Abstract
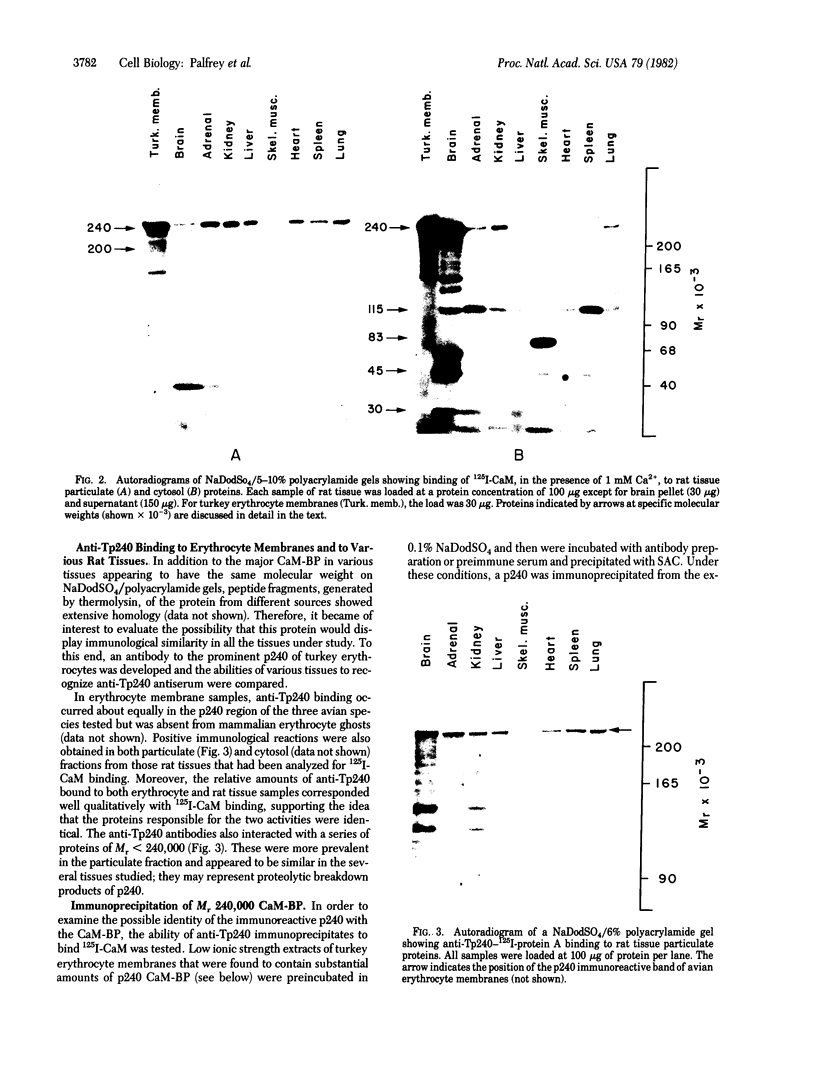
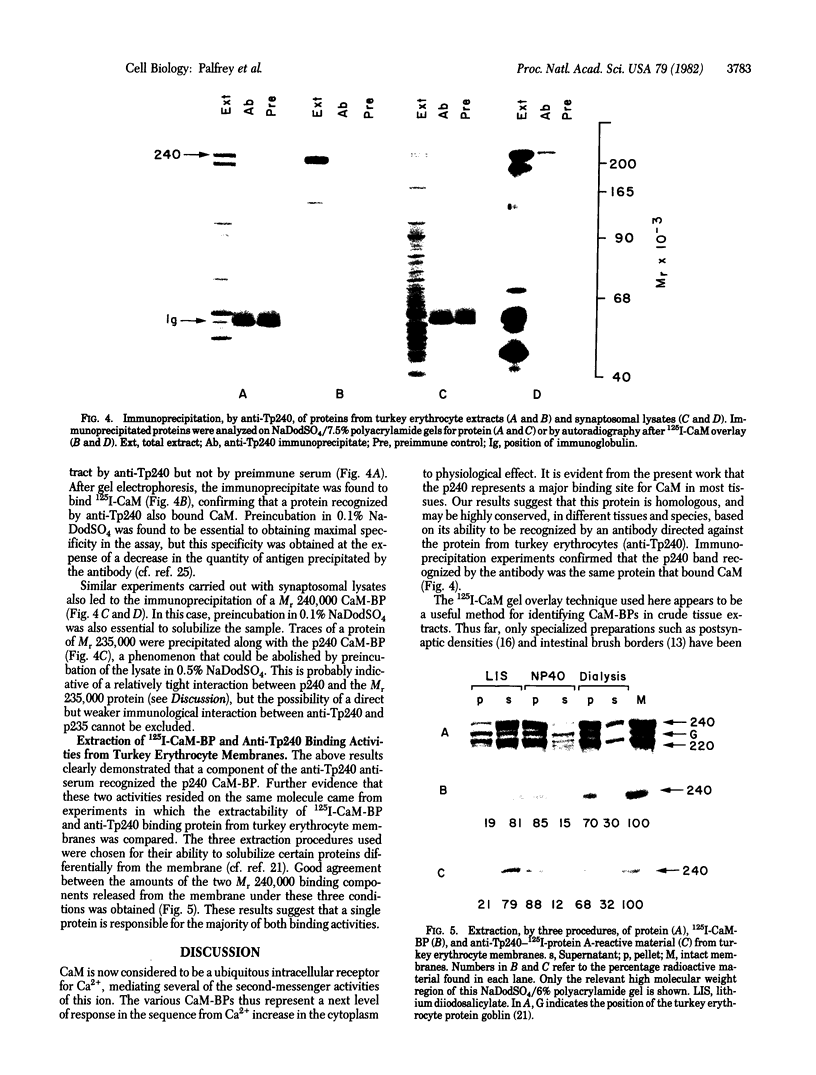
A major calmodulin-binding protein (CaM-BP) of Mr 240,000 was demonstrated in various rat tissues by using a 125I-labeled CaM gel overlay technique. This protein (designated p240) was detected in the particulate fraction and to a lesser extent in the cytosol of all tissues studied. Binding of CaM to p240 was completely dependent on Ca2+. A second, exclusively soluble, CaM-BP (Mr115,000) common to several tissues and a number of other CaM-BPs with a more restricted tissue distribution were also observed by using this technique. CaM binding to p240 occurred in high amounts in plasma membranes from avian erythrocytes but was absent from mammalian erythrocyte membranes. Antibodies prepared against turkey erythrocyte p240 (anti-Tp240) crossreacted with p240 in other tissues. Identity between the proteins recognized by anti-Tp240 and CaM was confirmed by demonstrating that 125I-labeled CaM could bind to p240 specifically immunoprecipitated from either rat brain or turkey erythrocytes by anti-Tp240. The p240 may be related to a previously described actin-binding protein and may represent a major site of action of CaM on the cytoskeleton.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Eisenberg E. Regulation and kinetics of the actin-myosin-ATP interaction. Annu Rev Biochem. 1980;49:921–956. doi: 10.1146/annurev.bi.49.070180.004421. [DOI] [PubMed] [Google Scholar]
- Alper S. L., Palfrey H. C., DeRiemer S. A., Greengard P. Hormonal control of protein phosphorylation in turkey erythrocytes. Phosphorylation by cAMP-dependent and Ca2+-dependent protein kinases of distinct sites in goblin, a high molecular weight protein of the plasma membrane. J Biol Chem. 1980 Nov 25;255(22):11029–11039. [PubMed] [Google Scholar]
- Beam K. G., Alper S. L., Palade G. E., Greengard P. Hormonally regulated phosphoprotein of turkey erythrocytes: localization to plasma membrane. J Cell Biol. 1979 Oct;83(1):1–15. doi: 10.1083/jcb.83.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K. Direct identification of specific glycoproteins and antigens in sodium dodecyl sulfate gels. Methods Enzymol. 1978;50:54–64. doi: 10.1016/0076-6879(78)50007-4. [DOI] [PubMed] [Google Scholar]
- Carlin R. K., Grab D. J., Siekevitz P. Function of a calmodulin in postsynaptic densities. III. Calmodulin-binding proteins of the postsynaptic density. J Cell Biol. 1981 Jun;89(3):449–455. doi: 10.1083/jcb.89.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroni P., Carafoli E. The Ca2+-pumping ATPase of heart sarcolemma. Characterization, calmodulin dependence, and partial purification. J Biol Chem. 1981 Apr 10;256(7):3263–3270. [PubMed] [Google Scholar]
- Chafouleas J. G., Dedman J. R., Munjaal R. P., Means A. R. Calmodulin. Development and application of a sensitive radioimmunoassay. J Biol Chem. 1979 Oct 25;254(20):10262–10267. [PubMed] [Google Scholar]
- Cheung W. Y. Cyclic 3',5'-nucleotide phosphodiesterase: pronounced stimulation by snake venom. Biochem Biophys Res Commun. 1967 Nov 30;29(4):478–482. doi: 10.1016/0006-291x(67)90508-6. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Glenney P., Osborn M., Weber K. An F-actin- and calmodulin-binding protein from isolated intestinal brush borders has a morphology related to spectrin. Cell. 1982 Apr;28(4):843–854. doi: 10.1016/0092-8674(82)90063-0. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Weber K. Calmodulin-binding proteins of the microfilaments present in isolated brush borders and microvilli of intestinal epithelial cells. J Biol Chem. 1980 Nov 25;255(22):10551–10554. [PubMed] [Google Scholar]
- Goelz S. E., Nestler E. J., Chehrazi B., Greengard P. Distribution of protein I in mammalian brain as determined by a detergent-based radioimmunoassay. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2130–2134. doi: 10.1073/pnas.78.4.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grand R. J., Perry S. V., Weeks R. A. Troponin C-like proteins (calmodulins) from mammalian smooth muscle and other tissues. Biochem J. 1979 Feb 1;177(2):521–529. doi: 10.1042/bj1770521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins R. C., Dahmus M. E. Rapid visualization of protein bands in preparative SDS-polyacrylamide gels. Anal Biochem. 1979 Mar;93(2):257–260. doi: 10.1016/s0003-2697(79)80148-7. [DOI] [PubMed] [Google Scholar]
- Huttner W. B., Greengard P. Multiple phosphorylation sites in protein I and their differential regulation by cyclic AMP and calcium. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5402–5406. doi: 10.1073/pnas.76.10.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivarie R. D., Jones P. P. A rapid sensitive assay for specific protein synthesis in cells and in cell-free translations: use of Staphylococcus aureus as an adsorbent for immune complexes. Anal Biochem. 1979 Aug;97(1):24–35. doi: 10.1016/0003-2697(79)90322-1. [DOI] [PubMed] [Google Scholar]
- Kakiuchi S., Sobue K., Fujita M. Purification of a 240 000 Mr calmodulin-binding protein from a microsomal fraction of brain. FEBS Lett. 1981 Sep 14;132(1):144–148. doi: 10.1016/0014-5793(81)80449-8. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Crouch T. H., Krinks M. H. Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6270–6273. doi: 10.1073/pnas.76.12.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levine J., Willard M. Fodrin: axonally transported polypeptides associated with the internal periphery of many cells. J Cell Biol. 1981 Sep;90(3):631–642. doi: 10.1083/jcb.90.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcum J. M., Dedman J. R., Brinkley B. R., Means A. R. Control of microtubule assembly-disassembly by calcium-dependent regulator protein. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3771–3775. doi: 10.1073/pnas.75.8.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means A. R., Tash J. S., Chafouleas J. G. Physiological implications of the presence, distribution, and regulation of calmodulin in eukaryotic cells. Physiol Rev. 1982 Jan;62(1):1–39. doi: 10.1152/physrev.1982.62.1.1. [DOI] [PubMed] [Google Scholar]
- Pershadsingh H. A., Landt M., McDonald J. M. Calmodulin-sensitive ATP-dependent Ca2+ transport across adipocyte plasma membranes. J Biol Chem. 1980 Oct 10;255(19):8983–8986. [PubMed] [Google Scholar]
- Schulman H., Greengard P. Ca2+-dependent protein phosphorylation system in membranes from various tissues, and its activation by "calcium-dependent regulator". Proc Natl Acad Sci U S A. 1978 Nov;75(11):5432–5436. doi: 10.1073/pnas.75.11.5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimo-Oka T., Watanabe Y. Stimulation of actomyosin Mg2+-ATPase activity by a brain microtubule-associated protein fraction. High-molecular-weight actin-binding protein is the stimulating factor. J Biochem. 1981 Nov;90(5):1297–1307. doi: 10.1093/oxfordjournals.jbchem.a133595. [DOI] [PubMed] [Google Scholar]
- Sobue K., Fujita M., Muramoto Y., Kakiuchi S. The calmodulin-binding protein in microtubules is tau factor. FEBS Lett. 1981 Sep 14;132(1):137–140. doi: 10.1016/0014-5793(81)80447-4. [DOI] [PubMed] [Google Scholar]
- Sobue K., Muramoto Y., Fujita M., Kakiuchi S. Calmodulin-binding protein of erythrocyte cytoskeleton. Biochem Biophys Res Commun. 1981 Jun 16;100(3):1063–1070. doi: 10.1016/0006-291x(81)91931-8. [DOI] [PubMed] [Google Scholar]
- Stewart A. A., Ingebritsen T. S., Manalan A., Klee C. B., Cohen P. Discovery of a Ca2+- and calmodulin-dependent protein phosphatase: probable identity with calcineurin (CaM-BP80). FEBS Lett. 1982 Jan 11;137(1):80–84. doi: 10.1016/0014-5793(82)80319-0. [DOI] [PubMed] [Google Scholar]
- Vincenzi F. F., Hinds T. R., Raess B. U. Calmodulin and the plasma membrane calcium pump. Ann N Y Acad Sci. 1980;356:232–244. doi: 10.1111/j.1749-6632.1980.tb29614.x. [DOI] [PubMed] [Google Scholar]
- Wang J. H., Sharma R. K., Huang C. Y., Chau V., Chock P. B. On the mechanism of activation of cyclic mucleotide phosphodiesterase by calmodulin. Ann N Y Acad Sci. 1980;356:190–204. doi: 10.1111/j.1749-6632.1980.tb29611.x. [DOI] [PubMed] [Google Scholar]