SUMMARY
The vertebrate body forms in an anterior-to-posterior progression, driven by a population of undifferentiated cells at the posterior-most end of the embryo. Recent studies have demonstrated that these undifferentiated cells are multipotent stem cells, suggesting that local signaling factors specify cell fate. However, the mechanism of cell fate specification during this process is unknown. Using a combination of single cell transplantation and newly developed cell-autonomous inducible Wnt inhibitor and activator transgenic zebrafish lines, we show that canonical Wnt signaling is continuously necessary and sufficient to specify mesoderm from a bipotential neural/mesodermal precursor. Surprisingly, we also find that Wnt signaling functions subsequently within the mesoderm to specify somites instead of posterior vascular endothelium. Our results demonstrate that dynamic local Wnt signaling cues specify germ layer contribution and mesodermal tissue type specification of multipotent stem cells throughout the formation of the early vertebrate embryonic body.
INTRODUCTION
A hallmark of vertebrate development is the continuous growth of the body at the posterior end during the period following gastrulation, resulting in embryos with widely divergent body lengths (Gomez et al., 2008; Martin and Kimelman, 2009). For much of the past century the dogma of vertebrate body formation postulated that the three germ layers are specified during gastrulation, and that the elaboration of the body builds upon this initial specification (Gont et al., 1993; Pasteels, 1939, 1942, 1943; Spofford, 1945). This was challenged by a study that lineage labeled groups of cells in the frog (Davis and Kirschner, 2000), and more recently by clonal labeling studies in the mouse, which indicates that a neural/mesodermal fate decision is continuously made within the tail bud (Tzouanacou et al., 2009). This growing body of literature has led to the prevailing model that a population of stem cells resides in the vertebrate tail bud, although only in the amniotes have experiments thus far been done to show that these cells have a self-renewing capacity (Wilson et al., 2009). How unspecified cells choose between these different germ layer fates as the body extends remains a critical unanswered question in vertebrate development (Wilson et al., 2009).
A major lineage of the tail bud stem cells is the mesodermally derived somites, which form in a sequential anterior to posterior fashion dependent upon a molecular clock and wave front mechanism (Dequéant and Pourquié, 2008; Holley, 2007; Lewis et al., 2009). Somites later differentiate to form skeletal muscle, bone, and dermis (Brand-Saberi and Christ, 2000). We previously demonstrated that the somite progenitor cells reside in the tail bud in a self-sustaining molecular niche consisting of high canonical (β-catenin dependent) Wnt signaling and low retinoic acid signaling (Martin and Kimelman, 2008, 2010). This molecular niche is maintained by an autoregulatory loop between the transcription factor Brachyury (Ntl and Bra in zebrafish) and canonical Wnt signaling. Although loss of Brachyury or Wnt signaling in whole embryos results in a failure to maintain mesodermal progenitors, thus causing a subsequent loss of somites, individual mesodermal progenitor cells in a wild-type environment do not require Brachyury function because the surrounding cells provide Wnt signals (Martin and Kimelman, 2008, 2010). This result suggested that Wnt signaling is the key factor maintaining mesodermal progenitor cells, and that the essential role for Brachyury is to sustain the Wnt signal among the somite progenitor cells throughout somitogenesis.
Canonical Wnt signaling plays multiple roles in embryogenesis that change dramatically depending on the embryonic stage (Schier and Talbot, 2005). Although Wnt signaling is needed for posterior development of the vertebrate embryonic body (Agathon et al., 2003; Galceran et al., 1999; Lekven et al., 2001; Martin and Kimelman, 2008; Shimizu et al., 2005; Takada et al., 1994), as well as for partitioning the somites (Aulehla et al., 2008), we reasoned that it could be the regulator of the ongoing neural/mesodermal fate decision within the tail bud. Because Wnt signaling is essential in early patterning, traditional loss of function studies cause severe phenotypes that preclude the analysis of postgastrulation phenotypes (Galceran et al., 1999; Lekven et al., 2001; Liu et al., 1999; Takada et al., 1994). In addition, the expression of multiple canonical Wnt ligands and secreted Wnt inhibitors in the tail bud of vertebrate embryos muddies the analysis of the overall role of Wnt signaling in tail bud stem cells.Wehave developed methods to circumvent these issues by creating heat-shock inducible cell-autonomous Wnt inhibitor or activator transgenic zebrafish lines, which allows us to determine the direct role of Wnt signaling in individual tail bud stem cells. Our results demonstrate that Wnt signaling is maintained in the tail bud of vertebrate embryos to direct two critical stem cell fate decision events. Initially, Wnt signaling specifies mesodermal fate from a bipotential neural/mesodermal stem cell, allowing the embryo to dynamically allocate cell fates as the body extends during the somitogenesis period. After the mesoderm has been specified, we find that Wnt signaling has a role in regulating a second fate decision between somite and posterior vascular fates.
RESULTS
Wnt Signaling Is Required Postgastrulation for the Continued Specification of Somites at the Expense of Spinal Cord
The study of the role of canonical Wnt signaling in posterior vertebrate development has previously been limited to the analysis of embryos with a constitutive loss of Wnt signaling from early stages of development (Galceran et al., 1999; Heasman et al., 2000; Lekven et al., 2001; Ramel and Lekven, 2004; Shimizu et al., 2005; Takada et al., 1994; Yang et al., 2002). Although this has been informative with respect to the gastrula stage role of Wnt signaling, the interpretation of the ongoing role of Wnt signaling after gastrulation is difficult due to the severe defects that gastrula stage loss of Wnt signaling causes. We bypassed early developmental defects and specifically tested the postgastrula requirement for Wnt signaling using a heat shock (HS) inducible secreted Wnt inhibitor line (HS:dkk1-EGFP, referred to here as HS:dkk1) (Stoick-Cooper et al., 2007). Although inhibiting Wnt signaling at the end of gastrulation caused a severe deficit in posterior somites, it surprisingly had only a very minor effect on the overall length of embryos, suggesting that posterior growth is not defective (Figures 1A–1D; see also Figure S1 available online). Instead, the majority of tissue that formed posterior to the truncated somitic tissue consisted of an enlarged neural tube. The change in fate that is observed after loss of Wnt signaling is preceded by a rapid loss of the mesodermal progenitor marker ntl, and a corresponding dramatic increase in the neural progenitor marker sox2 (Figures 1K–1N). These results suggest that zebrafish tail bud stem cells have the potential to become either mesoderm or neural tissue, and that Wnt signaling directly specifies mesoderm at the expense of neural tissue. Indeed, we find that cells in an arc along the posterior wall of the tail bud, a region we term the stem zone (SZ), coexpress both the mesodermal progenitor marker ntl (brachyury) (Schulte-Merker et al., 1994) and the neural progenitor marker sox2 (Graham et al., 2003) (Figures 1E–1G) in a region distinct from the cells committed to the paraxial mesodermal lineage, which are marked by the expression of tbx16/spadetail (Griffin et al., 1998; Ho and Kane, 1990) (Figures 1H–1J).
Figure 1. Canonical Wnt Signaling Promotes Mesodermal and Inhibits Neural Development during Postgastrulation Development.
(A–D) The HS:dkk1 transgenic line was used to inhibit canonical Wnt signaling during postgastrula stages. (A and B) myod expression in wild-type and transgenic embryos that were heat-shocked at bud stage and fixed at 24 hpf. In transgenic embryos, there is a severe truncation of the mesodermally derived somites (B, arrow), despite the overall length of the embryo being similar to wild-type embryos. (C and D) sox3 expression in wild-type and transgenic embryos. An enlarged neural tube is present in the posterior embryo, beginning where somites are absent (D, arrow).
(E–G) Cells within the stem zone (SZ; G, arrowhead) express both the mesodermal progenitor marker ntl and the neural progenitor marker sox2.
(H–J) The cell population expressing both ntl and sox2 is different than cells that have committed to the mesodermal lineage, as marked by tbx16 expression (J, arrowhead showing nonoverlapping expression of ntl in the SZ).
(K–N) Wild-type and HS:dkk1 transgenic embryos were heat-shocked at the 12-somite stage and fixed at the 16-somite stage (3 hr after the heat shock). (K and L) The expression of the mesodermal progenitor marker ntl is completely lost in the SZ of transgenic embryos, (M and N) whereas the expression of the neural progenitor marker sox2 is significantly expanded. The ntl notochord domain is unaffected by Wnt inhibition. Note the unique ventral sox2 domain (arrowheads). See also Figure S1.
Wnt Signaling Is Required Cell-Autonomously for the Specification of Somite Tissue Both Pre- and Postgastrulation
The loss of mesodermal tissue and expansion of neural tissue in response to Wnt inhibition could be due to a change in the fate decision of a bipotential population of stem cells or due to an expansion of a neural progenitor pool when the mesodermal progenitors are inhibited. In addition, Wnt signaling may be required directly within mesodermal cells, or may regulate a downstream secreted signaling pathway that is causing the observed phenotype. To distinguish between these possibilities, we created a cell-autonomous inducible Wnt inhibitor transgenic line (HS:TCFΔC) that allows us to inhibit Wnt signaling at specific times within individual cells. This new line produces the same phenotype as obtained with the secreted Wnt inhibitor Dkk1, and inhibits the expression of known Wnt target genes (Figure S2).
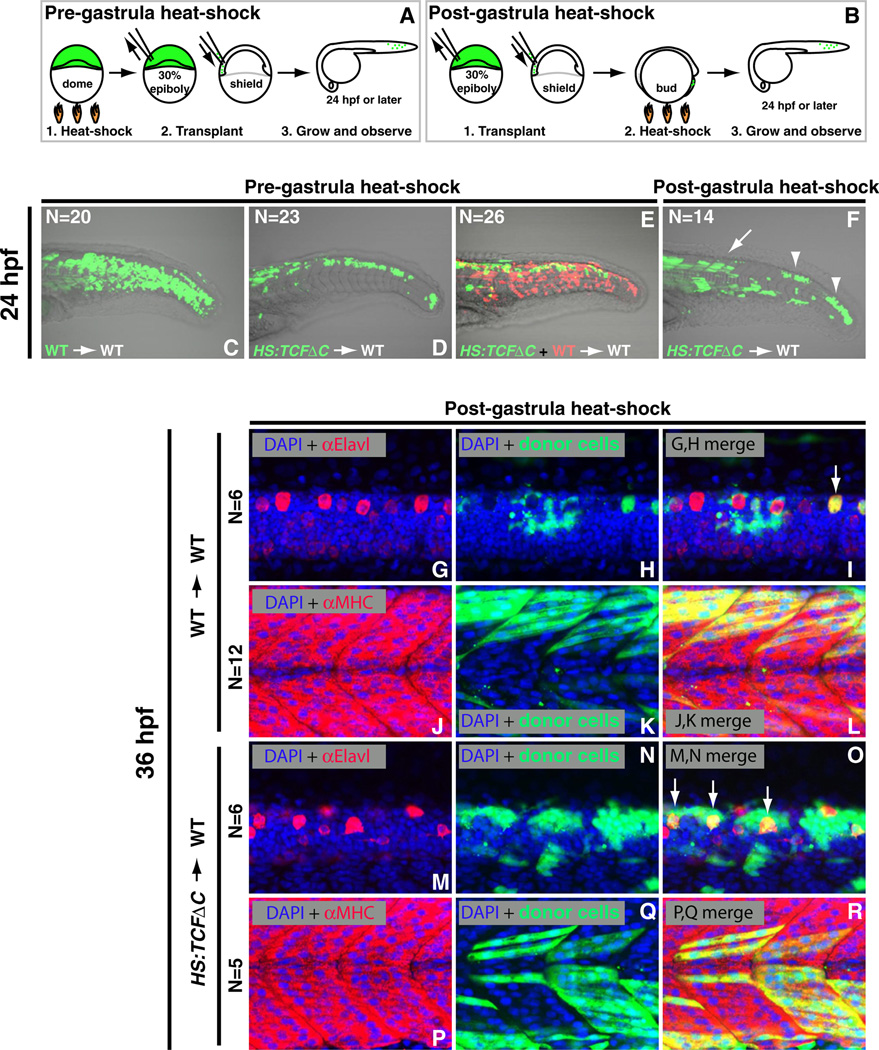
To specifically test the role of Wnt in mesodermal progenitors, control wild-type or HS:TCFΔC cells were transplanted into the ventral margin of shield stage embryos, which primarily populates posterior somites (Figures 2A and 2B) (Kimmel et al., 1990). Inhibition of Wnt signaling pregastrulation caused a dramatic conversion of cell fate from somites to spinal cord along the axis (Figures 2C–2E). Importantly, transfating also occurred when Wnt signaling was inhibited after gastrulation (Figure 2F), revealing an ongoing role of Wnt signaling in specifying mesodermal fate among bipotential neural/mesodermal stem cells. Transplanted cells that ended up in more anterior regions of the embryo, which differentiates early from the progenitor population, contributed to somites, whereas the later differentiating cells in the posterior regions became mostly neural (Figure 2F). This result demonstrates that Wnt inhibition does not block muscle differentiation per se but only affects the fate of cells that have not differentiated at the time Wnt signaling is inhibited. The assignment of cells to a neural or muscle fate based on morphology and position was confirmed using antibodies specific for the differentiated cell type (Figures 2G–2R).
Figure 2. Wnt Signaling Is Cell-Autonomously Required for Tail Bud Stem Cells to Join Somites throughout Body Formation.
(A and B) Experimental design for cell-transplants examining the pregastrula (A) and postgastrula (B) requirements for Wnt signaling. (C and D) Fluorescein dextran labeled donor cells were transplanted into the ventral margin of shield stage unlabeled wild-type host embryos. (C) Control cells contribute normally to posterior somites. (D) Cells from HS:TCFΔC donor embryos heat-shocked at the pregastrula (dome) stage before transplantation are unable to contribute to somites, and instead reside within the neural tube.
(E) Double transplantation of wild-type (red) and HS:TCFΔC (green) cells that were heat-shocked at dome stage and cotransplanted into the ventral margin of wild-type host embryos. Wild-type cells contribute normally to somites whereas cells lacking Wnt function cannot join the posterior somites and instead contribute to the neural tube.
(F) Host embryos containing fluorescein dextran labeled HS:TCFΔC cells transplanted into the ventral margin and heat-shocked at the postgastrula (bud) stage have an intermediate phenotype, with anterior cells contributing normally to somites (arrow) and posterior cells transfating to neural tissue (arrowheads).
(G–R) The ability of transplanted cells to differentiate into muscle or neurons was verified using antibodies directed against Myosin Heavy Chain or Elavl, respectively. (G–L) Wild-type cells labeled with fluorescein dextran (green) transplanted into the ventral margin of unlabeled wild-type host embryos and heat-shocked at bud stage primarily form muscle (L) with the occasional differentiated neuron (I, arrow), as indicated by the overlap of red fluorescent antibody staining and the green fluorescence of the transplanted donor cells. (M–R) HS:TCFΔC cells labeled with fluorescein dextran were transplanted into unlabeled wild-type host embryos and heat-shocked at bud stage. Transplanted cells in the posterior of the embryo differentiated as neurons (O) and not muscle (not shown), whereas the early differentiating cells in the anterior regions contributed to muscle (R). See also Figures S2 and S3.
Posterior mesodermal cells migrate down from the ventral margin to the vegetal pole where they undergo a morphological change to form the tail bud at the end of gastrulation as revealed by Kanki and Ho (1997). Potentially, pregastrula Wnt inhibition could alter the fate of these cells by changing their morphogenetic movements. However, the conversion in cell fate that occurs when individual cells lack Wnt signaling during the gastrula stages does not appear to be due to a gastrula stage change in morphogenesis as cotransplanted wild-type and HS:TCFΔC cells show no obvious differences at the end of gastrulation (Figure S3). It is not until mid-somitogenesis stages that differences in the morphogenesis of wild-type and Wnt loss of function cells becomes readily apparent, when wild-type cells have joined the hypoblast normally, but Wnt loss of function cells remain in the epiblast (Figure S3).
Single Cell Transplants Reveal that Wnt Signaling Specifies Mesoderm from a Bipotential Neural/ Mesodermal Stem Cell
To quantify transfating we developed an assay in which a single cell is transplanted into the ventral margin of a host embryo. Traditional cell transplantation in zebrafish involves transplanting a group of cells from donor to host embryo without the ability to precisely control the exact number of cells transplanted. Groups of transplanted cells will most often give rise to multiple tissue types in host embryos, making quantifiable fate analysis an extremely difficult and tedious process. Our method allows us to transplant exactly one cell into a host embryo (Figures 3A and 3B), which provides an easily quantifiable fate change assay because the descendants of a single transplanted cell almost always gives rise to a lineage restricted population of cells (Figures 3C and 3D). When a single cell is transplanted into the ventral margin of a shield stage host embryo, the clone originating from the donor cell most often contributes to the posterior somites (Figures 3C–3G). Wnt inhibition caused a decrease of somite clones and an increase in spinal cord clones both before and after gastrulation (Figure 3G). These results definitively demonstrate that Wnt signaling is the critical factor specifying mesodermal fate in a bipotential neural/mesodermal population of stem cells, and that this mechanism functions both before and after gastrulation. Because the size of the SZ constantly shrinks, it was difficult to obtain enough single cell transplants to examine the fate change using this method during later stages. However, both gene expression (Figure 1; Figure S4) and multicell transplants (Figure S4) revealed that Wnt continues to regulate the neural/mesodermal fate decision during somitogenesis.
Figure 3. A Single Cell Transplantation Assay Demonstrates that Wnt Signaling Specifies Mesodermal Fate in Bipotential Neural/Mesodermal Stem Cells throughout Body Formation.
(A and B) Host embryos were visualized under a fluorescent microscope immediately after the single cell transplant to verify that only one cell was transplanted (A, arrow). An overlay with the bright field image illustrates the position of the single cell in the ventral margin of the host embryo (B, arrow, animal pole of the embryo is to the top).
(C and D) Single cell transplantations into the ventral margin produce lineage restricted clones, primarily giving rise to clones within the somites (C) with some contributing only to the neural tube (D).
(E and F) Single HS:TCFΔC cells transplanted into wild-type host embryos and heat-shocked at bud stage produce morphologically normal muscle fibers (E) and spinal cord neurons (F).
(G) Quantification of one-cell transplants of control or HS:TCFΔC indicate that Wnt signaling is required continuously throughout development to specify mesoderm in a bipotential neural/mesodermal stem cell. Inhibiting Wnt signaling before or after gastrulation causes a significant decrease in clones that contribute to the somites and a significant increase in the clones that give rise to spinal cord neurons (p < 0.05 indicated by red asterisk). Nonmuscle mesoderm (see Figure 4) is not included in these graphs so the totals do not add up to 100%. See also Figure S4.
Wnt Signaling Specifies a Second Fate Decision within Tail Bud Mesodermal Progenitor Cells
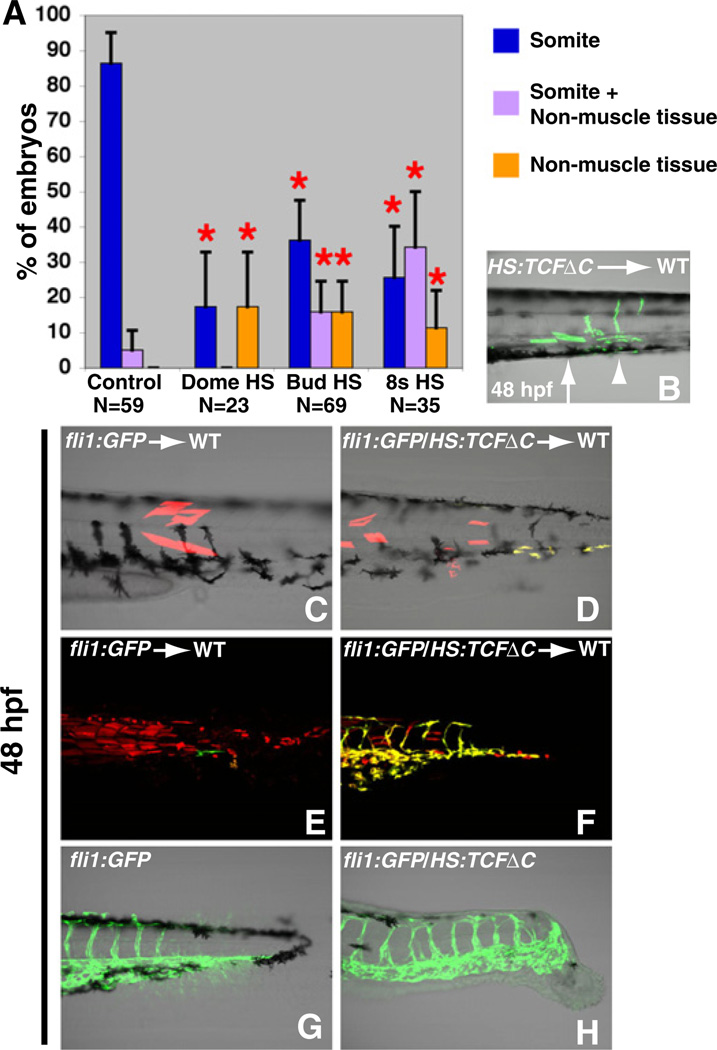
After mesodermal specific cells leave the SZ, they are thought to just produce somitic tissue (and notochord in amniotes) (Cambray and Wilson, 2002; Davis and Kirschner, 2000; Gont et al., 1993; Kanki and Ho, 1997; Wilson et al., 2009). We discovered from our single cell transplants that Wnt signaling regulates a subsequent fate decision within these cells. Inhibition of Wnt signaling postgastrulation caused a significant portion of cells to adopt a morphology that was clearly different from the muscle or neural cells (Figures 4A and 4B). Based on location and morphology, these cells appeared to be the endothelial cells of the dorsal aorta, cardinal vein, and intersomitic vessels. This was an extremely surprising result because the zebrafish endothelial mesoderm is thought to be specified during gastrulation (Zhong, 2005). To determine if Wnt inhibition was truly converting cells to a vascular fate, we used cells from the fli1:GFP line, which specifically labels the vascular endothelium (Lawson and Weinstein, 2002). Whereas single fli1:GFP cells transplanted into the ventral marginal zone mostly became muscle, single cells from a fli1:GFP/HS:TCFΔC cross that were heat-shocked at the 8-somite stage contributed progeny to the vascular endothelium (Figures 4C and 4D). This result identifies the tail bud mesodermal progenitors as a source of posterior vasculature.
Figure 4. Wnt Signaling Specifies Paraxial Fate in Bipotential Paraxial/Endothelial Mesodermal Progenitors.
(A) In the absence of Wnt signaling, there is a significant shift in the resident location of cells from the somites to the nonmuscle mesoderm (note that the data for the somite clones is the same as that presented in Figure 3G, data for neural clones can be found in Figure 3).
(B) An example of a single cell HS:TCFΔC transplant that was heat-shocked at the 8-somite stage, where several cells in the anterior of the clone have contributed normally to somites (arrow), whereas the cells in the posterior have contributed to a ventral mesoderm fate that appears to be vascular endothelium (arrowhead).
(C and D) Rhodamine-labeled single cells were transplanted from the indicated donor and heat shocked at bud stage. (D) Wnt inhibition causes some of the progeny of the single cell to express the vascular marker fli1 (seen as yellow from the overlap of GFP and rhodamine).
(E and F) Rhodamine-labeled cells were transplanted from the indicated donor and heat shocked at the 8-somite stage. (F) Wnt inhibition causes many of the transplanted cells to adopt a vascular fate instead of a muscle fate.
(G and H) fli1:GFP and fli1:GFP/HS:TCFΔC embryos were heat shocked at the 8-somite stage. (H) Wnt inhibition causes a large increase in the amount of posterior vasculature.
Because only a small number of transplanted single cells remain in the SZ by later stages due to the continual depletion of this zone, we also examined the results of Wnt inhibition when groups of cells were transplanted, again using the fli1:GFP line to mark the vasculature. When Wnt signaling was inhibited in transplanted cells after gastrulation, there was a significant expansion in both the percentage (100%, n = 17 for bud stage HS; 80%, n = 20 for 8-somite stage HS, compared to 61%, n = 46 for controls) of host embryos with donor cells contributing to the vasculature, as well as the amount of donor cells within individual host embryos that contributed to vascular tissue instead of somites (Figures 4E and 4F). Intriguingly, pregastrula Wnt inhibition produced the opposite effect, causing a significant decrease in the percentage of host embryos with donor cells contributing to vasculature (12%; n = 26 for dome stage HS), demonstrating that the somite/endothelial decision is not due to changes in early patterning, but instead regulates cell decisions in the postgastrula embryo. Wnt signaling also controls the fate of nontransplanted cells, because we saw a large increase in the amount of posterior vasculature along with a concomitant suppression of muscle cells when Wnt signaling was inhibited in embryonic cells that had not been transplanted (Figures 4G and 4H). In summary, our results demonstrate the ability of Wnt signaling to regulate a second fate decision among the progenitor cells, creating vasculature for the posterior of the embryo in a process we call secondary vascularization.
Tail Bud Mesodermal Progenitors Normally Contribute Cells to the Posterior Vasculature
We sought to determine the location of the bipotential somitic/endothelial mesodermal cells within the tail bud. Intriguingly, sox2, which is expressed in the bipotential mesodermal/neural precursors, is also expressed in a domain within the ventral tail bud (Figure 1M). This domain overlaps with ntl expression and is distinct from the developing lateral plate mesoderm (Figures 5A and 5B), the previously identified source of zebrafish embryonic vasculature (Jin et al., 2005). Importantly, the ventral sox2 expression expands when Wnt signaling is inhibited (Figure 1N). Labeling the ventral sox2 region using a photo-convertible fluorescent protein (Kaede) identifies this group of cells as progenitors of both the somites and the posterior vasculature (Figures 5C–5F). Labeling the posterior SZ indicates that cells from this population also give rise to posterior vasculature (Figures 5G–5J). Because the ventral domain originally derives from the SZ (data not shown), these results indicate that Wnt signaling first specifies the neural/mesodermal fate, and then a subset of the mesodermal cells make a somite/endothelial fate decision (Figure 7). These results indicate that new cells from the tail bud join the developing posterior vasculature, which along with single cell transplantation results (Figure 4A), rule out the possible hypothesis that the expansion of posterior vasculature in Wnt loss of function cells is due to an increase in the proliferation of endothelial progenitors specified during gastrulation. Intriguingly, both regions where fate decisions are occurring are marked by the expression of sox2.
Figure 5. Mesodermal Progenitors of the Tail Bud Normally Contribute to Posterior Endothelial Tissue.
(A) Double fluorescent in situ staining of gata1 (green, arrow) and sox2 (red, arrowhead) indicates that the sox2 positive cells of the tail bud are distinct from the gata1 expressing intermediate cell mass.
(B) Double fluorescent in situ staining of ntl (green) and sox2 (red) indicates that sox2 positive cells coexpress ntl in both the ventral domain (arrow) and in the SZ (arrowhead).
(C–J) fli1:gfp transgenic embryos were injected with nuclear localized kaede mRNA (C and D). A small population of cells were photoconverted from green to red in the region of the ventral sox2 expressing cells, (G and H) and in the SZ region of sox2 expressing cells. (E, F, I, and J) In both cases, cells gave rise to both somites and vascular endothelium (arrows indicate red nucleus in a cell with cytoplasmic GFP expression from the fli1:gfp).
Figure 7. A Model of Wnt Signaling Function in the Developmental Specification of Tail Bud Stem Cells.
Wnt signaling acts within a bipotential neural/mesodermal stem cell (yellow) to specify mesodermal fate, as well as in bipotential paraxial/endothelial mesodermal progenitors (red) to specify paraxial fate. Pink arrows indicate the movement of cells into the spinal cord or somite/endothelial tissue.
Activated Wnt Signaling Is Sufficient to Specify Somite Tissue at the Expense of Spinal Cord and Vascular Endothelium
Because loss of Wnt signaling cell-autonomously shifts cells to neural and vascular fates, we asked if a gain of Wnt function would shift cells in the opposite direction. To do this, we created a transgenic line carrying a heat-shock inducible cell-autonomous activator of the Wnt pathway using a form of β-catenin that is not subject to GSK3-induced degradation (Yost et al., 1996) (HS:caβcat). We found that activation of this transgene induced the expression of known Wnt target genes as expected (Figure S2). When control wild-type cells are transplanted into the ventral margin of wild-type host embryos, the majority of cells contribute to the posterior somites, but a portion of the cells often end up in the spinal cord (Figure 6A) or the posterior vasculature (Figure 4E). Activation of the Wnt signaling pathway in donor cells caused a significant reduction in the percentage of host embryos with donor cells in either spinal cord or posterior vasculature, with all the transplanted cells ending up in the somites (Figures 6B and 6C). This result indicates that canonical Wnt signaling is sufficient to drive multipotent tail bud stem cells into the somite cell fate.
Figure 6. Wnt Signaling Is Sufficient to Specify Paraxial Mesoderm in Multipotent Stem Cells.
(A and B) Fluorescein labeled wild-type or HS:caβcat cells were heat-shocked and then transplanted into the ventral margin of shield stage unlabeled wild-type embryos. (A) In control transplants, the majority of cells contribute to the posterior somites, but a small number also contribute to the posterior spinal cord. (B) In transplants with activated Wnt signaling, cells contribute to somites but not spinal cord.
(C) Host embryos containing transplanted cells with activated Wnt signaling have a significantly reduced likelihood of cells contributing to neural or endothelial fates (as determined by transplanting fli1:GFP or fli1:GFP/HS:caβcat), compared with controls (p < 0.05 indicated by red asterisk). See also Figure S2.
DISCUSSION
Despite the restricted expression of Wnt ligands in the tail bud of all vertebrate embryos, as well as in the posterior growth zone of a wide variety of bilaterian animals, the role that this critical signaling pathway is playing in the posterior stem/progenitor cell population has remained unclear (Martin and Kimelman, 2009; Petersen and Reddien, 2009). We have shown here that Wnt signaling dynamically regulates two critical decisions in the stem zone, allowing the embryo to constantly allocate cells to different fates as the embryo extends (Figure 7). Once this allocation is made along the neural/mesodermal lineage, downstream transcription factors such as Tbx6/Tbx16 and Sox2 lock in that fate decision to ensure that cells remain committed to a specific lineage (Chapman and Papaioannou, 1998; Row et al., 2011; Takemoto et al., 2011). Through the dynamic regulation of Wnt signaling at both the extracellular and intracellular level (van Amerongen and Nusse, 2009), we propose that this pathway ensures that posterior growth is tightly coupled to cell fate allocation, making certain that the body forms the correct proportion of each cell type as the body extends during the somite-forming stages.
The role of Wnt signaling in mesoderm and neural specification has been controversial (Li and Storey, 2011). Early studies in frogs have suggested that canonical Wnt signaling functions during gastrulation to induce neural tissue (Baker et al., 1999), whereas others have suggested that the gastrula stage role of Wnt signaling is to induce mesoderm (Schohl and Fagotto, 2003). In the mouse, loss of wnt3a function or its downstream signaling components results in a reduction in the number of somites that form, along with an expansion of neural tissue, including ectopic neural tubes where somites should have formed (Galceran et al., 1999; Takada et al., 1994). On the other hand, the tbx6 mutant, which also forms ectopic neural tubes in place of somites (Chapman and Papaioannou, 1998), exhibits an expansion of Wnt signaling (Takemoto et al., 2011). Given this result, and the ability of Wnt to positively regulate the neural progenitor marker sox2, it was recently proposed that Wnt must be downregulated for mesoderm specification (Takemoto et al., 2011). In all these previous studies, Wnt signaling was altered both during and after gastrulation, which complicates interpretation due to the complex and changing role of Wnt signaling during embryogenesis (Kimelman, 2006). Moreover, in these experiments, Wnt signaling was altered in all cells of the embryo, making it problematic to sort out direct versus indirect effects. Our method of temporal, single cell, cell-autonomous manipulation of Wnt signaling resolves the controversy regarding the role Wnt signaling in mesoderm specification, allowing us to definitively show that the function of Wnt is to specify mesoderm in bipotential neural/mesodermal cells throughout the process of body formation, both before and after gastrulation.
As described earlier, a long-standing debate has existed between the view that the most posterior end of the embryo is a mass of undifferentiated cells, and the view that germ layer fates are established during gastrulation. Lineage labeling studies, particularly in amphibians, have promoted the fixed fate view (Kanki and Ho, 1997; Pasteels, 1939, 1942, 1943; Spofford, 1945), and examination of gene expression in the modern era shows that the tail bud appears to be composed of unique neural and mesodermal domains (Beck and Slack, 1998; Cambray and Wilson, 2007; Gont et al., 1993; Knezevic et al., 1998). Yet our data shows that posterior progenitors located in the stem zone are multipotent, consistent with previous lineage labeling studies in other species (Davis and Kirschner, 2000; Tzouanacou et al., 2009), and coexpress both a mesodermal (ntl/brachyury) and a stem/neural gene (sox2). How can these differences be reconciled? First, the stem zone, which contributes cells to both the mesodermal and neural lineages (unpublished results) and coexpresses ntl and sox2, had not been described, and only became clear with the use of a fluorescent in situ hybridization technique. Second, progenitor cells undergo a limited amount of division in the lower vertebrates (Cooke, 1979; Kanki and Ho, 1997) (and our unpublished results), which must develop rapidly and so begin with a large number of cells at gastrulation. This provides a limited number of cases where the progeny of a cell can develop into two alternative fates. Third, and perhaps most significantly, most progenitor cells and their progeny are likely to appear to be lineage restricted because they are either in an environment of high Wnt, in which case they are likely to become mesodermal, or low Wnt, in which case they will adopt a neural fate. Only the cells at the borders are likely to be exposed to differential signaling environments, and only when they divide is there a possibility of the two daughters giving rise to different tissue types. In the mouse, which develops much more of the body from the tail bud and undergoes much more proliferation, the appearance of both germ layers from a single cell becomes more apparent (Tzouanacou et al., 2009). Our results demonstrate that the multipotential neural/mesodermal progenitor originated in the lower vertebrates, and continues to be used in amniotes.
In the course of our analysis we discovered a second and unexpected role for Wnt signaling, to guide mesodermal cells between the somite and vascular fates. Although the formation of secondary vascular endothelial cells from the stem zone was unexpected, intriguingly, single chick somite-derived cells that enter the limb have been shown to be bipotential, with daughter cells contributing to both limb muscle and endothelial cells under the influence of an unidentified signal (Kardon et al., 2002), suggesting a common mechanism is likely to occur in amniotes. Interestingly, it was previously noted that the well-studied zebrafish cloche mutant completely lacks trunk expression of the vascular markers fli1a, kdrl (flk1), and flt4, yet shows a number of cells expressing these markers within the tail, exactly within the region we propose is derived from the ventral somite/vascular bipotential cells marked by sox2 (Liao et al., 1997; Thompson et al., 1998). These cells do not go on to express the downstream marker tie1 (Liao et al., 1997), indicating that secondary vascularization bypasses an early need for cloche within the vascular lineage, but then depends on cloche in a later step to complete vascular differentiation. Thus, these caudal vascular cells are under a different form of genetic control than those specified during gastrulation. Why does secondary vascularization exist? In zebrafish, which develop very rapidly, much of the vasculature progenitors are established at the time of gastrulation and these cells gradually extend toward the posterior during somitogenesis. In zebrafish, we propose the secondary vascularization exists to ensure that the most caudal end of the embryo is completely vascularized by providing a secondary source of vascular cells derived from the tail bud. We suggest that this mechanism will be found in other species, especially those in which much of the posterior region derives from growth of the tail bud, allowing the vasculature to extend as the body does.
The fact that Wnt signaling is involved in at least two separate cell-fate decision events within distinct but very closely opposed regions of the tail bud indicates that this pathway is carefully fine-tuned to allow the proper allocation of stem cells to specific tissues. In addition to the continuous expression of Wnt ligands in the tail bud (Clements et al., 2009; Szeto and Kimelman, 2004; Weidinger et al., 2005), secreted Wnt antagonists are also present in localized regions of the tail bud (Hashimoto et al., 2000; Hsieh et al., 1999; Pézeron et al., 2006; Row and Kimelman, 2009; Tendeng and Houart, 2006). Other signaling pathways are also present in the tail bud, such as the Bmp pathway, which have very clear interactions with the Wnt pathway (Eivers et al., 2008; Row and Kimelman, 2009). Together, these factors are likely to affect the level and timing of the canonical Wnt signal that a particular cell is exposed to, which will in turn affect the fate of that cell. Future research elucidating these fine-tuning mechanisms within the tail bud will help us understand how stem cells are directed to specific fates during the formation of the vertebrate body.
EXPERIMENTAL PROCEDURES
In Situ Hybridization
Alkaline phosphate in situ hybridization was performed as described previously (Griffin et al., 1995). Fluorescent in situ hybridization followed the procedure of Dalgin and Prince (https://wiki.zfin.org/display/prot/Wholemount+Flourescence+In+Situ+Hybridization+and+Fluorescein+Tyramide+Conjugation).
Cell Transplantation
Control WIK/AB or transgenic embryos were injected with either 2% fluorescein dextran or 2% rhodamine dextran and transplanted into the ventral margin of shield stage uninjected WIK/AB host embryos using a CellTram (Eppendorf). One-cell transplants were done in a similar fashion except that the cells removed from donor embryos were repeatedly expelled into embryo media and sucked back into the transplant needle in order to mechanically separate individual cells. A single cell was then drawn up into the transplant needle and deposited in the ventral margin of host embryos. The embryos were visualized under a fluorescent dissecting microscope directly after transplantation to confirm that only one cell had been transplanted.
Generation of HS:TCFΔC Transgenic Zebrafish
Xenopus laevis TCF3 was PCR amplified using primers that create a C-terminal truncation (which eliminates the DNA binding domain of TCF3). The PCR fragment was cloned into the Hind3 and BstB1 sites of the CE-GFP vector, creating a C-terminal GFP fusion. This plasmid was digested with BamH1 and Not1 and inserted into the Tol2-hsp70 vector. The Tol2-hsp70-TCFΔC-GFP plasmid was used to create a stable transgenic line as previously described (Kawakami, 2004). The line is designated w74. All animal protocols used here were approved by the University of Washington Institutional Animal Care and Use Committee.
Generation of HS:caβcat Transgenic Zebrafish
Full-length Xenopus laevis β-catenin was mutated so that the last four of the five Ser and Thr residues of the GSK-3 recognition site were converted to Ala by PCR based mutagenesis, and inserted into the CS2MT vector, which adds six myc tags to the C terminus (Yost et al., 1996). A BamH1-NotI fragment was inserted into the Tol2-HS-GFP vector (Row and Kimelman, 2009), which has a bidirectional synthetic heat shock promoter (Bajoghli et al., 2004). The resulting vector expresses GFP from one side and caβcat-myc from the other side of the heat-shock promoter. This plasmid was used to create a stable transgenic line as previously described (Kawakami, 2004). The line is designated w75.
Transgenic Heat Shock Conditions
Transgenic lines were heat-shocked for 30 min by transferring embryos from 28.5°C to embryo media heated in a circulating water bath. The temperature was optimized for each transgenic line (40°C for HS:dkk1 and HS:TCFΔC, and 41°C for HS:caβcat).
Lineage Labeling
Tail bud lineage analysis was performed by injecting 100 pg of nuclear localized Kaede (Ando et al., 2002) mRNA into fli1-EGFP (Lawson and Weinstein, 2002) embryos. At the 12–14 somite stage nuclei in the tail bud were photoconverted from green to red on an Olympus Fluoview scanning confocal microscope using a 405 nm laser. The embryos were examined again when they were 48 hr old. Cells contributing to endothelial tissue were determined by the presence of a red nucleus in a cell containing cytoplasmic GFP produced by the fli1:EGFP transgenic line.
Immunohistochemistry
Embryos were fixed overnight in 4% paraformaldehyde at 4°C. Myosin Heavy Chain was detected using the monoclonal MF20 antibody (Developmental Studies Hybridoma Bank) at a 1:50 dilution. Elavl was detected using the monoclonal antibody 16A11 (Invitrogen) at a 1:700 dilution. Both primary antibodies were detected using an anti-mouse Alexa-488 conjugated secondary antibody (Invitrogen) at a 1:500 dilution.
Statistical Analysis
The statistical analysis of transplanted cell fate was determined by comparing the number of experimental embryos having cells contribute to a particular fate to the number of controls contributing to that same fate. The p value was determined using the χ2 test. Error bars for the cell fate data represent the 95% confidence interval for a single population proportion calculated without continuity correction.
Supplementary Material
ACKNOWLEDGMENTS
We thank Richard Row and Cort Bouldin for comments on the manuscript and Steve Hauschka and David Raible for antibodies. This work was supported by an NIH grant (GM079203) to D.K. and an American Cancer Society Fellowship (PF-07-048-01-DDC) to B.L.M.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes four figures and can be found with this article online at doi:10.1016/j.devcel.2011.11.001.
REFERENCES
- Agathon A, Thisse C, Thisse B. The molecular nature of the zebrafish tail organizer. Nature. 2003;424:448–452. doi: 10.1038/nature01822. [DOI] [PubMed] [Google Scholar]
- Ando R, Hama H, Yamamoto-Hino M, Mizuno H, Miyawaki A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc. Natl. Acad. Sci. USA. 2002;99:12651–12656. doi: 10.1073/pnas.202320599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulehla A, Wiegraebe W, Baubet V, Wahl MB, Deng C, Taketo M, Lewandoski M, Pourquié O. A beta-catenin gradient links the clock and wavefront systems in mouse embryo segmentation. Nat. Cell Biol. 2008;10:186–193. doi: 10.1038/ncb1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajoghli B, Aghaallaei N, Heimbucher T, Czerny T. An artificial promoter construct for heat-inducible misexpression during fish embryogenesis. Dev. Biol. 2004;271:416–430. doi: 10.1016/j.ydbio.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Baker JC, Beddington RS, Harland RM. Wnt signaling in Xenopus embryos inhibits bmp4 expression and activates neural development. Genes Dev. 1999;13:3149–3159. doi: 10.1101/gad.13.23.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck CW, Slack JM. Analysis of the developing Xenopus tail bud reveals separate phases of gene expression during determination and outgrowth. Mech. Dev. 1998;72:41–52. doi: 10.1016/s0925-4773(98)00015-x. [DOI] [PubMed] [Google Scholar]
- Brand-Saberi B, Christ B. Evolution and development of distinct cell lineages derived from somites. Curr. Top. Dev. Biol. 2000;48:1–42. doi: 10.1016/s0070-2153(08)60753-x. [DOI] [PubMed] [Google Scholar]
- Cambray N, Wilson V. Axial progenitors with extensive potency are localised to the mouse chordoneural hinge. Development. 2002;129:4855–4866. doi: 10.1242/dev.129.20.4855. [DOI] [PubMed] [Google Scholar]
- Cambray N, Wilson V. Two distinct sources for a population of maturing axial progenitors. Development. 2007;134:2829–2840. doi: 10.1242/dev.02877. [DOI] [PubMed] [Google Scholar]
- Chapman DL, Papaioannou VE. Three neural tubes in mouse embryos with mutations in the T-box gene Tbx6. Nature. 1998;391:695–697. doi: 10.1038/35624. [DOI] [PubMed] [Google Scholar]
- Clements WK, Ong KG, Traver D. Zebrafish wnt3 is expressed in developing neural tissue. Dev. Dyn. 2009;238:1788–1795. doi: 10.1002/dvdy.21977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. Cell number in relation to primary pattern formation in the embryo of Xenopus laevis. II. Sequential cell recruitment, and control of the cell cycle, during mesoderm formation. J. Embryol. Exp. Morphol. 1979;53:269–289. [PubMed] [Google Scholar]
- Davis RL, Kirschner MW. The fate of cells in the tailbud of Xenopus laevis. Development. 2000;127:255–267. doi: 10.1242/dev.127.2.255. [DOI] [PubMed] [Google Scholar]
- Dequéant ML, Pourquié O. Segmental patterning of the vertebrate embryonic axis. Nat. Rev. Genet. 2008;9:370–382. doi: 10.1038/nrg2320. [DOI] [PubMed] [Google Scholar]
- Eivers E, Fuentealba LC, De Robertis EM. Integrating positional information at the level of Smad1/5/8. Curr. Opin. Genet. Dev. 2008;18:304–310. doi: 10.1016/j.gde.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galceran J, Fariñas I, Depew MJ, Clevers H, Grosschedl R. Wnt3a−/− like phenotype and limb deficiency in Lef1(−/−)Tcf1(−/−) mice. Genes Dev. 1999;13:709–717. doi: 10.1101/gad.13.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez C, Ozbudak EM, Wunderlich J, Baumann D, Lewis J, Pourquié O. Control of segment number in vertebrate embryos. Nature. 2008;454:335–339. doi: 10.1038/nature07020. [DOI] [PubMed] [Google Scholar]
- Gont LK, Steinbeisser H, Blumberg B, de Robertis EM. Tail formation as a continuation of gastrulation: the multiple cell populations of the Xenopus tailbud derive from the late blastopore lip. Development. 1993;119:991–1004. doi: 10.1242/dev.119.4.991. [DOI] [PubMed] [Google Scholar]
- Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- Griffin K, Patient R, Holder N. Analysis of FGF function in normal and no tail zebrafish embryos reveals separate mechanisms for formation of the trunk and the tail. Development. 1995;121:2983–2994. doi: 10.1242/dev.121.9.2983. [DOI] [PubMed] [Google Scholar]
- Griffin KJ, Amacher SL, Kimmel CB, Kimelman D. Molecular identification of spadetail: regulation of zebrafish trunk and tail mesoderm formation by T-box genes. Development. 1998;125:3379–3388. doi: 10.1242/dev.125.17.3379. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Itoh M, Yamanaka Y, Yamashita S, Shimizu T, Solnica-Krezel L, Hibi M, Hirano T. Zebrafish Dkk1 functions in forebrain specification and axial mesendoderm formation. Dev. Biol. 2000;217:138–152. doi: 10.1006/dbio.1999.9537. [DOI] [PubMed] [Google Scholar]
- Heasman J, Kofron M, Wylie C. Beta-catenin signaling activity dissected in the early Xenopus embryo: a novel antisense approach. Dev. Biol. 2000;222:124–134. doi: 10.1006/dbio.2000.9720. [DOI] [PubMed] [Google Scholar]
- Ho RK, Kane DA. Cell-autonomous action of zebrafish spt-1 mutation in specific mesodermal precursors. Nature. 1990;348:728–730. doi: 10.1038/348728a0. [DOI] [PubMed] [Google Scholar]
- Holley SA. The genetics and embryology of zebrafish metamerism. Dev. Dyn. 2007;236:1422–1449. doi: 10.1002/dvdy.21162. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Kodjabachian L, Rebbert ML, Rattner A, Smallwood PM, Samos CH, Nusse R, Dawid IB, Nathans J. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature. 1999;398:431–436. doi: 10.1038/18899. [DOI] [PubMed] [Google Scholar]
- Jin SW, Beis D, Mitchell T, Chen JN, Stainier DY. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development. 2005;132:5199–5209. doi: 10.1242/dev.02087. [DOI] [PubMed] [Google Scholar]
- Kanki JP, Ho RK. The development of the posterior body in zebrafish. Development. 1997;124:881–893. doi: 10.1242/dev.124.4.881. [DOI] [PubMed] [Google Scholar]
- Kardon G, Campbell JK, Tabin CJ. Local extrinsic signals determine muscle and endothelial cell fate and patterning in the vertebrate limb. Dev. Cell. 2002;3:533–545. doi: 10.1016/s1534-5807(02)00291-5. [DOI] [PubMed] [Google Scholar]
- Kawakami K. Transgenesis and gene trap methods in zebrafish by using the Tol2 transposable element. Methods Cell Biol. 2004;77:201–222. doi: 10.1016/s0091-679x(04)77011-9. [DOI] [PubMed] [Google Scholar]
- Kimelman D. Mesoderm induction: from caps to chips. Nat. Rev. Genet. 2006;7:360–372. doi: 10.1038/nrg1837. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Warga RM, Schilling TF. Origin and organization of the zebrafish fate map. Development. 1990;108:581–594. doi: 10.1242/dev.108.4.581. [DOI] [PubMed] [Google Scholar]
- Knezevic V, De Santo R, Mackem S. Continuing organizer function during chick tail development. Development. 1998;125:1791–1801. doi: 10.1242/dev.125.10.1791. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- Lekven AC, Thorpe CJ, Waxman JS, Moon RT. Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev. Cell. 2001;1:103–114. doi: 10.1016/s1534-5807(01)00007-7. [DOI] [PubMed] [Google Scholar]
- Lewis J, Hanisch A, Holder M. Notch signaling, the segmentation clock, and the patterning of vertebrate somites. J. Biol. 2009;8:44. doi: 10.1186/jbiol145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RA, Storey KG. An emerging molecular mechanism for the neural vs mesodermal cell fate decision. Cell Res. 2011;21:708–710. doi: 10.1038/cr.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Bisgrove BW, Sawyer H, Hug B, Bell B, Peters K, Grunwald DJ, Stainier DY. The zebrafish gene cloche acts upstream of a flk-1 homologue to regulate endothelial cell differentiation. Development. 1997;124:381–389. doi: 10.1242/dev.124.2.381. [DOI] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat. Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- Martin BL, Kimelman D. Regulation of canonical Wnt signaling by Brachyury is essential for posterior mesoderm formation. Dev. Cell. 2008;15:121–133. doi: 10.1016/j.devcel.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BL, Kimelman D. Wnt signaling and the evolution of embryonic posterior development. Curr. Biol. 2009;19:R215–R219. doi: 10.1016/j.cub.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BL, Kimelman D. Brachyury establishes the embryonic mesodermal progenitor niche. Genes Dev. 2010;24:2778–2783. doi: 10.1101/gad.1962910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasteels J. La formation de la queue chez les Vertebres. Ann. Soc. Royale Zool. De Belgique. 1939;70:33–51. [Google Scholar]
- Pasteels J. New observations concerning the maps of presumptive areas of the young amphibian gastrula (Amblystoma and Discoglossus) J. Exp. Zool. 1942;89:255–281. [Google Scholar]
- Pasteels J. Proliferations et croissance dans la gastrulation et la formation de la queue des Vertebres. Arch. Biol. (Liege) 1943;54:1–51. [Google Scholar]
- Petersen CP, Reddien PW. Wnt signaling and the polarity of the primary body axis. Cell. 2009;139:1056–1068. doi: 10.1016/j.cell.2009.11.035. [DOI] [PubMed] [Google Scholar]
- Pézeron G, Anselme I, Laplante M, Ellingsen S, Becker TS, Rosa FM, Charnay P, Schneider-Maunoury S, Mourrain P, Ghislain J. Duplicate sfrp1 genes in zebrafish: sfrp1a is dynamically expressed in the developing central nervous system, gut and lateral line. Gene Expr. Patterns. 2006;6:835–842. doi: 10.1016/j.modgep.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Ramel MC, Lekven AC. Repression of the vertebrate organizer by Wnt8 is mediated by Vent and Vox. Development. 2004;131:3991–4000. doi: 10.1242/dev.01277. [DOI] [PubMed] [Google Scholar]
- Row RH, Kimelman D. Bmp inhibition is necessary for postgastrulation patterning and morphogenesis of the zebrafish tailbud. Dev. Biol. 2009;329:55–63. doi: 10.1016/j.ydbio.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Row RH, Maître JL, Martin BL, Stockinger P, Heisenberg CP, Kimelman D. Completion of the epithelial to mesenchymal transition in zebrafish mesoderm requires Spadetail. Dev. Biol. 2011;354:102–110. doi: 10.1016/j.ydbio.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schier AF, Talbot WS. Molecular genetics of axis formation in zebrafish. Annu. Rev. Genet. 2005;39:561–613. doi: 10.1146/annurev.genet.37.110801.143752. [DOI] [PubMed] [Google Scholar]
- Schohl A, Fagotto F. A role for maternal beta-catenin in early mesoderm induction in Xenopus. EMBO. J. 2003;22:3303–3313. doi: 10.1093/emboj/cdg328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Merker S, van Eeden FJ, Halpern ME, Kimmel CB, Nüsslein-Volhard C. no tail (ntl) is the zebrafish homologue of the mouse T (Brachyury) gene. Development. 1994;120:1009–1015. doi: 10.1242/dev.120.4.1009. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Bae YK, Muraoka O, Hibi M. Interaction of Wnt and caudal-related genes in zebrafish posterior body formation. Dev. Biol. 2005;279:125–141. doi: 10.1016/j.ydbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Spofford WR. Observations on the posterior part of the neural plate in Ambylstoma. J. Exp. Zool. 1945;99:33–52. doi: 10.1002/jez.1401070106. [DOI] [PubMed] [Google Scholar]
- Stoick-Cooper CL, Weidinger G, Riehle KJ, Hubbert C, Major MB, Fausto N, Moon RT. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007;134:479–489. doi: 10.1242/dev.001123. [DOI] [PubMed] [Google Scholar]
- Szeto DP, Kimelman D. Combinatorial gene regulation by Bmp and Wnt in zebrafish posterior mesoderm formation. Development. 2004;131:3751–3760. doi: 10.1242/dev.01236. [DOI] [PubMed] [Google Scholar]
- Takada S, Stark KL, Shea MJ, Vassileva G, McMahon JA, McMahon AP. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev. 1994;8:174–189. doi: 10.1101/gad.8.2.174. [DOI] [PubMed] [Google Scholar]
- Takemoto T, Uchikawa M, Yoshida M, Bell DM, Lovell-Badge R, Papaioannou VE, Kondoh H. Tbx6-dependent Sox2 regulation determines neural or mesodermal fate in axial stem cells. Nature. 2011;470:394–398. doi: 10.1038/nature09729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tendeng C, Houart C. Cloning and embryonic expression of five distinct sfrp genes in the zebrafish Danio rerio. Gene Expr. Patterns. 2006;6:761–771. doi: 10.1016/j.modgep.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Thompson MA, Ransom DG, Pratt SJ, MacLennan H, Kieran MW, Detrich HW, 3rd, Vail B, Huber TL, Paw B, Brownlie AJ, et al. The cloche and spadetail genes differentially affect hematopoiesis and vasculogenesis. Dev. Biol. 1998;197:248–269. doi: 10.1006/dbio.1998.8887. [DOI] [PubMed] [Google Scholar]
- Tzouanacou E, Wegener A, Wymeersch FJ, Wilson V, Nicolas JF. Redefining the progression of lineage segregations during mammalian embryogenesis by clonal analysis. Dev. Cell. 2009;17:365–376. doi: 10.1016/j.devcel.2009.08.002. [DOI] [PubMed] [Google Scholar]
- van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- Weidinger G, Thorpe CJ, Wuennenberg-Stapleton K, Ngai J, Moon RT. The Sp1-related transcription factors sp5 and sp5-like act downstream of Wnt/beta-catenin signaling in mesoderm and neuroectoderm patterning. Curr. Biol. 2005;15:489–500. doi: 10.1016/j.cub.2005.01.041. [DOI] [PubMed] [Google Scholar]
- Wilson V, Olivera-Martinez I, Storey KG. Stem cells, signals and vertebrate body axis extension. Development. 2009;136:1591–1604. doi: 10.1242/dev.021246. [DOI] [PubMed] [Google Scholar]
- Yang J, Tan C, Darken RS, Wilson PA, Klein PS. Beta-catenin/Tcf-regulated transcription prior to the midblastula transition. Development. 2002;129:5743–5752. doi: 10.1242/dev.00150. [DOI] [PubMed] [Google Scholar]
- Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- Zhong TP. Zebrafish genetics and formation of embryonic vasculature. Curr. Top. Dev. Biol. 2005;71:53–81. doi: 10.1016/S0070-2153(05)71002-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.