Abstract
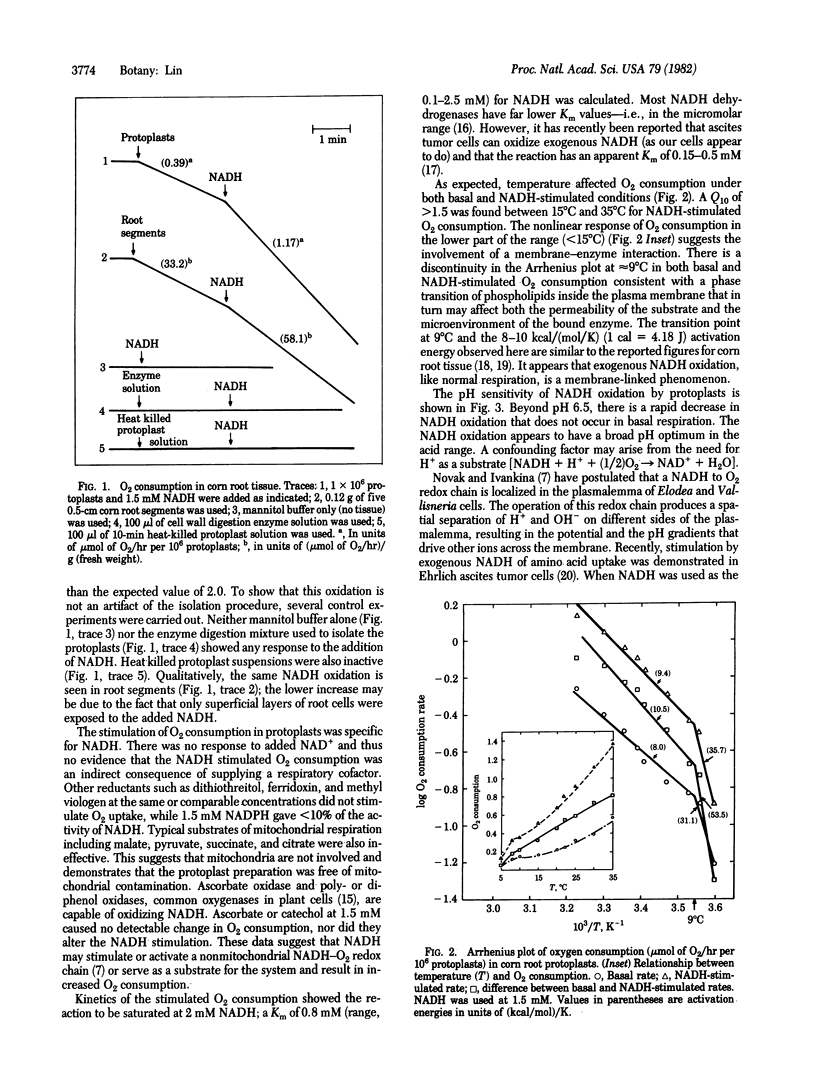
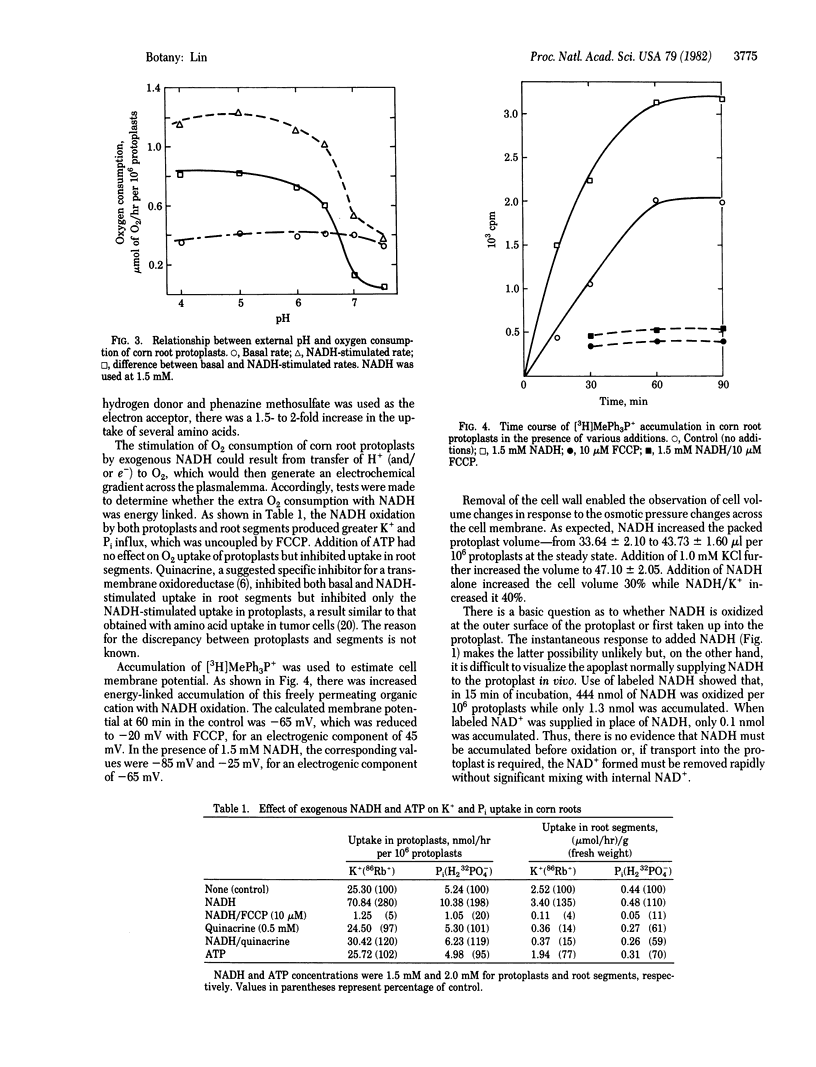
Addition of 1.5 mM NADH tripled the O2 consumption in corn root protoplasts. The stimulation was temperature and pH dependent, specific to NADH, and accompanied by a 2- to 3-fold increase in K+ and Pi uptake into protoplasts. The increase in ion uptake was not due to the accumulation of NADH into protoplasts. The effect of exogenous NADH on O2 consumption and ion uptake was also evident in corn root segments but to a lesser extent. A 20-mV hyperpolarization of protoplast membrane potential occurred on addition of NADH and was abolished by the uncoupler carbonyl cyanide p-trifluoromethoxyphenylhydrazone. Increases in cell volume of 30% and 40% were detected in response to NADH/H+ and NADH/H+/K+, respectively. The data are discussed in terms of a transmembrane redox reaction and the possibility that some part of the energy-linked ion transport may be driven by a NADH → O2 electron-transport system in the plasmalemma.
Keywords: NADH oxidation, ion transport
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brain R. D., Freeberg J. A., Weiss C. V., Briggs W. R. Blue light-induced Absorbance Changes in Membrane Fractions from Corn and Neurospora. Plant Physiol. 1977 May;59(5):948–952. doi: 10.1104/pp.59.5.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaney R. L., Brown J. C., Tiffin L. O. Obligatory reduction of ferric chelates in iron uptake by soybeans. Plant Physiol. 1972 Aug;50(2):208–213. doi: 10.1104/pp.50.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman J. M., Lafayette P. R., Gronewald J. W., Hanson J. B. Effect of ATPase inhibitors on cell potential and k influx in corn roots. Plant Physiol. 1980 Jun;65(6):1139–1145. doi: 10.1104/pp.65.6.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry J. M., Mackellar W., Morré D. J., Crane F. L., Jacobsen L. B., Schirrmacher V. Evidence for a plasma membrane redox system on intact ascites tumor cells with different metastatic capacity. Biochim Biophys Acta. 1981 Jan 14;634(1):11–18. doi: 10.1016/0005-2728(81)90123-7. [DOI] [PubMed] [Google Scholar]
- Crane F. L., Löw H. NADH oxidation in liver and fat cell plasma membranes. FEBS Lett. 1976 Oct 1;68(2):153–156. doi: 10.1016/0014-5793(76)80425-5. [DOI] [PubMed] [Google Scholar]
- Day D. A., Wiskich J. T. The oxidation of malate and exogenous reduced nicotinamide adenine dinucleotide by isolated plant mitochondria. Plant Physiol. 1974 Jan;53(1):104–109. doi: 10.1104/pp.53.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesaitis A. J., Heners P. R., Hertel R. Characterization of a Membrane Fraction Containing a b-type Cytochrome. Plant Physiol. 1977 May;59(5):941–947. doi: 10.1104/pp.59.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W. Corn Root Protoplasts: ISOLATION AND GENERAL CHARACTERIZATION OF ION TRANSPORT . Plant Physiol. 1980 Oct;66(4):550–554. doi: 10.1104/pp.66.4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W. Inhibition of anion transport in corn root protoplasts. Plant Physiol. 1981 Aug;68(2):435–438. doi: 10.1104/pp.68.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W. Potassium and Phosphate Uptake in Corn Roots: Further Evidence for an Electrogenic H/K Exchanger and an OH/Pi Antiporter. Plant Physiol. 1979 May;63(5):952–955. doi: 10.1104/pp.63.5.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löw H., Crane F. L. Redox function in plasma membranes. Biochim Biophys Acta. 1978 Jul 31;515(2):141–161. doi: 10.1016/0304-4157(78)90002-3. [DOI] [PubMed] [Google Scholar]
- MITCHELL P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961 Jul 8;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- Rubinstein B. Use of lipophilic cations to measure the membrane potential of oat leaf protoplasts. Plant Physiol. 1978 Dec;62(6):927–929. doi: 10.1104/pp.62.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S., Kawasaki T. The involvement of the membrane oxidoreduction system in stimulating amino acid uptake in Ehrlich ascites tumor cells. Biochim Biophys Acta. 1981 Jun 22;644(2):192–200. doi: 10.1016/0005-2736(81)90375-8. [DOI] [PubMed] [Google Scholar]


