Abstract
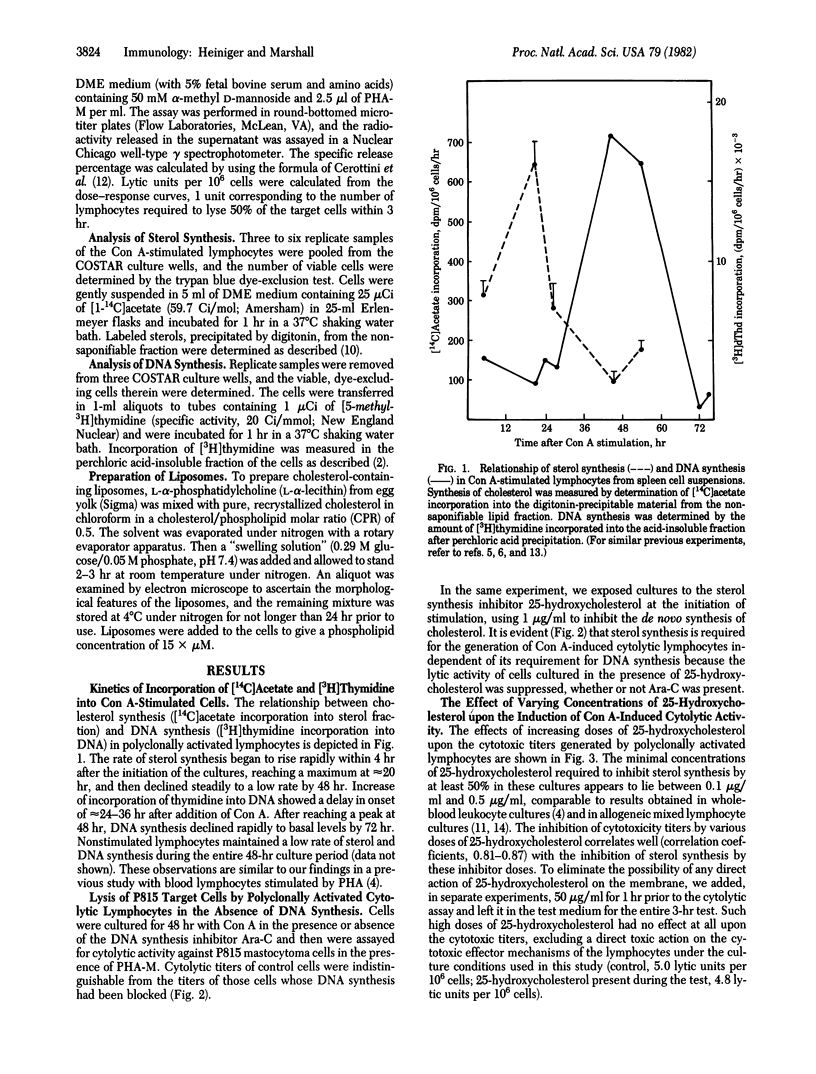
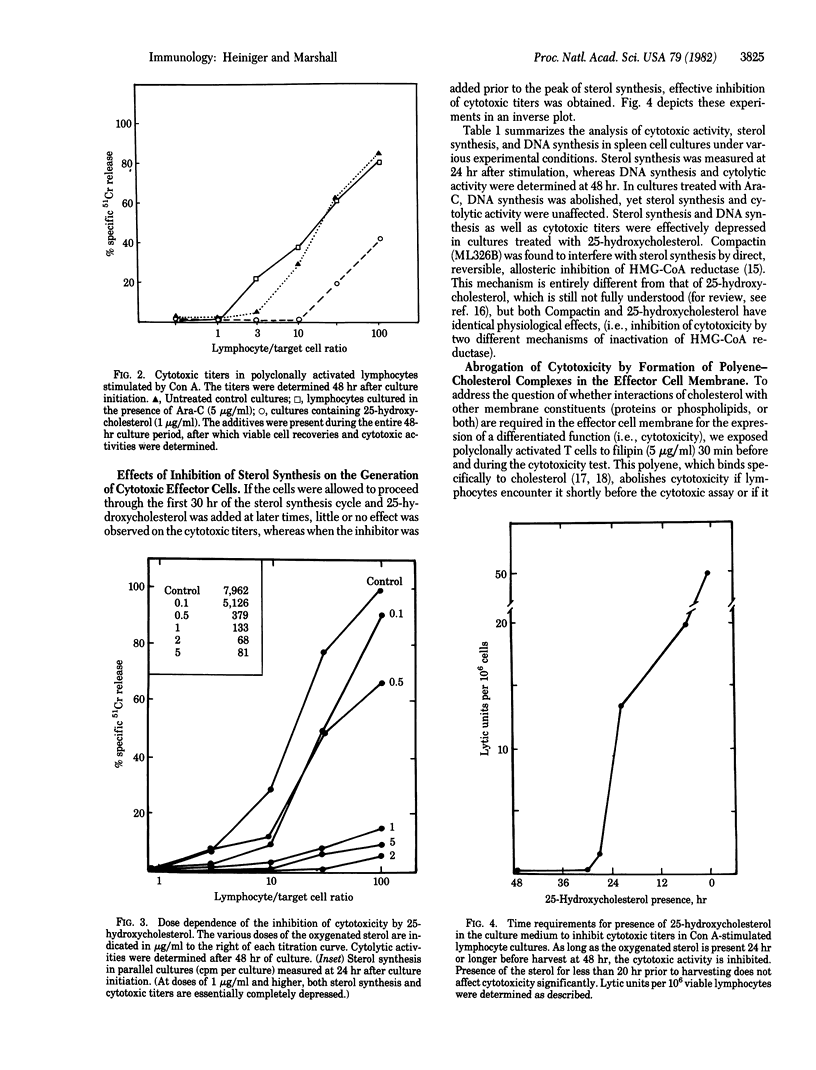
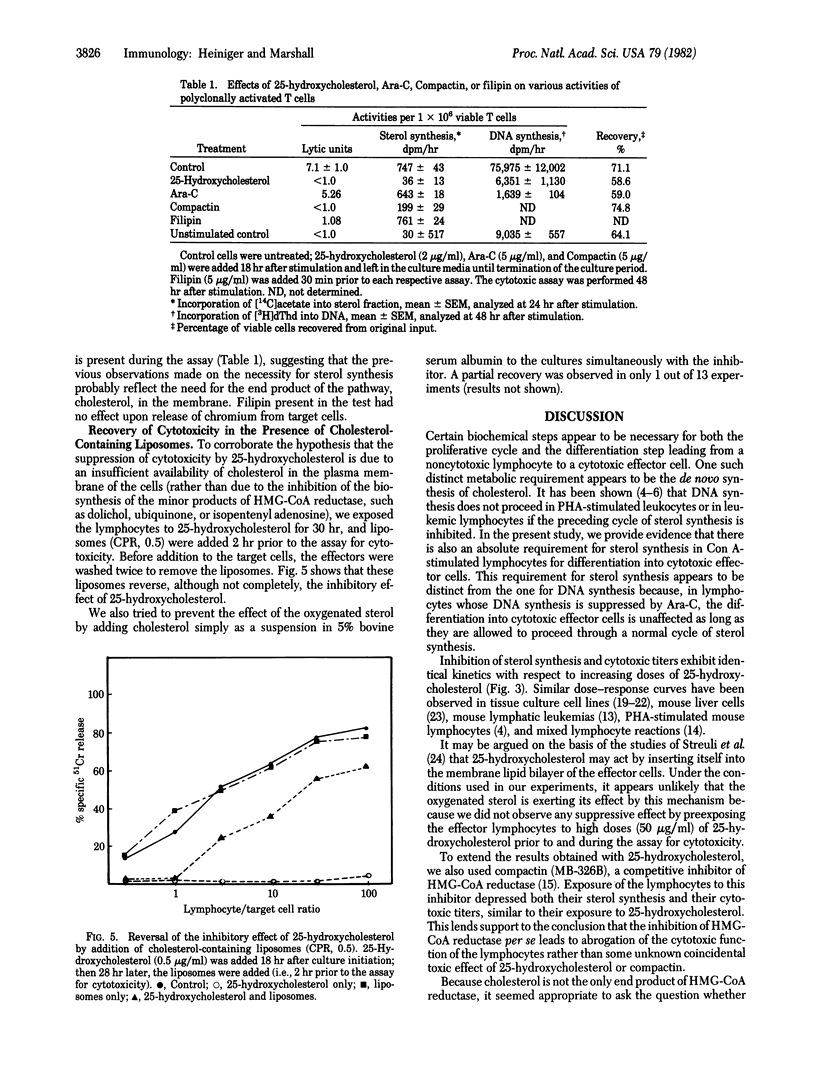
The kinetics of sterol synthesis and DNA synthesis in polyclonally activated, concanavalin A-stimulated spleen cell cultures were analyzed. Inhibition of DNA synthesis by 1-beta-D-arabinofuranosylcytosine (Ara-C) did not abrogate the formation of cytotoxic effector cells. However, inhibition of sterol synthesis by 25-hydroxycholesterol inhibited formation of cytotoxic effector cells as well as cellular proliferation. The inhibition of cytotoxicity correlated well with the dose of 25-hydroxycholesterol administered and was dependent on the time of administration. The agent had to be present when sterol synthesis occurred normally during the time lapse before DNA synthesis began. Compactin had the same effect as 25-hydroxycholesterol. The effects of inhibition of sterol biosynthesis on cytotoxicity could be counteracted by addition of cholesterol-containing liposomes. Based on these experiments, the links between proliferation and differentiation in lymphocytes are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevan M. J., Cohn M. Cytotoxic effects of antigen- and mitogen-induced T cells on various targets. J Immunol. 1975 Feb;114(2 Pt 1):559–565. [PubMed] [Google Scholar]
- Cerottini J. C., Engers H. D., Macdonald H. R., Brunner T. Generation of cytotoxic T lymphocytes in vitro. I. Response of normal and immune mouse spleen cells in mixed leukocyte cultures. J Exp Med. 1974 Sep 1;140(3):703–717. doi: 10.1084/jem.140.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. W., Cavenee W. K., Kandutsch A. A. Sterol synthesis in variant Chinese hamster lung cells selected for resistance to 25-hydroxycholesterol. Cross-resistance to 7-ketocholesterol, 20alpha-hydroxycholesterol, and serum. J Biol Chem. 1979 Feb 10;254(3):715–720. [PubMed] [Google Scholar]
- Chen H. W., Heiniger H. J., Kandutsch A. A. Relationship between sterol synthesis and DNA synthesis in phytohemagglutinin-stimulated mouse lymphocytes. Proc Natl Acad Sci U S A. 1975 May;72(5):1950–1954. doi: 10.1073/pnas.72.5.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. W., Heiniger H. J., Kandutsch A. A. Stimulation of sterol and DNA synthesis in leukemic blood cells by low concentrations of phytohemagglutinin. Exp Cell Res. 1977 Oct 15;109(2):253–262. doi: 10.1016/0014-4827(77)90004-0. [DOI] [PubMed] [Google Scholar]
- Dabrowski M. P., Peel W. E., Thomson A. E. Plasma membrane cholesterol regulates human lymphocyte cytotoxic function. Eur J Immunol. 1980 Nov;10(11):821–827. doi: 10.1002/eji.1830101105. [DOI] [PubMed] [Google Scholar]
- Endo A., Tsujita Y., Kuroda M., Tanzawa K. Inhibition of cholesterol synthesis in vitro and in vivo by ML-236A and ML-236B, competitive inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Eur J Biochem. 1977 Jul 1;77(1):31–36. doi: 10.1111/j.1432-1033.1977.tb11637.x. [DOI] [PubMed] [Google Scholar]
- Heiniger H. J., Brunner K. T., Cerottini J. C. Cholesterol is a critical cellular component for T-lymphocyte cytotoxicity. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5683–5687. doi: 10.1073/pnas.75.11.5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiniger H. J., Kandutsch A. A., Chen H. W. Depletion of L-cell sterol depresses endocytosis. Nature. 1976 Oct 7;263(5577):515–517. doi: 10.1038/263515a0. [DOI] [PubMed] [Google Scholar]
- Heiniger H. J., Taylor B. A., Hards E. J., Meier H. Heritability of the phytohemagglutinin responsiveness of lymphocytes and its relationship to leukemogenesis. Cancer Res. 1975 Mar;35(3):825–831. [PubMed] [Google Scholar]
- Heiniger H. J., Wolf J. M., Chen H. W., Meier H. A micromethod for lymphoblastic transformation of mouse lymphocytes from peripheral blood. Proc Soc Exp Biol Med. 1973 May;143(1):6–11. doi: 10.3181/00379727-143-37242. [DOI] [PubMed] [Google Scholar]
- James M. J., Kandutsch A. A. Regulation of hepatic dolichol synthesis by beta-hydroxy-beta-methylglutaryl coenzyme A reductase. J Biol Chem. 1980 Sep 25;255(18):8618–8622. [PubMed] [Google Scholar]
- Kandutsch A. A., Chen H. W. Inhibition of sterol synthesis in cultured mouse cells by 7alpha-hydroxycholesterol, 7beta-hydroxycholesterol, and 7-ketocholesterol. J Biol Chem. 1973 Dec 25;248(24):8408–8417. [PubMed] [Google Scholar]
- Kandutsch A. A., Saucier S. E. Prevention of cyclic and triton-induced increases in hydroxymethylglutaryl coenzyme A reductase and sterol synthesis by puromycin. J Biol Chem. 1969 May 10;244(9):2299–2305. [PubMed] [Google Scholar]
- Kolakowski D., Malina R. M. Spatial ability, throwing accuracy and man's hunting heritage. Nature. 1974 Oct 4;251(5474):410–412. doi: 10.1038/251410a0. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Less R. K. Primary generation of cytolytic T lymphocytes in the absence of DNA synthesis. J Exp Med. 1979 Jul 1;150(1):196–201. doi: 10.1084/jem.150.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman A. W., Spielvogel A. M., Wong R. G. Polyene antibiotic - sterol interaction. Adv Lipid Res. 1976;14:127–170. [PubMed] [Google Scholar]
- Schroeder F., Bieber L. L. Effects of Filipin and cholesterol on housefly, Musca domestica L., and wax moth, Galleria mellonella L. Chem Biol Interact. 1972 Mar;4(4):239–249. doi: 10.1016/0009-2797(72)90019-1. [DOI] [PubMed] [Google Scholar]
- Schroeder F., Holland J. F., Bieber L. L. Fluorometric investigations of the interaction of polyene antibiotics with sterols. Biochemistry. 1972 Aug 1;11(16):3105–3111. doi: 10.1021/bi00766a026. [DOI] [PubMed] [Google Scholar]
- Schroepfer G. J., Jr, Parish E. J., Chen H. W., Kandutsch A. A. Inhibition of sterol biosynthesis in L cells and mouse liver cells by 15-oxygenated sterols. J Biol Chem. 1977 Dec 25;252(24):8975–8980. [PubMed] [Google Scholar]
- Streuli R. A., Chung J., Scanu A. M., Yachnin S. Inhibition of human lymphocyte E-rosette formation by oxygenated sterols. J Immunol. 1979 Dec;123(6):2897–2902. [PubMed] [Google Scholar]
- Wang T., Sheppard J. R., Foker J. E. Rise and fall of cyclic AMP required for onset of lymphocyte DNA synthesis. Science. 1978 Jul 14;201(4351):155–157. doi: 10.1126/science.208147. [DOI] [PubMed] [Google Scholar]
- de Kruijff B., Demel R. A. Polyene antibiotic-sterol interactions in membranes of Acholeplasma laidlawii cells and lecithin liposomes. 3. Molecular structure of the polyene antibiotic-cholesterol complexes. Biochim Biophys Acta. 1974 Feb 26;339(1):57–70. doi: 10.1016/0005-2736(74)90332-0. [DOI] [PubMed] [Google Scholar]


