inhibitors, possibly to control bystander damage of the photoreceptors.
Compared to WT, the CFH-deficient mice displayed most of the same changes in complement expression with age with one notable difference: there was no age-dependent increased neuroretinal expression of Cd59a. The murine gene encoding CD59 is duplicated; Cd59a represents the main regulator of membrane-attack complex (MAC) assembly and is widely expressed, while Cd59b is expressed only in testis.17 In agreement with our data, the human retina, rat, and murine eyecups have abundant CD59a expression in the photoreceptor and outer plexiform layers, while the RPE expression is weak.9,18–21 Collectively, this might explain the visual functional deficits and morphological changes in the Cfh−/− mouse retina with age. Specifically, lack of CFH caused increased deposition of C3 around the photoreceptor outer segments in the neuroretina22 corresponding to the expression pattern of CD59a in WT mice.18
Experimental laser photocoagulation of Bruch's membrane in mice results in choroidal neovascularization (CNV) via complement activation. Thus, C3 and MAC were reported deposited in sites of experimental CNV, and loss of complement function or delivery of CD59a reduced CNV formation in studies using this model.23–26 Notably, laser-induced acute complement activation included a transient decrease in CD59a expression.24 Thus, expression of CD59a may decrease in the context of increased complement activation.
In ARPE19 cells, CD59 expression was shown to increase in response to T cell-derived mediators.27 Primary cultures of human RPE cells also upregulated CD59 expression in response to TNFα or IL1β, but cultured murine RPE cells failed to do so.18 Further, oxidative stress increased expression of CD59 in an epithelial cell line derived from human lung,28 whereas the expression was decreased in ARPE19.29 LPS induced upregulation of CD59 in human monocyte-derived dendritic cells30 and in human monocytes,31 whereas murine bone marrow-derived macrophages stimulated with IFNγ and LPS or immune complex and LPS downregulated expression of Cd59a.32 It is not known, whether these differences are caused by different stimulation, demands for costimulation, or actually reflect differences in the regulation of CD59 between human and mouse. Together, the reports do support a tissue-specific regulation of CD59, which may be a consequence of complex control at the transcriptional level.33
Although the total loss of CFH-function in Cfh−/− mice does not accurately reflect the relative loss of function in the AMD risk-conferring CFHY402H genotype, similarities have been reported. As such, it was shown that RPE from donors with the CFHHH402 genotype had a tendency to decreased expression of CD59 compared with RPE from the CFHYY402 donors,18 while there was an increased content of MAC in human RPE/choroid from individuals with the CFHHH402 genotype.34 And in a gene expression study on a few donor eyes, a tendency to decreased expression of CD59 in retina and RPE/choroid from AMD patients was shown.9 Notably, several studies have demonstrated increased plasma levels of complement regulators and split-products in AMD patients, in particular with the CFHHH402 genotype,5–8 which thus might act to decrease ocular expression of CD59 and/or increase ocular deposition of MAC. In support of this, systemic virus infection in mice resulting in acute complement activation induced a transient 2-fold decrease in Cd59a expression in RPE/choroid (Faber C. unpublished data) and a decreased density of CD59 on peripheral monocytes have been reported in patients with neovascular AMD.35 Collectively, the retinal damage in AMD could be the result of a local decrease in CD59 expression in response to increased systemic complement activation.
Increased density of MAC in human choroid has previously been associated with loss of RPE and AMD severity.36 Unlike MAC and the negative regulators MCP/CD46, CFH, vitronectin, and clusterin, CD59 is one of a few complement-related molecules, which, to the best of our knowledge, has not been identified in drusen.3,4,9 While negative results have to be interpreted with caution, it does support our hypothesis that down-regulation of CD59 is an early event in AMD pathogenesis.
Deficiency of CFH was inconsequential to hepatic expression of the complement genes tested. Specifically, Cd59a was upregulated with age both in WT and Cfh−/− mice, thus validating that the lack of neuroretinal upregulation of Cd59a is not caused by a systemic defect of Cd59a regulation in the Cfh−/− mice. Unlike the RPE/choroid, the hepatocytes did not increase expression of C3 and Cfb, which suggests that ocular expression of complement genes is influenced by changes in the local microenvironment. This might include accumulation of waste products from the visual cycle37 or oxidative damage.38 Nevertheless, hepatic complement gene expression probably also changes in response to stimuli, accordingly it has been reported that expression of several complement genes, including C3 and CFB, is higher in adult compared with fetal human liver.9
In summary, we have shown that the age-related increased expression of complement in RPE/choroid is upheld by an increased neuroretinal expression of negative regulators. The lack of neuroretinal upregulation of Cd59 with age constitutes the main difference between WT and Cfh−/− mice, which might explain the functional deficits and altered retinal morphology of aged Cfh−/− mice.
Supplementary Material
Footnotes
Supported by Lundbeckfonden (MHN); The Danish Eye Research Foundation (CF); Svend Helge Arvid Schrøder og Hustru Ketty Lydia Larsen Schrøders Fond (CF); Mindefonden for lærerinde Karen Svankjær Yde (CF) and Aase og Ejnar Danielsens Fond (CF); the Medical Research Council (SEM); and the Wellcome Trust (grant reference 090669, SEM).
These authors contributed equally to the work presented here and should therefore be regarded as equivalent authors.
Disclosure: C. Faber, None; J. Williams, None; H.B. Juel, None; J. Greenwood, None; M.H. Nissen, None; S.E. Moss, None
References
- 1.la Cour M, Kiilgaard JF, Nissen MH. Age-related macular degeneration: epidemiology and optimal treatment. Drugs Aging. 2002;19:101–133 [DOI] [PubMed] [Google Scholar]
- 2.Coleman HR, Chan CC, Ferris FL III, Chew EY. Age-related macular degeneration. Lancet. 2008;372:1835–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L, Clark ME, Crossman DK, et al. Abundant lipid and protein components of drusen. PLoS ONE. 2010;5:e10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crabb JW, Miyagi M, Gu X, et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:14682–14687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scholl HPN, Issa PC, Walier M, et al. Systemic complement activation in age-related macular degeneration. PLoS ONE. 2008;3:e2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Machalinska A, Dziedziejko V, Mozolewska-Piotrowska K, Karczewicz D, Wiszniewska B, Machalinski B. Elevated plasma levels of C3a complement compound in the exudative form of age-related macular degeneration. Ophthalmic Res. 2009;42:54–59 [DOI] [PubMed] [Google Scholar]
- 7.Sivaprasad S, Adewoyin T, Bailey TA, et al. Estimation of systemic complement C3 activity in age-related macular degeneration. Arch Ophthalmol. 2007;125:515–519 [DOI] [PubMed] [Google Scholar]
- 8.Reynolds R, Hartnett ME, Atkinson JP, Giclas PC, Rosner B, Seddon JM. Plasma complement components and activation fragments: associations with age-related macular degeneration genotypes and phenotypes. Invest Ophthalmol Vis Sci. 2009;50:5818–5827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson DH, Radeke MJ, Gallo NB, et al. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res. 2009;29:95–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu H, Chen M, Forrester JV. Para-inflammation in the ageing retina. Prog Retin Eye Res. 2009;28:348–368 [DOI] [PubMed] [Google Scholar]
- 11.Chen H, Liu B, Lukas TJ, Neufeld AH. The aged retinal pigment epithelium/choroid: a potential substratum for the pathogenesis of age-related macular degeneration. PLoS ONE. 2008;3:e2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen M, Muckersie E, Robertson M, Forrester JV, Xu H. Up-regulation of complement factor B in retinal pigment epithelial cells is accompanied by complement activation in the aged retina. Exp Eye Res. 2008;87:543–550 [DOI] [PubMed] [Google Scholar]
- 13.Chen M, Muckersie E, Forrester JV, Xu H. Immune activation in retinal aging: a gene expression study. Invest Ophthalmol Vis Sci. 2010;51:5888–5896 [DOI] [PubMed] [Google Scholar]
- 14.Luo C, Chen M, Xu H. Complement gene expression and regulation in mouse retina and retinal pigment epithelium/choroid. Mol Vis. 2011;17:1588–1597 [PMC free article] [PubMed] [Google Scholar]
- 15.Mattapallil MJ, Wawrousek EF, Chan CC, et al. The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes. Invest Ophthalmol Vis Sci. 2012;53:2921–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pickering MC, Cook HT, Warren J, et al. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet. 2002;31:424–428 [DOI] [PubMed] [Google Scholar]
- 17.Baalasubramanian S, Harris CL, Donev RM, et al. CD59a is the primary regulator of membrane attack complex assembly in the mouse. J Immunol. 2004;173:3684–3692 [DOI] [PubMed] [Google Scholar]
- 18.Yang P, Tyrrell J, Han I, Jaffe GJ. Expression and modulation of RPE cell membrane complement regulatory proteins. Invest Ophthalmol Vis Sci. 2009;50:3473–3481 [DOI] [PubMed] [Google Scholar]
- 19.Bora NS, Gobleman CL, Atkinson JP, Pepose JS, Kaplan HJ. Differential expression of the complement regulatory proteins in the human eye. Invest Ophthalmol Vis Sci. 1993;34:3579–3584 [PubMed] [Google Scholar]
- 20.Sohn JH, Kaplan HJ, Suk HJ, Bora PS, Bora NS. Chronic low level complement activation within the eye is controlled by intraocular complement regulatory proteins. Invest Ophthalmol Vis Sci. 2000;41:3492–3502 [PMC free article] [PubMed] [Google Scholar]
- 21.Vogt SD, Barnum SR, Curcio CA, Read RW. Distribution of complement anaphylatoxin receptors and membrane-bound regulators in normal human retina. Exp Eye Res. 2006;83:834–840 [DOI] [PubMed] [Google Scholar]
- 22.Coffey PJ, Gias C, McDermott CJ, et al. Complement factor H deficiency in aged mice causes retinal abnormalities and visual dysfunction. Proc Natl Acad Sci U S A. 2007;104:16651–16656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cashman SM, Ramo K, Kumar-Singh RA. Non membrane-targeted human soluble CD59 attenuates choroidal neovascularization in a model of age related macular degeneration. PLoS ONE. 2011;6:e19078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bora NS, Kaliappan S, Jha P, et al. CD59, a complement regulatory protein, controls choroidal neovascularization in a mouse model of wet-type age-related macular degeneration. J Immunol. 2007;178:1783–1790 [DOI] [PubMed] [Google Scholar]
- 25.Bora PS, Sohn JH, Cruz JMC, et al. Role of complement and complement membrane attack complex in laser-induced choroidal neovascularization. J Immunol. 2005;174:491–497 [DOI] [PubMed] [Google Scholar]
- 26.Bora NS, Jha P, Lyzogubov VV, et al. Recombinant membrane-targeted form of CD59 inhibits the growth of choroidal neovascular complex in mice. J Biol Chem. 2010;285:33826–33833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juel HB, Kaestel C, Folkersen LW, et al. Retinal pigment epithelial cells upregulate expression of complement factors after co-culture with activated T cells. Exp Eye Res. 2011;92:180–188 [DOI] [PubMed] [Google Scholar]
- 28.Thurman JM, Renner B, Kunchithapautham K, Holers VM, Rohrer B. Aseptic injury to epithelial cells alters cell surface complement regulation in a tissue specific fashion. Adv Exp Med Biol. 2010;664:151–158 [DOI] [PubMed] [Google Scholar]
- 29.Thurman JM, Renner B, Kunchithapautham K, et al. Oxidative stress renders retinal pigment epithelial cells susceptible to complement-mediated injury. J Biol Chem. 2009;284:16939–16947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li K, Fazekasova H, Wang N, et al. Expression of complement components, receptors and regulators by human dendritic cells. Mol Immunol. 2011;48:1121–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christmas SE, De La Mata Espinosa C, Halliday D, Buxton CA, Cummerson JA, Johnson PM. Levels of expression of complement regulatory proteins CD46, CD55 and CD59 on resting and activated human peripheral blood leucocytes. Immunology. 2006;119:522–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo C, Chen M, Madden A, Xu H. Expression of complement components and regulators by different subtypes of bone marrow-derived macrophages. Inflammation. 2012;35:1448–1461 [DOI] [PubMed] [Google Scholar]
- 33.Martin BK. Transcriptional control of complement receptor gene expression. Immunol Res. 2007;39:146–159 [DOI] [PubMed] [Google Scholar]
- 34.Mullins RF, Dewald AD, Streb LM, Wang K, Kuehn MH, Stone EM. Elevated membrane attack complex in human choroid with high risk complement factor H genotypes. Exp Eye Res. 2011;93:565–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh A, Faber C, Falk M, Nissen MH, Hviid TV, Sorensen TL. Altered expression of CD46 and CD59 on leukocytes in neovascular age-related macular degeneration. Am J Ophthalmol. 2012;154:193–199 [DOI] [PubMed] [Google Scholar]
- 36.Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers Drusen as biomarkers of immune-mediated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20:705–732 [DOI] [PubMed] [Google Scholar]
- 37.Zhou J, Kim SR, Westlund BS, Sparrow JR. Complement activation by bisretinoid constituents of RPE lipofuscin. Invest Ophthalmol Vis Sci. 2009;50:1392–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hollyfield JG, Perez VL, Salomon RG. A hapten generated from an oxidation fragment of docosahexaenoic acid is sufficient to initiate age-related macular degeneration. Mol Neurobiol. 2010;41:290–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.