Abstract
Purpose.
In human retinal degeneration, rod photoreceptors reactively sprout neurites. The mechanism is unknown in part because of the paucity of animal models displaying this feature of human pathology. We tested the role of cAMP and opsin in sprouting by tiger salamander rod cells, photoreceptors that can produce reactive growth.
Methods.
In vitro systems of isolated photoreceptor cells and intact neural retina were used. cAMP signaling was manipulated with nucleotide analogues, enzyme stimulators, agonists for adenosine and dopamine receptors, and the opsin agonist, β-ionone. Levels of cAMP were determined by radioimmunoassay, and protein levels by Western blot and quantitative immunocytochemistry. Neuritic growth was assayed by image analysis and conventional and confocal microscopy.
Results.
cAMP analogues and stimulation of adenylyl cyclase (AC) directly or through G-protein–coupled receptors resulted in significant increases in neuritic growth of isolated rod, but not cone, cells. The signaling pathway included protein kinase A (PKA) and phosphorylation of the transcription factor cAMP response element-binding protein (pCREB). Opsin, a G-linked receptor, is present throughout the plasmalemma of isolated cells; its activation also induced sprouting. In neural retina, rod sprouting was significantly increased by β-ionone with concomitant increases in cAMP, pCREB, and synaptic proteins. Notably, opsin stimulated sprouting only when mislocalized to the plasmalemma of the rod cell body.
Conclusions.
cAMP causes neuritic sprouting in rod, but not cone, cells through the AC-PKA-CREB pathway known to be associated with synaptic plasticity. We propose that in retinal disease, mislocalized rod opsin gains access to cAMP signaling, which leads to neuritic sprouting.
cAMP-PKA-CREB signaling stimulates neuritic sprouting by adult rod cells in vitro. This signaling is initiated by opsin if the protein is mislocalized to the plasmalemma of the inner segment. Thus, in retinal disease, activation of mislocalized opsin may contribute to aberrant sprouting by rod cells.
Introduction
Sprouting of new neuronal processes by adult nerve cells is an injury-induced phenomenon expressed in both the peripheral and central nervous systems (CNS). New growth comes from both injured and uninjured, neighboring cells.1 Injury-induced sprouting has been described after lesion of the spinal cord and brain as well as in a number of neurodegenerative diseases including Alzheimer's,2,3 multiple sclerosis,4 and retinitis pigmentosa (RP).5 For humans, perhaps the most comprehensive descriptions of sprouting have come from studies of the retina, where the cell type producing the sprouts can be easily identified because of its well-known histology and the abundance of cell type–specific markers. Multiple types of RP show long neuritic sprouts emerging from rod photoreceptors5,6 and from the second- and third-order horizontal and amacrine cells.7 For rod cells, sprouting has also been observed in age-related macular degeneration (AMD),8 after laser damage,9 and after reattachment of a detached retina, a mechanical injury.10,11
Understanding the mechanisms leading to sprouting may increase our ability to improve functional recovery after injury to the CNS. It is thought that sprouting from rod cells, for instance, inhibits restoration of normal visual function after repair of a detached retina.10 Strategies to control photoreceptor neuritic growth, therefore, could be useful not only to reduce sprouting in degenerative disease and mechanical injury in order to preserve the normal retinal circuitry but also to increase sprouting after transplantation of photoreceptor cells back into degenerate retinas, a strategy that continues to be developed to restore sight in the blind.
Unfortunately, despite the ubiquitous nature of this reactive growth in human retina and the potential therapeutic importance, little is known about the mechanisms that produce nerve cell sprouting. An obstacle to progress is the lack of adequate animal models with which to experiment. For the retina, photoreceptor sprouting has not been observed, with one exception, in any of the numerous mouse models of retinal degeneration. In the rd1 mouse, cone cell sprouting does occur.12 Sprouting from cone cells is, however, relatively rare in human retinal disease and injury.7 Rod cell sprouting has been observed in an amphibian model of a common form of RP, created by transgenic expression of a mutation in the rod opsin gene.13 It has also been observed in genetically linked retinal degeneration of the pig and cat,14,15 and in reattachment of cat retina.10
In this report, we use two in vitro models, one of isolated retinal cells and the other of the intact neural retina, from an amphibian—the adult tiger salamander. Adult photoreceptors placed in culture sprout spontaneously. We can manipulate their sprouting with exogenous drugs. In this way, we have previously determined that sprouting by cone cells depends on increases in cGMP.16 Here, we examine the role of cAMP, a well-established signaling molecule in pathways leading to the regeneration of adult projection neurons17 and synaptic plasticity,18 and opsin, a G-protein–linked transmembrane protein, in sprouting by rod photoreceptors. We demonstrate not only that rod cell sprouting is cAMP dependent through modulation of the activity of adenylyl cyclase (AC) but also that the process increases phosphorylation of the cAMP response element-binding protein (CREB). Further, we suggest how cAMP is increased in retinal degeneration and in the reattached retina, to produce sprouting in the intact retina. We demonstrate that the mechanism includes activation of the rod photopigment opsin, a pathway previous explored for rod cell death.19 Opsin commonly mislocalized in disease and injury and thus can gain access to the AC-protein kinase A (PKA)-CREB pathway. For retinal disease and injury, the level of cAMP may be key to whether rod cells die or produce sprouts.
Retina is a well-established model for the CNS. Understanding the mechanism of sprouting here, therefore, may contribute to a deeper understanding of sprouting in neurons as well as how mislocalized or novel membrane components can contribute to disease processes in nerve and other tissues. These results have been previously reported in part at meetings of the Association for Researchers in Vision and Ophthalmology and the Society for Neuroscience.
Methods
Animals
Retinal tissue and cells were obtained from adult, aquatic-phase tiger salamanders (Ambystoma tigrinum, 18–25 cm in length; Charles Sullivan, Nashville, TN) and maintained at 5°C on a 12-hour light/12-hour dark cycle. Animals were adapted to the light cycle for at least 7 days before use. All protocols were approved by the Institutional Animal Care and Use Committee at New Jersey Medical School and adhere to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Chemicals and Antibodies
Forskolin (FSK), 2-p-(2-carboxyethyl) phenethylamino-5′-N-ethylcarboxamidoadenosine hydrochloride hydrate (CGS-21680, CGS), and β-ionone (Sigma, St. Louis, MO) were dissolved in 100% dimethyl sulfoxide (DMSO, Sigma) and diluted with medium to obtain their final concentration in 0.1% DMSO. Also, 9-(tetrahydro-2-furanyl)-9H-purin-6-amine (SQ22536), propidium iodide (PI), (-)-quinpirole (QPR, Sigma), adenosine-3′,5′-cyclic monophosphorothioate (Sp-cAMPS; Calbiochem, La Jolla, CA), and other reagents were dissolved in Millipore deionized water (Millipore, Billerica, MA). The salamander-specific antibody Sal-1 was generously provided by P. MacLeish (Morehouse School of Medicine, Atlanta, GA), and the 4D2 antibody against rod opsin was a gift from R. Molday (University of British Columbia, Vancouver, British Columbia, Canada). Antibodies against synaptophysin 1 (SYSY, Göttingen, Germany), CREB and phosphorylated CREB (pCREB) (Cell Signaling, Danvers, MA), synaptic vesicle protein 2 (SV2; Developmental Studies Hybridoma Bank, Iowa City, IA), α-tubulin (Sigma), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Santa Cruz Biotechnology, Santa Cruz, CA) were used. The molecular weights of the bands revealed by these antibodies in Western blots of salamander retinas were consistent with the antigens recognized in other species. Alex Fluor (AF) conjugated antibodies (Invitrogen, Carlsbad, CA) or horseradish peroxidase (HPR) conjugated antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) and the VECTASTAIN Elite ABC kit (VECTOR Laboratories, Burlingame, CA) were used as secondaries to label primary antibodies.
Cell and Tissue Culture
Retinal dissociation was performed as described previously.16,20 Briefly, animals were decapitated, pithed, and enucleated. For retinal cell culture, the neural retina was digested for 45 minutes in a Ringer's solution containing papain. After rinsing and trituration, the cell suspension was plated onto glass coverslips coated with Sal-1 antibody. Culture dishes were maintained in a dark, humidified incubator at 10°C in serum-free medium containing 108 mM NaCl, 2.5 mM KCl, 2 mM HEPES, 1 mM NaHCO3, 1.8 mM CaCl2, 0.5 mM NaH2PO4, 1 mM NaHCO3, 24 mM glucose, 0.5 mM MgCl2, 1 mM Na pyruvate, 7% medium 199, 1× MEM vitamin mix, 0.1× MEM essential amino acids, 0.1× MEM nonessential amino acids, 2 mM glutamine, 2 μg/mL bovine insulin, 1 μg/mL transferrin, 5 mM taurine, 0.8 μg/mL thyroxin, 10 μg/mL gentamicin, and 1 mg/mL BSA (pH 7.7). For retinal tissue culture, one-half of the detached retina was placed on a small piece of sterilized 3M filter paper (Whatman, Florham Park, NJ) with the ganglion cell layer facing the filter paper; the filter paper was placed in 1 mL culture medium and cultured in the same conditions described above. Cell or tissue cultures or eyeballs were fixed for a minimum of 2 hours with 4% paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.4.
Retinal Sections
Fixed retinal tissue was rinsed with 5% sucrose, placed in 30% (wt/vol) sucrose/PBS, embedded in the Tissue-Tek embedding medium, Optimum Cutting Temperature (OCT) Compound (Miles Inc., Elkhart, IN), for approximately 2 hours at room temperature, and sectioned with a cryostat (Leica, Allendale, NJ) at 35 μm. The sections were transferred to gelatin-coated slides and stored at −20°C.
Immunocytochemistry
For fluorescence immunolabeling, cultured cells or retinal sections were washed with PBS three times and incubated in blocking buffer (0.3% Triton X-100, 10% goat serum, 2mM Na3VO4, in 1× TBS) for 30 minutes at 25°C to block nonspecific binding and permeabilize the plasma membrane. Then, the specimens were labeled with polyclonal and/or monoclonal antibodies diluted in blocking buffer at 1:50 overnight at 4°C, followed by three rinses with PBS-T (0.3% Triton X-100 in PBS) and one incubation with blocking buffer for 1 hour at 25°C. Secondary antibodies conjugated with AF 488, AF 594, AF 647 (1:100; Invitrogen), and/or PI (0.1 mg/mL) were applied in the blocking buffer for 1 hour at 25°C in the dark. Cultures were covered with antifade medium (90% glycerol, 10% PBS, and 2.5% [wt/vol] 1,4-diazobicyclo[2,2,2]octane) to prevent bleaching and stored in the dark at 4°C. The retinal sections were covered with Fluoromount-G (SouthernBiotech, Birmingham, AL) and sealed under coverglass with colorless nail protector. Immunolabeling was examined with conventional fluorescence (Axiovert 135; Carl Zeiss, Thornwood, NY) using a 40× objective and/or a confocal microscope (LSM510; Carl Zeiss) equipped with a 40× water immersion objective.
For immunolabeling using the avidin-biotin complex (ABC), cultured cells were pretreated with 0.5% H2O2 in PBS for 10 minutes and washed three times with PBS to inhibit endogenous peroxidase activity before incubation with the blocking buffer, and then labeled with rabbit monoclonal anti-pCREB antibody overnight at 4°C. After three rinses with PBS-T and incubation with blocking buffer for 1 hour at 25°C, the cells were incubated with biotinylated goat-anti-rabbit secondary antibody (1:100) in blocking buffer for 1 hour at 25°C. The anti-pCREB antibody was visualized by using avidin-biotinylated HRP complex developed with stable diaminobenzidene (DAB; Sigma). To make quantitative comparisons possible, untreated/control cultures were processed exactly the same as treated cultures, including length of time, temperature, agitation, and light conditions of DAB development. Then the labeled cells were subject to fluorescence immunolabeling as described above to stain rod cells with mouse monoclonal anti-opsin antibody. The labeled cells were imaged with brightfield and fluorescence microscopy and preserved in antifade medium at 4°C.
Analysis of Cell Growth and Densitometry
The analyses of growth were performed as described.21,22 Briefly, a charge-coupled device (CCD) camera was used to capture images of ABC-labeled photoreceptors under brightfield microscopy, cone cells under phase-contrast microscopy, or rod cells under fluorescence microscopy. The photoreceptors were identified by morphology (cell shape, growth pattern, and presence of an ellipsoid), and rod cell identification was confirmed by rod opsin staining. Only rod cells without outer segments were used in the analysis to reduce variability. However, no attempt was made to distinguish between rod cells that had lost and those that had retained their synaptic terminals after isolation. Rod cells in both conditions produced neuritic sprouts. Neurites of 5 μm or longer, originating from the cell body, were counted as main processes. Varicosities of 0.5 μm or greater in diameter were counted. The length of the longest neurite was also measured with Image Pro 5.0 (Media Cybernetics, Inc., Bethesda, MD). For densitometric analysis, the Quantity One software (Version 4.6; Bio-Rad, Hercules, CA) was used to examine nuclei labeled for pCREB. All data collection on cell growth and immunocytochemistry was done blind or double blind.
Statistical significance of differences was calculated by the unpaired Student's t-test or the Mann-Whitney rank sum test, using SigmaPlot (Version 11; SPSS Inc., Chicago, IL). Significance was considered to be achieved at P < 0.05. The results were plotted in either SigmaPlot or Excel (Office 2003; Microsoft, Redmond, WA), and the data were expressed as mean + SEM.
Western Blot Analysis
Retinas were triturated and lysed for 30 minutes in ice-cold 1× TBS (Tris-buffered saline) with 1% Triton X-100, 1 mM Na3VO4, 1 mM DTT (dithiothreitol), 10 μg/mL leupeptin, 10 μg/mL aprotinin, and 1 mM phenylmethylsulfonyl fluoride. The cell lysate was mixed with 1 volume of 2× Laemmli sample buffer (Sigma) and boiled for 5 minutes, followed by 10 minutes centrifugation at 21,130g (Eppendorf, Westbury, NY) at 25°C to clarify the solution. Proteins were separated on a 10% Tris-HCl polyacrylamide gel at 200 V or NuPAGE Novex 3% to 8% Tris-acetate gel at 150V (Invitrogen) along with molecular weight markers (Full-Range RPN800E; Amersham Biosciences, Piscataway, NJ; and Precision Plus Protein prestained Standards, Bio-Rad). Separated proteins were electrophoretically transferred to a nitrocellulose membrane, and the latter was treated with 5.0% nonfat milk in TBS buffer for 1 hour at room temperature. This was followed by incubating with anti-pCREB (1:1000), anti-CREB (1:1000), anti-opsin (1:300), anti-SV2 (1:300), anti-synaptophysin (1:1000), anti-α-tubulin, or anti-GAPDH (1:6000) in TBS buffer containing 2.5% nonfat milk and 0.05% Tween-20, overnight at 4°C. After extensive washing with PBS-0.05% Tween-20 (vol/vol), incubation was carried out with corresponding goat anti-mouse or goat anti-rabbit IgG linked to HRP at room temperature for 1 hour. Blots were developed on Denville Blue Bio film with ECL reagent (Amersham). For quantification, target protein bands were normalized with the density of GAPDH bands on the same gel and compared with control groups.
Radioimmunoassay (RIA)
For cultured cells, the cell density was determined before the cells were lysed in 215 μL medium containing 2.3% perchloric acid (PCA). For cultured retinas, each retina was frozen in liquid nitrogen, pulverized into powder, and solubilized in 100 μL HEPES (50 mM, pH 8), followed by adding 7.5 μL of 33% PCA. The lysed cell or tissue extracts were collected and stored at −20°C. RIA was conducted using techniques developed for salamander retina as previously described.16 Briefly, 15 μL or 7.5 μL of 10 N KOH was added to each sample for neutralization. Protein concentration was determined using Bradford Dye Reagent according to the manufacturer's instruction (Bio-Rad). cAMP or cGMP in samples and standards was acetylated with a triethylamine and acetic anhydride mixture. Samples were diluted (usually 1:2) for the assay. 125I-labeled cAMP or cGMP (20,000 cpm) and 20 μL of anti-cAMP or anti-cCMP antibody (1:3333) were added to samples, and the standards and complexes were precipitated by centrifugation after overnight incubation at 4°C with polyethylene glycol (PEG)-8000. Radioactivity was determined by a Cobra II Auto-Gamma counter (PerkinElmer, Wellesley, MA). The concentration of cAMP or cGMP was determined by referring to the respective standard curve.
Analysis of Rod Cell Sprouting in Intact Retina
After 7 days culture, retina samples were sectioned, immunolabeled for rod opsin as described above, and examined with confocal microscopy. For quantitation, digitized images were acquired from sections of the entire extent of the retina and manually aligned to create a complete retinal montage using Adobe Photoshop 7.01 (Adobe Systems Inc., San Jose, CA). Rod neurites that penetrated from the outer plexiform layer into the inner nuclear layer for more than 5 μm were regarded as sprouts. Neurites were visible because of opsin immunolabel. Some opsin appears in the plasmalemma of rod cells outside the outer segments even in normal cells.23,24 Thus, to confirm inclusion of all sprouts, even those that may not have been heavily labeled for opsin, selected sections were saturated in the channel used for opsin immunolabeling. The total number of sprouts from one retina section was divided by the total retinal length to yield a normalized number, which was used to compare the effects of different drugs on rod cell sprouting.
Results
Effect of cAMP Analogues on Neuritic Growth by Photoreceptors
cAMP analogues were applied to the salamander retinal culture system.20 In this system, retinal neurons are isolated from adult, aquatic-phase animals, and thus photoreceptors are fully differentiated as they would be in retinal injury and most diseases. Soon after plating, rod and cone cells extend filopodia. Some of these filopodia differentiate over a few days into neurites filled with microtubules and form presynaptic varicosities filled with synaptic vesicles.25 These neuritic processes mimic the appearance of neuritic sprouts seen in the diseased or injured human retina.
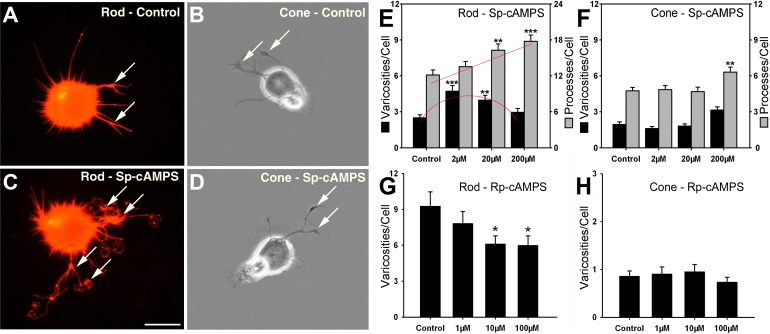
Retinal cultures containing predominately photoreceptors were maintained in serum-free medium with 2, 20, and 200 μM Sp-cAMPS. Sp-cAMPS is a highly membrane-permeable, phosphodiesterase (PDE)-resistant analogue.26,27 After 3 days, rod and cone cells were individually examined for the number of neuritic processes, the length of the longest process, and the number of presynaptic varicosities, as previously described.16 For rod cells, 20 and 200 μM Sp-cAMPS increased the number of processes by 34.0% (P = 0.002) and 46.3% (P < 0.001), respectively. The length of the longest process was also increased significantly at all concentrations; moreover, the effect of Sp-cAMPS on the number and length of processes was highly correlated (R2 = 0.88). Sp-cAMPS (2 and 20 μM) also increased the number of varicosities by 89.6% (P < 0.001) and 59.8% (P = 0.002), respectively (Figs. 1A, 1C, 1E). The increase in the number of processes of rod cells was linear, whereas the increase in the number of varicosities was a bell-shaped curve. Varicosities therefore have a maximal cAMP concentration beyond which their formation becomes inhibited. In contrast, for cone cells, 2 and 20 μM Sp-cAMPS caused almost no change in both the number of processes and varicosities. However, 200 μM Sp-cAMPS caused a significant increase in the number of processes by 33.1% (P = 0.002), but an insignificant increase in the number of varicosities (Figs. 1B, 1D, 1F).
Figure 1. .
cAMP analogues modulate neuritic growth of rod and cone cells after 3 days in vitro. (A) An untreated rod cell labeled for rod opsin; neuritic processes with presynaptic varicosities (arrows) have grown in culture. (B) An untreated cone cell under phase-contrast microscopy; varicosities (arrows) occur on neuritic growth. (C) A rod cell treated with Sp-cAMPS (2 μM) has numerous processes and varicosities (arrows); (D) A cone cell treated with Sp-cAMPS (2 μM) forms neuritic processes and varicosities (arrows) but appears similar to the control cone cell. (E) For rod cells, 2 and 20 μM Sp-cAMPS increased the number of varicosities, whereas 20 and 200 μM Sp-cAMPS increased the number of processes. Red lines indicate shape of the response. (F) Cone cells show significant changes only in the number of processes and only when treated with 200 μM Sp-cAMPS. (G) Ten and one hundred micromolar Rp-cAMPS, an inhibitor of PKA, decreased varicosities of rod cells. (H) Cone cells show no significant difference in varicosities with Rp-cAMPS treatment. (E, F) n = 800 cells; 16 cultures, 2 animals. (G, H) n = 400 cells; 16 cultures, 1 animal. *P < 0.05; **P < 0.01; ***P < 0.001; Scale bar = 20 μm.
The above results indicate that both rod and cone cells respond to the activation of the cAMP signaling pathway. The stimulation of neuritic growth in cone cells, however, required a high concentration of Sp-cAMPS (200 μM), suggesting that the neuritic growth of rod cells is more sensitive than that of cone cells to increased levels of cAMP. We have previously shown that another purine cyclic nucleotide signaling pathway, cGMP-dependent signaling, plays a more important role in stimulation of neuritic growth for cone cells.16 Thus, the cAMP signaling pathway appears to be primarily working in rod photoreceptors.
Elevation of intracellular cAMP activates PKA.28 Therefore, we applied the nonhydrolyzable cAMP analogue Rp-cAMPS to inhibit PKA activity and to test for inhibition of neuritic development. For rod cells, 10 and 100 μM Rp-cAMPS caused decreases in the number of varicosities by 34.2% (P = 0.012) and 35.4% (P = 0.014), respectively (Fig. 1G), compared with the 0.1% DMSO-treated controls. In contrast, for cone cells, treatment of Rp-cAMPS for 3 days at 1, 10, and 100 μM caused no significant change in the number of varicosities (Fig. 1H). The same concentrations of Rp-cAMPS caused no change in retinal cell density, indicating that the effect of Rp-cAMPS on neuritic growth was not a result of cytotoxicity (see Supplementary Material and Supplementary Fig. S1A, http://www.iovs.org/content/53/10/6355/suppl/DC1). Thus, neuritic growth in rod cells, but not in cones, requires PKA activity.
Modulation of Adenylyl Cyclase Affects the Neuritic Growth by Photoreceptors
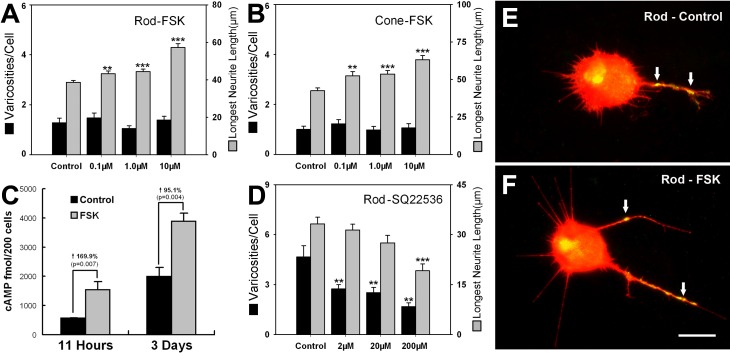
Adenylyl cyclase catalyzes the conversion of adenosine triphosphate (ATP) into cAMP. At least nine closely related AC isoforms have been cloned and characterized.29 Photoreceptors have been reported to contain AC1,30,31 although the presence of other types cannot be ruled out. Diterpene FSK, an alkaloid extracted from the herb Coleus forskohlii, activates almost all forms of the enzyme except AC9 and the newly described testis form.29 Forskolin was applied to retinal cultures for 3 days. Because process number and longest length were highly correlated in experiments with the Sp-cAMPS analogue, we tested only the length of the longest processes in this and subsequent experiments. At 0.1, 1, and 10 μM, FSK significantly increased the longest neuritic length of rod cells by 11.8% (P < 0.01), 14.7% (P < 0.001), and 48.0% (P < 0.001), respectively, and of cone cells by 23.2% (P < 0.01), 26% (P < 0.001), and 48.6% (P < 0.001), respectively. However, FSK at these concentrations failed to significantly affect the varicosity numbers of rod or cone cells (Figs. 2A, 2B, 2E, 2F). Radioimmunologic analysis showed that intracellular cAMP of retinal cells was upregulated by 1 μM FSK after 11 hours and 3 days of treatment; by approximately 100% in the latter case (Fig. 2C). These cAMP concentrations are presumed to be higher than those reached with cAMP analogues and thus perhaps had a nonspecific effect on cone cells. Additionally, although cGMP levels were much lower than cAMP, there were significant increases in cGMP in experiments both with and without IBMX, a phosphodiesterase inhibitor (see Supplementary Material and Supplementary Fig. S1B, http://www.iovs.org/content/53/10/6355/suppl/DC1). Increased cGMP could have stimulated cone cell growth.
Figure 2. .
FSK, an activator of, and SQ22536, an inhibitor of, AC activity, affect photoreceptor growth after 3 days in vitro. (A, B) 0.1, 1, and 10 μM FSK increased the longest neuritic length but not varicosities of rod and cone cells (n = 814 cells; 16 cultures, 2 animals). (C) Radioimmunoassay showed that intracellular cAMP was upregulated by 1 μM FSK after 11 hours and 3 days of treatment (n = 16 cultures, 3 animals). (D) SQ22536 reduced varicosity formation by rod cells at all doses and process number at 200 μM (n = 400 cells; 8 cultures, 1 animal). (E, F) Rod cells immunolabeled for opsin (red) and synaptophysin (green) from a control culture (0.1% DMSO) and a culture treated with 10 μM FSK. Opsin is present throughout the plasma membrane; synaptophysin is present in small varicosities along the neurites (arrows) and in the Golgi apparatus. **P < 0.01; ***P < 0.001; Scale bar = 20 μm.
In contrast to increased growth with FSK, SQ22536, an adenylyl cyclase inhibitor,32 significantly reduced varicosity number in rod cells at all doses and process number at 200 μM (Fig. 2D). SQ22536, however, did not significantly affect either the number of varicosities or processes of cone cells at any dose (see Supplementary Material and Supplementary Fig. S1C, http://www.iovs.org/content/53/10/6355/suppl/DC1).
Thus, these results, like those from the use of analogues, suggest that the modulation of neuritic outgrowth of rod cells is more sensitive to changes in the cAMP signaling pathway than that of cone cells.
Modulation of Adenylyl Cyclase by Activation of the Adenosine or Dopamine Receptor
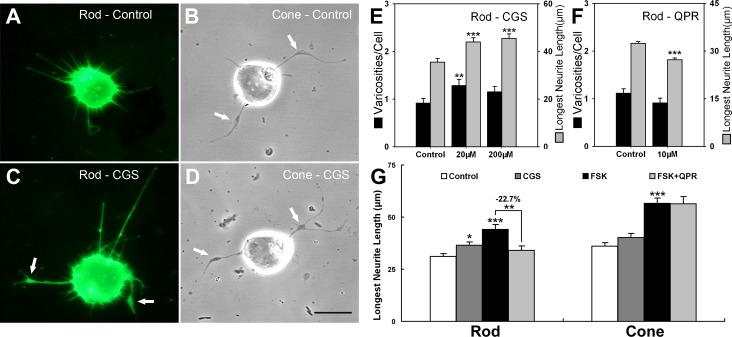
To avoid the possible nonspecific effects of high levels of cAMP resulting from long-term treatment with analogues or FSK, and to stimulate cAMP signaling more physiologically, we tested neurotransmitters known to affect cAMP levels. Histochemical and pharmacologic evidence suggest that the actions of adenosine on photoreceptors are mediated by A2a receptors and linked to stimulation of adenylyl cyclase.33–36 Therefore, an adenosine A2a receptor agonist, CGS-21680 (CGS),37 was applied to retinal cell cultures for 3 days. The agonist significantly increased the length of the longest neurite of rod cells by 23.8% (P < 0.001) and 28.3% (P < 0.001) at 20 and 200 μM, respectively. The varicosity development of rod cells was increased by 40.1% (P = 0.001) at 20 μM (Figs. 3A, 3C, 3E). The change in neuritic length was linear, whereas the varicosities data were suggestive of a bell-shaped curve. For cone cells, CGS showed no clear effects at all doses (Figs. 3B, 3D, 3G).
Figure 3. .
Agonists to neurotransmitter receptors—CGS-21680, an adenosine A2a receptor agonist, and QPR, a D2–4 receptor agonist—which modulate AC activity, affect rod cell growth after 3 days. (A, B) Control rod (opsin-immunolabeled) and cone (phase-contrast) cells. (C, D) Rod and cone cells treated with CGS (20 μM). Arrows indicate varicosities. (E) In rod cells, CGS increased the length of neuritic processes and, at the lower concentration, the number of presynaptic varicosities (see arrows in C), while there was no clear effect on the growth of cone cells (see arrows in B, D). (A–E: n = 472 cells; 12 cultures, 2 animals). (F) Rod cells treated with QPR show reduced neuritic growth compared with the control group (0.1% DMSO) (n = 1018 cells; 24 cultures, 4 animals). (G) Neurotransmitter agonists modulated FSK-stimulated growth of rod but not cone cells. Cells were treated with 0.1% DMSO (control), CGS (20 μM), FSK (10 μM), or FSK + QPR (10 + 10 μM). The longest neuritic length of rod cells treated with FSK + QPR was 22.7% lower than FSK treatment alone and was close to the baseline level; there was no inhibition of growth in cone cells treated with FSK + QPR compared with the group treated with FSK alone. CGS treatment also increased rod but not cone growth but to a lesser extent than FSK (n = 353 cells; 12 cultures, 2 animals). *P < 0.05; **P < 0.01; ***P < 0.001; Scale bar = 20 μm.
It has been shown that activation of D2–4 dopamine receptor decreases cAMP levels in photoreceptors.31,38,39 Retinal cultures were treated with QPR, a dopamine D2/D3/D4 agonist,40 to examine for inhibition of photoreceptor growth. Ten micromolar QPR caused a significant decrease in neuritic length of rod cells by 15.7% (P < 0.001, Fig. 3F) but did not significantly affect the growth of cone cells (the longest neuritic length was decreased by 6.1%, P = 0.29). To further confirm the effect of QPR on rod cell sprouting, 10 μM FSK plus 10 μM QPR were used to treat cultures for 3 days. Neuritic growth of rod cells treated with FSK + QPR was downregulated by 22.7% (P < 0.01) compared with that of FSK treatment alone (Fig. 3G). Cone cells were not affected by either adenosine or dopamine. Thus, modulation of AC by the neurotransmitter receptors for adenosine increased, and for dopamine decreased, aspects of rod cell neuritic development as expected if rod neuritic growth were cAMP-dependent (see Supplementary Material and Supplementary Table S1 for summary, http://www.iovs.org/content/53/10/6355/suppl/DC1).
Forskolin and Transmitter Agonists Modulate CREB Phosphorylation
One of the major effects of the activation of the cAMP signaling pathway is phosphorylation of nuclear transcription factors by PKA. The transcription factor CREB is the best-characterized nuclear protein mediating transcriptional stimulation by cAMP.41 We used either FSK or transmitter agonists to test if modulation of CREB phosphorylation occurred in salamander photoreceptors coincidentally with changes in neuritic growth.
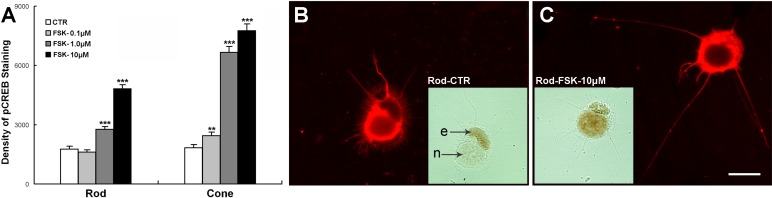
Isolated photoreceptors treated with FSK for 3 days were analyzed for levels of pCREB using densitometry of ABC-HRP immunocytochemistry. FSK increased the density of pCREB immunolabel in both rod and cone cells in a dose-dependent manner (Fig. 4A). One and ten micromolar FSK significantly increased the pCREB level of rod cells by 56.3% and 172.6% (Figs. 4A–C), respectively, and also correspondingly increased the neuritic length of rod cells by 14.7% and 48.0% but failed to significantly affect the varicosity number as described above (Fig. 2A). Similarly, FSK at all doses increased the pCREB level as well as the neuritic length of cone cells (Fig. 2B). The levels of pCREB immunolabeling were lower in rod than in cone cells. This may be due to the faster rise and fall of pCREB in rod than in cone cells (Figs. 5A, 5B) so that at 3 days pCREB levels in rod cells had already begun to subside. Nonetheless, increases in the length of neuritic sprouts by FSK correlate with activation of the pCREB signaling pathway in both rod and cone cells.
Figure 4. .
Forskolin upregulates pCREB in rod and cone cells in 3-day cultures. (A) Rod and cone cells were treated with 0.1% DMSO (control), or 0.1, 1.0, or 10 μM FSK. pCREB immunolabel in the rod cells treated with 1.0 or 10 μM FSK and in all the cone cell groups increased (n = 480 cells; 16 cultures, 1 animal; **P < 0.01; ***P < 0.001). (B) A control rod cell with a few neurites labeled for rod opsin (red) and pCREB (inset), which lightly labeled the nucleus (n). Label in the ellipsoid (e) is nonspecific. (C) A rod cell treated with FSK showing long neurites labeled with rod opsin and dense nuclear pCREB labeling (inset). Scale bar = 20 μm.
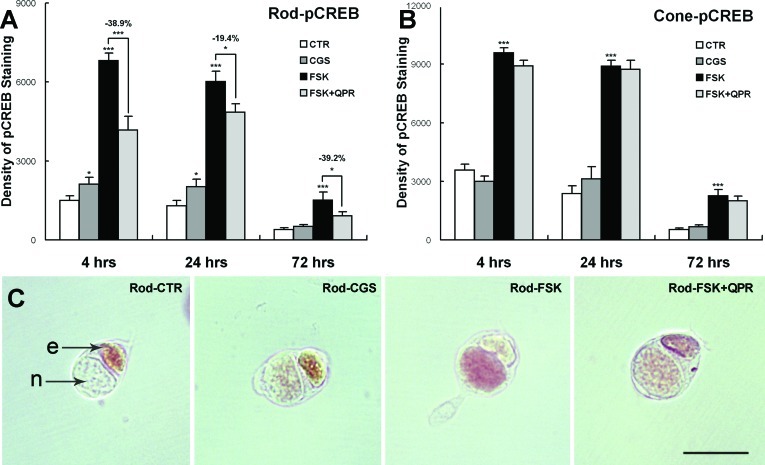
Figure 5. .
Modulation of AC activity through adenosine and dopamine changes pCREB immunolabeling of 4-, 24-, and 72-hour cultures. (A) For rod cells, nuclear pCREB was increased with 20 μM CGS (at 4 and 24 hours) and 10 μM FSK treatments compared with the control group (0.1% DMSO) but was reduced with FSK + 10 μM QPR compared with the FSK-treated group. (B) For cone cells, significant changes in pCREB occurred only with FSK. (A, B) n = 1057 cells; 18 cultures, 2 animals; *P < 0.05; ***P < 0.001). (C) ABC-HRP immunocytochemistry for pCREB. Label is present in nuclei (n) of rod cells after 4 hours in vitro. Ellipsoids show nonspecific labeling (e). Scale bar = 20 μm.
The effects of adenosine A2a receptor agonist CGS (20 μM) and dopamine D2 receptor agonist QPR (10 μM) on pCREB signaling in photoreceptors were also determined (Fig. 5). CGS moderately increased pCREB protein levels in rod cells by 41.7% (P < 0.05) and 56.4% (P < 0.05) at 4 and 24 hours, respectively, while there were no significant changes of pCREB levels in cone cells at any time point. Moreover, rod cells treated with FSK + QPR (10 μM + 10 μM) had lower pCREB levels than those treated with FSK alone at all time points (Fig. 5A). No significant differences in pCREB levels were present in cone cells treated with FSK + QPR compared with FSK alone (Fig. 5B). Thus, cAMP signaling, evidenced by phosphorylation of CREB, could be modulated through the adenosine or dopamine receptor in rod but not in cone cells. Additionally, changes in pCREB occurred relatively quickly, already by 4 hours after FSK or receptor-agonist application.
Activation of Mislocalized Opsin by β-Ionone Promotes Neuritic Growth by Rod Cells
If activation of the cAMP-PKA-pCREB pathway is responsible for rod cell sprouting, what would promote stimulation of this pathway in vivo? Adenosine is present in the retina, but its effects can be countered by dopamine. Thus, although these neurotransmitters may have a role, there are likely to be other factors more central to cAMP-dependent signaling and neuritic growth in the diseased or injured retina. In a previous study on isolated rod photoreceptors, it was determined that activation of opsin, which spreads throughout the plasma membrane of cultured cells, promotes rod cell death by stimulation of adenylyl cyclase, an enzyme not normally accessible as a downstream target (see Supplementary Material and Supplementary Fig. S2, http://www.iovs.org/content/53/10/6355/suppl/DC1).19 So-called “mislocalized opsin,” appearing on the plasma membrane of all parts of the rod cell, is seen not only in isolated cultured cells but in virtually all types of retinal degeneration (see Discussion) and after injury such as detachment and reattachment.10,42 In the course of this previous study, preliminary data indicated that cells that did not die had increased neuritic growth.
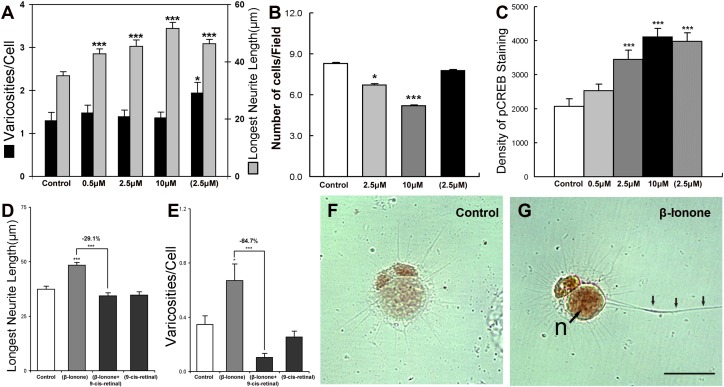
Exogenous application of β-ionone activates rod opsin and its associated G protein, transducin (Gt).43 β-ionone also binds to cone opsin. It works as an inverse agonist, depressing transduction signaling in the red-sensitive cones, 85% of the salamander cone cells,44 but activating the blue- and ultraviolet-sensitive salamander cone cells.45,46 Salamander retinal cells were continuously treated with 0.5, 2.5, and 10 μM β-ionone for 3 days, or treated with 2.5 μM β-ionone for 1 day and then cultured for another 2 days in control medium. For rod cells, treatment with β-ionone at all concentrations significantly increased the length of the longest neurite after 3 days culture. In addition, both the number of presynaptic varicosities and neuritic length of the rod cells treated with 2.5 μM β-ionone for 1 day and cultured for another 2 days were significantly increased by 50.1% (P < 0.05) and 31.6% (P < 0.001), respectively (Fig. 6A). In contrast, the results for cone cells in all β-ionone–treated groups were not significant (see Supplementary Material and Supplementary Fig. S1D, http://www.iovs.org/content/53/10/6355/suppl/DC1). Cell death, previously seen with β-ionone treatment,19 was also examined. Rod cell numbers were reduced by 19.0% (P < 0.05) with 2.5 μM β-ionone, and by 37.2% (P < 0.001) with the treatment of 10 μM β-ionone for 3 days. Cells treated with 2.5 μM β-ionone for only 1 day did not show significant cell death (Fig. 6B).
Figure 6. .
β-ionone, a rod opsin agonist, increases neuritic growth and CREB phosphorylation in rod cells. (A) Rod cells treated with β-ionone show increases of the longest neuritic length in all treatment groups (continuous treatment at noted concentrations for 3 days) and an increase in varicosities with pulse-treatment of 2.5 μM β-ionone (2.5 μM) (24-hour treatment followed by 2 days of control medium) (n = 500 cells; 10 cultures, 1 animal). (B) Cell death was induced by β-ionone. Rod cell number was reduced by 19.0% with 2.5 μM β-ionone, and by 37.2% with 10 μM β-ionone after 3 days. However, cells treated with pulsed-2.5 μM β-ionone did not show any cell death (n = 167 fields; 8 cultures, 1 animal). (C) The density of pCREB immunolabel of rod cells treated with 2.5, 10, or pulsed 2.5 μM β-ionone was higher than that of control group by 66.4%, 98.4%, and 92.0%, respectively (n = 500 cells; 10 cultures, 1 animal). (D, E) Rod cells treated with both β-ionone and 9-cis-retinal for 1 day and then cultured 2 more days in the dark show a decrease in neuritic outgrowth and varicosity formation compared with cultures treated with β-ionone alone. Treatment with 9-cis-retinal in the dark did not change neuritic development compared with the control (n = 734 cells; 20 cultures, 2 animals). (F, G) ABC-HRP immunocytochemistry for pCREB in control and in pulsed-2.5 μM β-ionone–treated rod cells show denser nuclear pCREB labeling and a longer neurite (triple arrows) with β-ionone than in control conditions. *P < 0.05; ***P < 0.001; Scale bar = 20 μm.
The protein level of pCREB was determined in the same cultures by immunolabeling. Rod cells treated with 2.5 or 10 μM β-ionone showed higher density of pCREB staining than the control group by 66.4% (P < 0.001) and 98.4% (P < 0.001), respectively, while cells pulse-treated with 2.5 μM β-ionone showed a dramatic upregulation of pCREB protein level by 92.0% (P < 0.001) (Figs. 6C, 6F, 6G). In cone cells, the 10 μM β-ionone– treated group and 2.5-μM pulse-treated group showed moderate (33% and 36%; P < 0.05) increases of pCREB staining compared with that of the control group treated with 0.1% DMSO. This may be due in part to the activation of opsin in a subpopulation of cones.46 Thus, β-ionone activates the cAMP-PKA pathway as evidenced by pCREB upregulation. However, rod cells are much more responsive than cone cells to the treatment of β-ionone. Indeed, treatment of β-ionone at 2.5 μM for 1 day was enough to almost double pCREB immunolabel density and increase both neuritic length and the development of presynaptic varicosities in rod cells.
Finally, to ensure that the effect of β-ionone was specific for opsin, cultures were kept in the dark and treated with β-ionone in the presence of an excess of 9-cis-retinal, an additional chromophore for opsin, which activates opsin in the presence of light. Treatment with 9-cis-retinal alone in the dark had no effect on neuritic development of rod cells. The stimulatory effect of β-ionone on rod cell outgrowth was significantly inhibited by 9-cis-retinal, reducing the length of the longest neurite by 29.1% (P < 0.001) and the number of varicosities by 84.7% (P < 0.001) (Figs. 6D, 6E). These results indicate that the promotion of neurite growth of rod cells by β-ionone is primarily through the specific activation of opsin.
Rod Cell Sprouting in Cultured Neural Retina
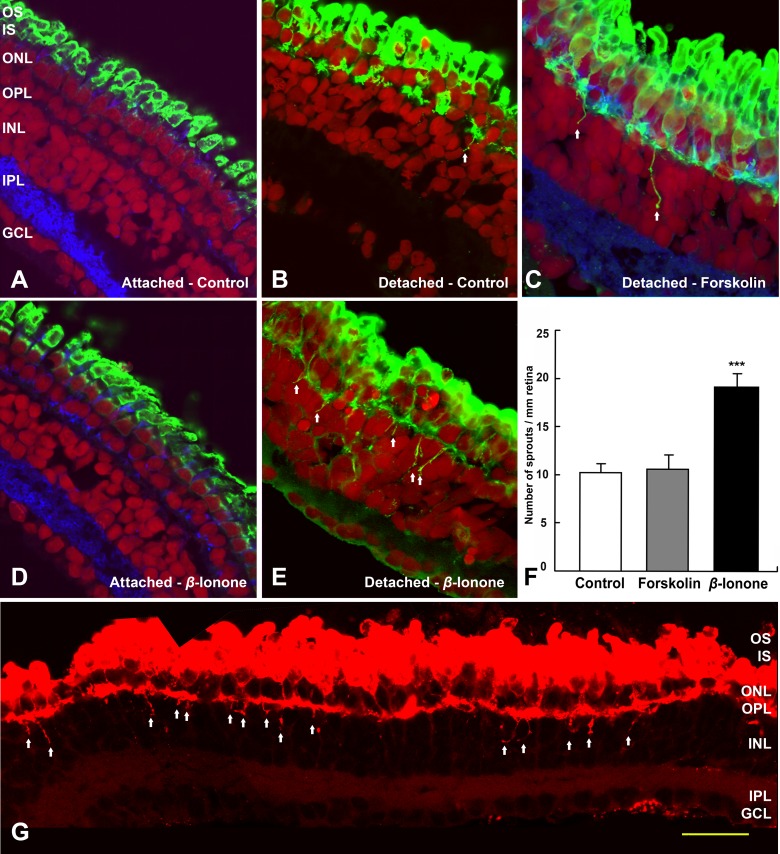
If activating opsin present on the plasma membrane of the cell soma and synaptic terminal of isolated cells increases neuritic sprouting, does opsin activation also increase sprouting in the intact neural retina? It has previously been reported that when neural retina is detached from the underlying retinal pigmented epithelium (RPE), the opsin photopigment spreads into the plasma membranes of the rod cell soma and even up to the synaptic terminal.10 For salamander, neural retina that was isolated from the eyeball and cultured on a piece of filter paper had much more mislocalization of opsin than retina cultured inside the eyeball (Figs. 7A, 7B). This agrees with previous findings. Detached retina with mislocalized opsin showed some sprouting (Fig. 7B). Somewhat surprising, however, was that activating the cAMP pathway in detached retinas with 10 μM FSK did not show a significant increase in sprouting (Fig. 7C). FSK presumably boosted AC activity in all cell types presenting a complex scenario. This complexity may include changes in glia as well as neurons and inhibitory as well as stimulatory signals. Alternatively, FSK may have influenced (i.e., reduced) the amount of mislocalization by preserving retinal structure.47 In contrast, retinas treated with 10 μM β-ionone showed increased sprouting as expected but only if the retina was detached and thus had abundant mislocalized opsin (Fig. 7D attached; Fig. 7E detached). Indeed, detached retinas treated with 10 μM β-ionone had an 87.2% increase in rod neurites penetrating more than 5 μm into the inner nuclear layer per millimeter of retina compared with untreated detached control retinas (Fig. 7F; P < 0.001). Thus, although some sprouting is present when opsin is mislocalized, both opsin mislocalization and exogenous activation must occur for significant sprouting to develop (see Supplementary Material and summary in Supplementary Table S2, http://www.iovs.org/content/53/10/6355/suppl/DC1).
Figure 7. .
Rod cell sprouting in the detached but intact retina is increased by β-ionone after 7 days. (A) Salamander retina cultured in the eyecup show no rod opsin mislocalization and no rod cell sprouting. (B) Neural retina detached from the RPE show rod opsin mislocalization and some neuritic sprouting (arrows). (C) Detached neural retina cultured with FSK show rod opsin mislocalization and a little neuritic sprouting (arrows). (D) Retina cultured in the eyecup and treated with β-ionone show no rod opsin mislocalization and no sprouting. (E) Detached neural retina cultured with β-ionone show both rod opsin mislocalization and robust neuritic spouting (arrows). (A–E) Opsin, green; synaptophysin, blue; nuclei, red; ***P < 0.001). (F) Quantification of rod cell sprouting, from retinal sections immunolabeled for opsin after 7 days culture. The total number of sprouting neurites from a single retinal section was divided by total retinal length to yield a normalized number of sprouts. Treatment of detached retina with 10 μM FSK showed no significant increase in the number of rod cell sprouts compared with untreated, detached retina. In contrast, 10 μM β-ionone dramatically increased, by 87.2%, rod sprouts (n = 5 sections, 2 animals). (G) Detached but intact retina treated with 10 μM β-ionone. Images were reassembled into a complete retinal montage for each section; sprouts longer than 5 μm are indicated by arrows. Scale bar = 50 μm.
Activation of CREB Correlates with Synthesis of Synaptic Proteins
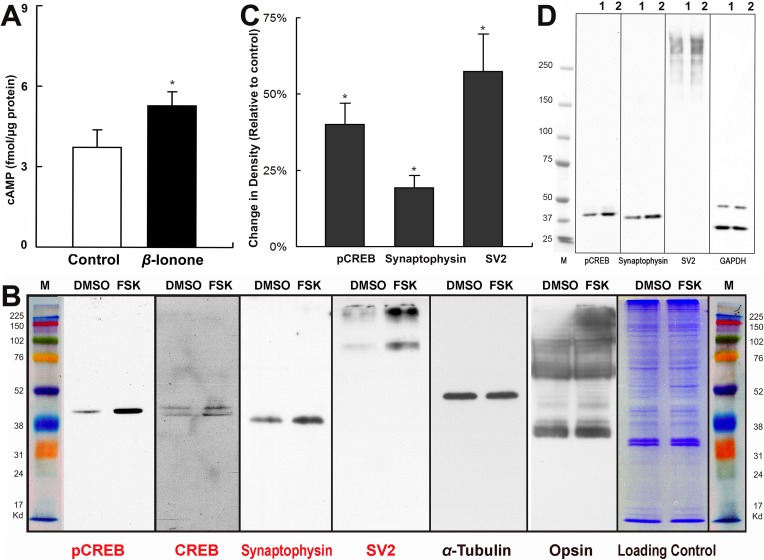
Assuming that activation of opsin in the intact neural retina works through activation of adenylyl cyclase, then cAMP should be elevated in retinas treated with β-ionone. Indeed, neural retinas treated with β-ionone had significantly higher levels of cAMP after 24 hours, as determined by radioimmunoassay (Fig. 8A). The increase in cAMP concentration supports our scheme whereby activated opsin stimulates AC (see Supplementary Material and Supplementary Fig. S2, http://www.iovs.org/content/53/10/6355/suppl/DC1).
Figure 8. .
β-ionone increases expression of pCREB, synaptophysin, and SV2. (A) cAMP was upregulated in radioimmunoassays of intact neural retina by β-ionone after 24 hours (n = 10 retinas, 5 animals; *P < 0.05). (B) In Western blots of salamander retina treated with FSK for 4 hours, pCREB, CREB, synaptophysin, and SV2 were elevated, while tubulin and opsin showed no change (repeated twice). (C, D) Western blots of salamander retina treated with β-ionone for 24 hours. The density of target protein bands were normalized with the density of GAPDH and compared with control. The protein levels of pCREB, synaptophysin, and SV2 were increased in the β-ionone–treated group (lane 1, DMSO; lane 2, β-ionone; n = 5 blots, 6 retinas, 3 animals).
To determine possible cAMP-dependent changes in protein synthesis, two retinas isolated from the same animal were treated with FSK (10 μM) and 0.1% DMSO, respectively, for 4 hours, followed by Western blot analysis of CREB and the pCREB targets, synaptophysin and synaptic vesicle protein 2 (SV2) (see CREB Target Gene Database; provided in the public domain at http://natural.salk.edu/CREB). pCREB, CREB, synaptophysin, and SV2 were all elevated, while α-tubulin and opsin did not change (Fig. 8B, representative of two experiments). Therefore, cAMP elevation resulted in modulation of CREB, which in turn appeared to target specific, synaptic proteins that contained the CRE element in their gene promoter. Similarly, treatment of neural retinas with β-ionone for 24 hours significantly increased the protein levels of pCREB (40.1%), synaptophysin (19.3%), and SV2 (57.4%) in Western blots (Figs. 8C, 8D).
Thus, the effect of β-ionone in the intact retina was identical to results on isolated cells, increased neuritic growth and expression of pCREB, and supports a mechanism where activation of mislocalized opsin stimulates the AC-PKA-pCREB pathway. Phosphorylation of CREB in turn promoted increased expression of proteins involved in neuritic differentiation.
Discussion
Virtually all human retinal degenerations and injuries describe the presence of the rod photopigment, opsin in abnormal plasma membrane locations in rod photoreceptors, often throughout the plasma membranes of the inner segment, soma, and even the synaptic terminal. Concomitantly, rod cell sprouting is ubiquitously described in AMD,8 RP,5–7 and retinal reattachment.10,11 We are proposing that these phenomena are linked through the signaling molecule cAMP by suggesting that opsin, if active when abnormally located on the plasmalemma, will stimulate adenylyl cyclase activity and change gene expression through the transcription factor CREB (see Supplementary Material and Supplementary Fig. S2, http://www.iovs.org/content/53/10/6355/suppl/DC1). Presumably opsin is activated by retinal and light in degenerate retinas, although normal opsin on its own has a low rate of constitutive activity,48 which suggests that sprouting could occur even in retinas maintained in the dark. Moreover, in dark conditions, the presence of extracellular adenosine and increased intracellular calcium could work synergistically with opsin to increase AC activity.
In this report, we have experimentally produced sprouting in the normal retina by activating mislocalized opsin with β-ionone. The specific stimulation of rod opsin was dramatically more effective in producing rod neurites than application of forskolin to the entire retina. We suggest that stimulating ACs throughout the retina with forskolin produced a variety of effects, which either worked to maintain synaptic structure47 or inhibit sprouting. β-ionone in contrast specifically targets photoreceptors. The effect of forskolin on the photoreceptor cell may have been too generalized as well. Compartmentalization of PKA signaling is recognized as a feature of growth-cone activity49 and may also be necessary for synaptic sprouting. β-ionone applied to retina also upregulated the levels of pCREB and synaptic proteins. CREB stimulates a large array of genes but is recognized to be involved in synaptic plasticity.50 In rod cells, β-ionone–induced CREB phosphorylation correlated with increased neuritic growth and presynaptic varicosity formation in isolated cells as well as in the intact neural retina. Moreover, pCREB had increased by 24 hours in the intact retina, prior to sprouting. In addition, previous work has shown that opsin mislocalization can occur very rapidly. Fusion of inner and outer segment membranes, for instance, which allows mislocalization, occurs within minutes after detachment of the neural retina,51 and mislocalized opsin is present in the plasma membranes of rod cells 40 minutes after isolation.25 Thus, mislocalization is the initial event, followed by transcription events, and finally neuritic growth.
Our observations were made possible by use of the adult salamander. Robust sprouting similar to that observed in degenerate or reattached human retina is present in some other animal models as well including Xenopus13 and cat.10 A pig model of retinal degeneration also showed neuritic growth, albeit relatively short sprouts, from rod cells.15 Sprouting in human retina seems to coincide with increased pCREB: human retinas with AMD have increased pCREB immunolabel.52 Injured cat retina53 and, as shown here, salamander retina also have increased pCREB in photoreceptors. In contrast, rodent models of retinal degeneration do not show significant rod cell sprouting5 in spite of the fact that high cAMP has been reported for several mouse models of retinal degeneration54–57 and that dopamine does not reduce cAMP in the rd2 mouse, suggesting unusual AC activity.56 Increased pCREB has been reported for rat photoreceptors after penetrating trauma,58 but decreases in pCREB occur in the rd1 mouse retina.59 One can only speculate at this point why the rodent retinas do not display human pathology. It is known that metabolic rates of mice are very high. Do mice produce so much more cAMP that cells die before there is time to grow neuritic sprouts? Rapid cell death as an explanation for the lack of rod sprouting has been previously proposed.5 Reducing AC activity could test the link between cAMP and sprouting in mice. In the rd1 mice, long sprouts do form from cone cells,12 suggesting that retinal conditions in mice can support sprouting. Thus, not all animals are equally useful for examining retinal synaptic plasticity, at least with regard to rod terminals. The salamander, however, is able to mimic human rod cell reactive change.
Mislocalization of rod opsin is key to the process we describe. This is evidenced by the lack of sprouting in the presence of opsin activation when there was little opsin mislocalization (i.e., when the neural retina was retained next to the RPE). Rod opsin is normally targeted to and retained in the membranes of the photosensitive outer segment through a C-terminal targeting sequence and by the presence of a periciliary barrier mechanism.60,61 Rod opsin is thought to mislocalize to inner segment plasma membranes for primarily three reasons: (1) the targeting machinery for opsin is faulty, and the opsin protein is misdirected to inner segment plasma membrane; (2) the barrier between the base of the cilium and the inner segment is changed, allowing backflow from the outer segment into the inner segment plasma membrane; or (3) there is fusion of the inner and outer segment membranes at regions lateral to the base of the cilium, allowing backflow of opsin from the outer into the inner segment by circumventing the ciliary barrier. In animal models, retinal degenerations associated with mutation of proteins in the targeting processes, including mutations of the opsin C-terminal (type-1 RP), show mislocalized opsin.62 However, it also appears that as outer segments degenerate, even if the targeting mechanisms have not been directly affected (in type-2 RP and in mutations in proteins other than opsin, for instance), opsin will appear in high concentrations in the plasma membrane of the inner segment/cell body.23,24,63 Although some damage to the outer segment seems necessary, inner-segment immunolabel for opsin can occur in the presence of intact cilia,64 suggesting that obvious breakdown in the ciliary transport and barrier mechanisms may not be involved. Moreover, in the presence of degeneration, rod opsin continues to be synthesized, at least for awhile, providing more protein that can mislocalize.65–67 Finally, mechanical injury, such as detachment, can promote fusion of the inner and outer segments to allow backflow of opsin from outer to inner segment membranes,46,51 and mislocalization occurs.42 Similar fusion has been seen in genetic retinal disease with accompanying opsin mislocalization.68 Thus, in virtually all situations in which rod cell outer segments become compromised, regardless of the specific mutation or injury, abnormal amounts of opsin appear on inner segment, cell body, and/or synaptic terminal plasma membrane. We are proposing that this mislocalized opsin leads to sprouting. However, there is one situation in which mislocalized opsin does not immediately produce notable sprouting (excluding the lack of sprouting in rodent models of retinal degeneration): although detachment causes mislocalization of opsin, sprouting is not observed in the detached retina.10 It is only when the retina is reattached that sprouting occurs, although only in retinal areas that display mislocalized opsin.69 A possible explanation is that when the retina is detached, rod cell metabolism slows,70 and there is a downregulation of the visual cycle genes, presumably reducing the production of the chromophore, 11-cis-retinal.71 Moreover, any retinal produced by the RPE may not reach the rod cells, so there is little opsin activation. Once there is reattachment, retinal has better access to opsin in the inner segment, cell body, and synaptic terminal, and opsin bound to retinal can now be activated by light. In our case, mislocalized opsin produced by detachment was experimentally activated by infusion of the opsin agonist, β-ionone. Without the opsin agonist, only a small amount of sprouting occurred in detached retina.
We have shown that the mechanisms for rod and cone cell sprouting are distinct. Rod cells depended on increases in cAMP, with cone cells responding very little to increased cAMP. Cone cells depend on increases in cGMP.16 Thus, cone cell sprouting in rd1 mice, which contain a mutation in the cGMP-specific phosphodiesterase, is presumably a result of increased cGMP (see Discussion in Ref. 16). In contrast, rod cell neuritic growth is inhibited by cGMP.16 Some increases in growth and pCREB immunolabel were seen in cone cells with forskolin, but the cAMP levels in this case were high, increasing the possibility of some cross-talk between cAMP and cGMP pathways.72 In addition, forskolin produced some increase in cGMP, which could directly promote cone cell growth. No cone responses were detected with the use of adenosine agonists, which arguably produce lower and more physiologic levels of cAMP, or with PKA inhibition. The low sensitivity of cone cells to cAMP, compared with that of rod cells, has also been documented for teleost photoreceptor elongation and contraction in light and dark.73 In summary, the separation of rod and cone sprouting into cAMP- and cGMP-dependent mechanisms is supported by our present and previous results. Furthermore, mislocalization of cone opsin would not be expected to stimulate soluble guanylyl cyclase, the cGMP-producing enzyme, because it is not a G-protein–coupled enzyme. The presence of more than one signaling pathway for sprouting of these sensory neurons supports the reports of contradictory results regarding the effects of cAMP on mature neurons in vitro.74 And, it is illustrative of a general condition in the nervous system—that the potential for, and presumably mechanism of, regenerative growth is cell-type specific. This is true not only in mammals75 but also in amphibians, in spite of their reputation as prodigious regenerators, where certain nerve cell types grow after injury and others do not.76
For rod cells, our results suggest that the levels of intracellular cAMP are critical. For length of the longest process, growth was dose dependent, increasing with increasing stimulation of AC whether by forskolin or neurotransmitter-receptor agonist. But, for the rod cell presynaptic varicosities, the relationship was more complex, suggesting a bell-shaped curve, with optimal varicosity development at a specific concentration, and reduction in development with increased dose. This is similar to the effects of cGMP on presynaptic varicosity development on cone cell processes in the salamander.16 Concentration dependency for development of new varicosities may presage rod cell death. Indeed, in our cultures, β-ionone stimulation of sprouting also produced significant cell death. As cAMP levels increase, a lethal concentration appears to be reached. Thus, we suggest that, like calcium,77 cAMP is both a survival/growth promoter and a death mediator so that whether a rod cell sprouts or dies is related to the level of intracellular cAMP. Cyclic AMP levels in turn may be related to levels of mislocalized opsin, as rod cell death is related to levels of mislocalized opsin.78
The nature of the link between mislocalized rod opsin and AC stimulation may be multifaceted. In tests on stimulation of mislocalized opsin as a cause of cell death in isolated salamander rod cells,19 we found that pertussis toxin reduced cell death, indicating that a Gi protein, like transducin, was involved. Moreover light-stimulation of rhodopsin in cell-free systems can enhance AC activity in a transducin-dependent manner.79,80 Chinese hamster ovary cells expressing bovine rhodopsin showed inhibition of AC in the presence of light; however, the AC isotype in question appeared to be Type VI,81 not Type I, the primary isotype in photoreceptors.30,31 Since our publication, there have been mixed reports in cold-blooded vertebrate models, testing our idea that G-protein activation by mislocalized opsin is involved in rod cell death. In Xenopus, our model was examined by creating a transgenic animal that expressed normal rod opsin as well as opsin with a double mutation at Q350ter (equivalent to Q344ter in mammals, to cause mislocalization) and K296R (to prevent 11-cis–retinal binding and therefore transducin activation).13 Because the double mutation did not appear to reduce degeneration, the authors concluded that activation of transducin by mislocalized opsin was not a factor in rod cell death. Their conclusion seems plausible if mutated protein was the only type of opsin on the membranes of the inner segment/synaptic terminal. Immunocytochemical analysis of normal opsin localization was done at only one time point and without quantitative controls for immunolabel density. Wild-type opsin is transported to the plasmalemma of inner segments in transgenic mice with Q344ter or S334ter mutated rhodopsin.62,82 Moreover, from other animal studies (see above), elevated amounts of normal opsin in the inner segment would be expected as outer segments degenerate. Mislocalized normal opsin could have interacted with transducin and contributed, in parallel with other causes like overexpression of opsin,83 to cell death in the Xenopus model. In ovl zebra fish with a retinal degeneration caused by a mutant ciliary gene, which therefore displays opsin mislocalization, both transducin and AC activity were shown to contribute to rod cell death84 in a pathway identical to our initial proposed pathway.19 In murine retina, Nakao et al.84 also demonstrated that rod cell death in rd10 mice was AC dependent. In another mouse study, a Q344ter-induced retinal degeneration was created on a rho −/− background to minimize retinal degeneration by rhodopsin overexpression.85 In these retinas, opsin is mutated at the C-terminal and thus missorts to the inner segment but still interacts with transducin. Degeneration was exacerbated by light. Additionally, the authors demonstrated that mislocalized opsin was capable of light activation in vivo and that cell death was reduced in animals bred on a Trα −/− background, indicating dependence on transducin signaling, supporting our model. In our study,19 and in this latter study,85 there was the recognition that other G proteins could be involved. For us, for instance, pertussis toxin did not completely prevent rod cell death. Other opsin family members in retinal ganglion cells and invertebrate photoreceptors interact with a Gq protein to cause an increase in calcium through activation of phospholipase C.86,87 Calcium/calmodulin in turn can stimulate photoreceptor AC type 1. Rod cells in fact contain a homolog of Gαq called Gα11.88 Additionally, it is likely that cAMP mechanisms are not the only pathways contributing to rod cell regenerative growth. In retinal ganglion cells, for example, increased cAMP combined with controlled inflammation and decreased PTEN activity determine the length of new regenerating processes and work through separate signaling cascades.89
Cyclic AMP has been recognized as a critical signaling molecule for nerve process regeneration and synaptic plasticity in the adult.17,90 We have tested the premise that cAMP must be involved in reactive sprouting as well. However, our previous data16 and this report clearly demonstrate that not all neurons use cAMP-dependent pathways to sprout. Moreover, the way that cAMP is increased in a neuron is likely to be cell-type specific, allowing cell-specific activation of cAMP-dependent pathways. In the retina, for instance, β-ionone could be used to specifically stimulate sprouting of rod photoreceptors after transplantation when the immature precursors, as well as mature, isolated rod cells (depending on what cells are used for transplantation), have mislocalized opsin.19,91 Finally, our results emphasize the importance of nerve cell polarity and compartmentalization by describing how a mislocalized membrane protein is linked to reactive cell behaviors.
Supplementary Material
Acknowledgments
The helpful comments on the manuscript by Peter Alfinito are gratefully acknowledged.
Footnotes
Supported by National Institutes of Health Grants EY012031 (ET-A) and GM007640 (AB), and the F. M. Kirby Foundation (ET-A and AB).
Disclosure: J. Wang, None; N. Zhang, None; A. Beuve, None; E. Townes-Anderson, None
References
- 1.Kerschensteiner M, Bareyre FM, Buddeberg BS, et al. Remodeling of axonal connections contributes to recovery in an animal model of multiple sclerosis. J Exp Med. 2004;200:1027–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masliah E, Hansen L, Mallory M, Albright T, Terry RD. Abnormal brain spectrin immunoreactivity in sprouting neurons in Alzheimer disease. Neurosci Lett. 1991;129:1–5 [DOI] [PubMed] [Google Scholar]
- 3.Geddes JW, Anderson KJ, Cotman CW. Senile plaques as aberrant sprout-stimulating structures. Exp Neurol. 1986;94:767–776 [DOI] [PubMed] [Google Scholar]
- 4.Dahl D, Perides G, Bignami A. Axonal regeneration in old multiple sclerosis plaques: immunohistochemical study with monoclonal antibodies to phosphorylated and non-phosphorylated neurofilament proteins. Acta Neuropathol. 1989;79:154–159 [DOI] [PubMed] [Google Scholar]
- 5.Li ZY, Kljavin IJ, Milam AH. Rod photoreceptor neurite sprouting in retinitis pigmentosa. J Neurosci. 1995;15:5429–5438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.John SK, Smith JE, Aguirre GD, Milam AH. Loss of cone molecular markers in rhodopsin-mutant human retinas with retinitis pigmentosa. Mol Vis. 2000;6:204–215 [PubMed] [Google Scholar]
- 7.Fariss RN, Li ZY, Milam AH. Abnormalities in rod photoreceptors, amacrine cells, and horizontal cells in human retinas with retinitis pigmentosa. Am J Ophthalmol. 2000;129:215–223 [DOI] [PubMed] [Google Scholar]
- 8.Gupta N, Brown KE, Milam AH. Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Exp Eye Res. 2003;76:463–471 [DOI] [PubMed] [Google Scholar]
- 9.Xiao M, Sastry SM, Li ZY, et al. Effects of retinal laser photocoagulation on photoreceptor basic fibroblast growth factor and survival. Invest Ophthalmol Vis Sci. 1998;39:618–630 [PubMed] [Google Scholar]
- 10.Lewis GP, Charteris DG, Sethi CS, Leitner WP, Linberg KA, Fisher SK. The ability of rapid retinal reattachment to stop or reverse the cellular and molecular events initiated by detachment. Invest Ophthalmol Vis Sci. 2002;43:2412–2420 [PubMed] [Google Scholar]
- 11.Sethi CS, Lewis GP, Fisher SK, et al. Glial remodeling and neural plasticity in human retinal detachment with proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 2005;46:329–342 [DOI] [PubMed] [Google Scholar]
- 12.Fei Y. Cone neurite sprouting: an early onset abnormality of the cone photoreceptors in the retinal degeneration mouse. Mol Vis. 2002;8:306–314 [PubMed] [Google Scholar]
- 13.Tam BM, Xie G, Oprian DD, Moritz OL. Mislocalized rhodopsin does not require activation to cause retinal degeneration and neurite outgrowth in Xenopus laevis. J Neurosci. 2006;26:203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chong NH, Alexander RA, Barnett KC, Bird AC, Luthert PJ. An immunohistochemical study of an autosomal dominant feline rod/cone dysplasia (Rdy cats). Exp Eye Res. 1999;68:51–57 [DOI] [PubMed] [Google Scholar]
- 15.Li ZY, Wong F, Chang JH, et al. Rhodopsin transgenic pigs as a model for human retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1998;39:808–819 [PubMed] [Google Scholar]
- 16.Zhang N, Beuve A, Townes-Anderson E. The nitric oxide-cGMP signaling pathway differentially regulates presynaptic structural plasticity in cone and rod cells. J Neurosci. 2005;25:2761–2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hannila SS, Filbin MT. The role of cyclic AMP signaling in promoting axonal regeneration after spinal cord injury. Exp Neurol. 2008;209:321–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038 [DOI] [PubMed] [Google Scholar]
- 19.Alfinito PD, Townes-Anderson E. Activation of mislocalized opsin kills rod cells: a novel mechanism for rod cell death in retinal disease. Proc Natl Acad Sci U S A. 2002;99:5655–5660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacLeish PR, Townes-Anderson E. Growth and synapse formation among major classes of adult salamander retinal neurons in vitro. Neuron. 1988;1:751–760 [DOI] [PubMed] [Google Scholar]
- 21.Fontainhas AM, Townes-Anderson E. RhoA and its role in synaptic structural plasticity of isolated salamander photoreceptors. Invest Ophthalmol Vis Sci. 2008;49:4177–4187 [DOI] [PubMed] [Google Scholar]
- 22.Zhang N, Townes-Anderson E. Regulation of structural plasticity by different channel types in rod and cone photoreceptors. J Neurosci. 2002;22:7065–7079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nir I, Papermaster DS. Immunocytochemical localization of opsin in degenerating photoreceptors of RCS rats and rd and rds mice. Prog Clin Biol Res. 1989;314:251–264 [PubMed] [Google Scholar]
- 24.Roof DJ, Adamian M, Hayes A. Rhodopsin accumulation at abnormal sites in retinas of mice with a human P23H rhodopsin transgene. Invest Ophthalmol Vis Sci. 1994;35:4049–4062 [PubMed] [Google Scholar]
- 25.Mandell JW, MacLeish PR, Townes-Anderson E. Process outgrowth and synaptic varicosity formation by adult photoreceptors in vitro. J Neurosci. 1993;13:3533–3548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothermel JD, Parker Botelho LH. A mechanistic and kinetic analysis of the interactions of the diastereoisomers of adenosine 3′, 5′-(cyclic)phosphorothioate with purified cyclic AMP-dependent protein kinase. Biochem J. 1988;251:757–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dostmann WR, Taylor SS, Genieser HG, Jastorff B, Doskeland SO, Ogreid D. Probing the cyclic nucleotide binding sites of cAMP-dependent protein kinases I and II with analogs of adenosine 3′, 5′-cyclic phosphorothioates. J Biol Chem. 1990;265:10484–10491 [PubMed] [Google Scholar]
- 28.Taylor SS, Kim C, Vigil D, et al. Dynamics of signaling by PKA. Biochim Biophys Acta. 2005;1754:25–37 [DOI] [PubMed] [Google Scholar]
- 29.Hanoune J, Defer N. Regulation and role of adenylyl cyclase isoforms. Annu Rev Pharmacol Toxicol. 2001;41:145–174 [DOI] [PubMed] [Google Scholar]
- 30.Xia Z, Choi EJ, Wang F, Blazynski C, Storm DR. Type I calmodulin-sensitive adenylyl cyclase is neural specific. J Neurochem. 1993;60:305–311 [DOI] [PubMed] [Google Scholar]
- 31.Jackson CR, Chaurasia SS, Zhou H, Haque R, Storm DR, Iuvone PM. Essential roles of dopamine D4 receptors and the type 1 adenylyl cyclase in photic control of cyclic AMP in photoreceptor cells. J Neurochem. 2009;109:148–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lippe C, Ardizzone C. Actions of vasopressin and isoprenaline on the ionic transport across the isolated frog skin in the presence and the absence of adenyl cyclase inhibitors MDL12330A and SQ22536. Comp Biochem Physiol C. 1991;99:209–211 [DOI] [PubMed] [Google Scholar]
- 33.Kvanta A, Seregard S, Sejersen S, Kull B, Fredholm BB. Localization of adenosine receptor messenger RNAs in the rat eye. Exp Eye Res. 1997;65:595–602 [DOI] [PubMed] [Google Scholar]
- 34.Rey HL, Burnside B. Adenosine stimulates cone photoreceptor myoid elongation via an adenosine A2-like receptor. J Neurochem. 1999;72:2345–2355 [DOI] [PubMed] [Google Scholar]
- 35.Alfinito PD, Alli R, Townes-Anderson E. Adenosine A(2a) receptor-mediated inhibition of rod opsin mRNA expression in tiger salamander. J Neurochem. 2002;83:665–672 [DOI] [PubMed] [Google Scholar]
- 36.Iuvone PM, Valenciano AI, Alonso-Gomez AI. Adenosine: a circadian modulator of melatonin biosynthesis in Xenopus photoreceptor cells. Invest Ophthalmol Vis Sci. 2000;41:S112–S112 [Google Scholar]
- 37.Jarvis MF, Schulz R, Hutchison AJ, Do UH, Sills MA, Williams M. [3H]CGS 21680, a selective A2 adenosine receptor agonist directly labels A2 receptors in rat brain. J Pharmacol Exp Ther. 1989;251:888–893 [PubMed] [Google Scholar]
- 38.Cohen AI, Blazynski C. Dopamine and its agonists reduce a light-sensitive pool of cyclic AMP in mouse photoreceptors. Vis Neurosci. 1990;4:43–52 [DOI] [PubMed] [Google Scholar]
- 39.Iuvone PM, Ivanova TN, Alonso-Gomez AL. Dopamine D4 receptors regulate intracellular calcium concentration in cultured chicken cone photoreceptor cells: relationship to dopamine receptor-mediated inhibition of cAMP formation. Brain Res. 2008;1207:111–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel S, Chapman KL, Marston D, Hutson PH, Ragan CI. Pharmacological and functional characterisation of dopamine D4 receptors in the rat retina. Neuropharmacology. 2003;44:1038–1046 [DOI] [PubMed] [Google Scholar]
- 41.Rosenberg D, Groussin L, Jullian E, Perlemoine K, Bertagna X, Bertherat J. Role of the PKA-regulated transcription factor CREB in development and tumorigenesis of endocrine tissues. Ann N Y Acad Sci. 2002;968:65–74 [DOI] [PubMed] [Google Scholar]
- 42.Lewis GP, Erickson PA, Anderson DH, Fisher SK. Opsin distribution and protein incorporation in photoreceptors after experimental retinal detachment. Exp Eye Res. 1991;53:629–640 [DOI] [PubMed] [Google Scholar]
- 43.Kefalov VJ, Carter Cornwall M, Crouch RK. Occupancy of the chromophore binding site of opsin activates visual transduction in rod photoreceptors. J Gen Physiol. 1999;113:491–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sherry DM, Bui DD, Degrip WJ. Identification and distribution of photoreceptor subtypes in the neotenic tiger salamander retina. Vis Neurosci. 1998;15:1175–1187 [DOI] [PubMed] [Google Scholar]
- 45.Jin J, Crouch RK, Corson DW, Katz BM, MacNichol EF, Cornwall MC. Noncovalent occupancy of the retinal-binding pocket of opsin diminishes bleaching adaptation of retinal cones. Neuron. 1993;11:513–522 [DOI] [PubMed] [Google Scholar]
- 46.Spencer M, Detwiler PB, Bunt-Milam AH. Distribution of membrane proteins in mechanically dissociated retinal rods. Invest Ophthalmol Vis Sci. 1988;29:1012–1020 [PubMed] [Google Scholar]
- 47.Khodair MA, Zarbin MA, Townes-Anderson E. Cyclic AMP prevents retraction of axon terminals in photoreceptors prepared for transplantation: an in vitro study. Invest Ophthalmol Vis Sci. 2005;46:967–973 [DOI] [PubMed] [Google Scholar]
- 48.Cornwall MC, Fain GL. Bleached pigment activates transduction in isolated rods of the salamander retina. J Physiol. 1994;480(pt 2):261–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han J, Han L, Tiwari P, Wen Z, Zheng JQ. Spatial targeting of type II protein kinase A to filopodia mediates the regulation of growth cone guidance by cAMP. J Cell Biol. 2007;176:101–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Josselyn SA, Nguyen PV. CREB synapses and memory disorders: past progress and future challenges. Curr Drug Targets CNS Neurol Disord. 2005;4:481–497 [DOI] [PubMed] [Google Scholar]
- 51.Townes-Anderson E. Intersegmental fusion in vertebrate rod photoreceptors: rod cell structure revisited. Invest Ophthalmol Vis Sci. 1995;36:1918–1933 [PubMed] [Google Scholar]
- 52.Beltran WA, Allore HG, Johnson E, et al. CREB1/ATF1 activation in photoreceptor degeneration and protection. Invest Ophthalmol Vis Sci. 2009;50:5355–5363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geller SF, Lewis GP, Fisher SK. FGFR1, signaling, and AP-1 expression after retinal detachment: reactive Muller and RPE cells. Invest Ophthalmol Vis Sci. 2001;42:1363–1369 [PubMed] [Google Scholar]
- 54.Lolley RN, Schmidt SY, Farber DB. Alterations in cyclic AMP metabolism associated with photoreceptor cell degeneration in the C3H mouse. J Neurochem. 1974;22:701–707 [DOI] [PubMed] [Google Scholar]
- 55.Weiss ER, Hao Y, Dickerson CD, et al. Altered cAMP levels in retinas from transgenic mice expressing a rhodopsin mutant. Biochem Biophys Res Commun. 1995;216:755–761 [DOI] [PubMed] [Google Scholar]
- 56.Nir I, Haque R, Iuvone PM. Regulation of cAMP by light and dopamine receptors is dysfunctional in photoreceptors of dystrophic retinal degeneration slow(rds) mice. Exp Eye Res. 2001;73:265–272 [DOI] [PubMed] [Google Scholar]
- 57.Traverso V, Bush RA, Sieving PA, Deretic D. Retinal cAMP levels during the progression of retinal degeneration in rhodopsin P23H and S334ter transgenic rats. Invest Ophthalmol Vis Sci. 2002;43:1655–1661 [PubMed] [Google Scholar]
- 58.Harada T, Imaki J, Hagiwara M, et al. Phosphorylation of CREB in rat retinal cells after focal retinal injury. Exp Eye Res. 1995;61:769–772 [DOI] [PubMed] [Google Scholar]
- 59.Paquet-Durand F, Azadi S, Hauck SM, Ueffing M, van Veen T, Ekstrom P. Calpain is activated in degenerating photoreceptors in the rd1 mouse. J Neurochem. 2006;96:802–814 [DOI] [PubMed] [Google Scholar]
- 60.Deretic D. A role for rhodopsin in a signal transduction cascade that regulates membrane trafficking and photoreceptor polarity. Vision Res. 2006;46:4427–4433 [DOI] [PubMed] [Google Scholar]
- 61.Nachury MV, Seeley ES, Jin H. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu Rev Cell Dev Biol. 2010;26:59–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sung CH, Makino C, Baylor D, Nathans J. A rhodopsin gene mutation responsible for autosomal dominant retinitis pigmentosa results in a protein that is defective in localization to the photoreceptor outer segment. J Neurosci. 1994;14:5818–5833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ishiguro S, Fukuda K, Kanno C, Mizuno K. Accumulation of immunoreactive opsin on plasma membranes in degenerating rod cells of rd/rd mutant mice. Cell Struct Funct. 1987;12:141–155 [DOI] [PubMed] [Google Scholar]
- 64.Nir I, Sagie G, Papermaster DS. Opsin accumulation in photoreceptor inner segment plasma membranes of dystrophic RCS rats. Invest Ophthalmol Vis Sci. 1987;28:62–69 [PubMed] [Google Scholar]
- 65.Nir I, Agarwal N, Sagie G, Papermaster DS. Opsin distribution and synthesis in degenerating photoreceptors of rd mutant mice. Exp Eye Res. 1989;49:403–421 [DOI] [PubMed] [Google Scholar]
- 66.Agarwal N, Nir I, Papermaster DS. Opsin synthesis and mRNA levels in dystrophic retinas devoid of outer segments in retinal degeneration slow (rds) mice. J Neurosci. 1990;10:3275–3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schalken JJ, Janssen JJ, Sanyal S, Hawkins RK, de Grip WJ. Development and degeneration of retina in rds mutant mice: immunoassay of the rod visual pigment rhodopsin. Biochim Biophys Acta. 1990;1033:103–109 [DOI] [PubMed] [Google Scholar]
- 68.Marc RE, Murry RF, Fisher SK, Linberg KA, Lewis GP. Amino acid signatures in the detached cat retina. Invest Ophthalmol Vis Sci. 1998;39:1694–1702 [PubMed] [Google Scholar]
- 69.Lewis GP, Sethi CS, Linberg KA, Charteris DG, Fisher SK. Experimental retinal reattachment: a new perspective. Mol Neurobiol. 2003;28:159–175 [DOI] [PubMed] [Google Scholar]
- 70.Mervin K, Valter K, Maslim J, Lewis G, Fisher S, Stone J. Limiting photoreceptor death and deconstruction during experimental retinal detachment: the value of oxygen supplementation. Am J Ophthalmol. 1999;128:155–164 [DOI] [PubMed] [Google Scholar]
- 71.Rattner A, Toulabi L, Williams J, Yu H, Nathans J. The genomic response of the retinal pigment epithelium to light damage and retinal detachment. J Neurosci. 2008;28:9880–9889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sunahara RK, Beuve A, Tesmer JJ, Sprang SR, Garbers DL, Gilman AG. Exchange of substrate and inhibitor specificities between adenylyl and guanylyl cyclases. J Biol Chem. 1998;273:16332–16338 [DOI] [PubMed] [Google Scholar]
- 73.Burnside B, Basinger S. Retinomotor pigment migration in the teleost retinal pigment epithelium. II. Cyclic-3′, 5′-adenosine monophosphate induction of dark-adaptive movement in vitro. Invest Ophthalmol Vis Sci. 1983;24:16–23 [PubMed] [Google Scholar]
- 74.Mattson MP, Taylor-Hunter A, Kater SB. Neurite outgrowth in individual neurons of a neuronal population is differentially regulated by calcium and cyclic AMP. J Neurosci. 1988;8:1704–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Benfey M, Bunger UR, Vidal-Sanz M, Bray GM, Aguayo AJ. Axonal regeneration from GABAergic neurons in the adult rat thalamus. J Neurocytol. 1985;14:279–296 [DOI] [PubMed] [Google Scholar]
- 76.Lyon MJ, Stelzner DJ. Tests of the regenerative capacity of tectal efferent axons in the frog, Rana pipiens. J Comp Neurol. 1987;255:511–525 [DOI] [PubMed] [Google Scholar]
- 77.Kater SB, Mills LR. Regulation of growth cone behavior by calcium. J Neurosci. 1991;11:891–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lopes VS, Jimeno D, Khanobdee K, et al. Dysfunction of heterotrimeric kinesin-2 in rod photoreceptor cells and the role of opsin mislocalization in rapid cell death. Mol Biol Cell. 2010;21:4076–4088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bitensky MW, Wheeler MA, Rasenick MM, et al. Functional exchange of components between light-activated photoreceptor phosphodiesterase and hormone-activated adenylate cyclase systems. Proc Natl Acad Sci U S A. 1982;79:3408–3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Muschietti JP, Bianchini GM, Martinetto HE, et al. Reconstitution of a light-stimulated adenylate cyclase from retina and Neurospora crassa preparations: characterization of the heterologous systems using normal and degenerative retinas. Eur J Biochem. 1989;185:205–210 [DOI] [PubMed] [Google Scholar]
- 81.Dickerson CD, Weiss ER. The coupling of pertussis toxin-sensitive G proteins to phospholipase A2 and adenylyl cyclase in CHO cells expressing bovine rhodopsin. Exp Cell Res. 1995;216:46–50 [DOI] [PubMed] [Google Scholar]
- 82.Concepcion F, Mendez A, Chen J. The carboxyl-terminal domain is essential for rhodopsin transport in rod photoreceptors. Vision Res. 2002;42:417–426 [DOI] [PubMed] [Google Scholar]
- 83.Tan E, Wang Q, Quiambao AB, et al. The relationship between opsin overexpression and photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2001;42:589–600 [PubMed] [Google Scholar]
- 84.Nakao T, Tsujikawa M, Notomi S, Ikeda Y, Nishida K. The role of mislocalized phototransduction in photoreceptor cell death of retinitis pigmentosa. PLoS One. 2012;7:e32472 Available at: http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0032472. Accessed July 19, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Concepcion F, Chen J. Q344ter mutation causes mislocalization of rhodopsin molecules that are catalytically active: a mouse model of Q344ter-induced retinal degeneration. PLoS One. 2010;5:e10904 Available at: http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0010904. Accessed July 19, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Terakita A, Tsukamoto H, Koyanagi M, Sugahara M, Yamashita T, Shichida Y. Expression and comparative characterization of Gq-coupled invertebrate visual pigments and melanopsin. J Neurochem. 2008;105:883–890 [DOI] [PubMed] [Google Scholar]
- 87.Yau KW, Hardie RC. Phototransduction motifs and variations. Cell. 2009;139:246–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peng YW, Rhee SG, Yu WP, et al. Identification of components of a phosphoinositide signaling pathway in retinal rod outer segments. Proc Natl Acad Sci U S A. 1997;94:1995–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kurimoto T, Yin Y, Omura K, et al. Long-distance axon regeneration in the mature optic nerve: contributions of oncomodulin, cAMP, and pten gene deletion. J Neurosci. 2010;30:15654–15663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kandel ER. The molecular biology of memory storage: a dialog between genes and synapses. Biosci Rep. 2001;21:565–611 [DOI] [PubMed] [Google Scholar]
- 91.Nir I, Cohen D, Papermaster DS. Immunocytochemical localization of opsin in the cell membrane of developing rat retinal photoreceptors. J Cell Biol. 1984;98:1788–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.