Abstract
Background
Reduced amplitude of the P300 event-related potential in auditory oddball tasks may characterize schizophrenia (SZ), but is also reported in bipolar disorder. Similarity of auditory processing abnormalities between these diagnoses is uncertain given the frequent combination of both psychotic and nonpsychotic patients in bipolar samples; abnormalities may be restricted to psychosis. In addition, typically only latency and amplitude of brain responses at selected sensors and singular time-points are used to characterize neural responses. Comprehensive quantification of brain activations involving both spatio-temporal and time-frequency analyses could better identify unique auditory oddball responses among patients with different psychotic disorders.
Methods
Sixty SZ, 60 bipolar I with psychosis (BPP), and 60 healthy subjects (H) were compared on neural responses during an auditory oddball task using multi-sensor electroencephalography. Principal components analysis was used to reduce multi-sensor data prior to evaluating group differences on voltage and frequency of neural responses over time.
Results
Linear discriminant analysis revealed five variables that best differentiated groups: (i) late beta activity to standard stimuli and (ii) late beta/gamma activity to targets discriminated BPP from other groups; (iii) mid-latency theta/alpha activity to standards and (iv) target-related voltage at the late N2 response discriminated both psychosis groups from H; and (v) target-related voltage during early N2 discriminated BPP from H.
Conclusions
Although the P300 significantly differentiated psychotic groups from H, it did not uniquely discriminate groups beyond the above variables. No variable uniquely discriminated SZ, perhaps indicating utility of this task for studying psychosis-associated neurophysiology generally and BPP specifically.
Keywords: ERP, auditory oddball, psychosis, time-frequency, P300, PCA
The P300 event-related potential (ERP), a positive voltage event centered on central-parietal sensors, is associated with working memory, context updating, and target recognition during visual and auditory oddball tasks (1). During auditory oddball processing, reduced amplitude of the P300 ERP has been a robust finding in the schizophrenia literature (2-8). A similar deviation from normal has been reported among bipolar disorder patients, although this is less frequently investigated (6,9-12). Supplementing its possible usefulness for understanding disease risk are reports of P300 abnormalities among clinically normal first-degree relatives of schizophrenia (13-18) and bipolar disorder patients (19).
P300 deviations among clinically normal family members of affected probands suggest that auditory oddball processing is associated with constitutional liability for illness. A twin study meta-analysis on P300 responses indicated relatively high aggregate heritability estimates among healthy participants (50-60%) (20). Evidence of genetic contribution to P300 variance further supports proposals of endophenotype status for these variables (21-22).
Endophenotypes should be relatively specific to persons with a disease and those at increased genetic risk for that disease (23). One study reported differences in P300 topographies between schizophrenia versus bipolar patients (more posterior abnormalities for schizophrenia and more anterior abnormalities for bipolar patients) (11), but most investigations focus on only one patient group, making direct comparison of P300 abnormalities difficult. P300 abnormalities may be endophenotypes for psychotic disorders generally rather than risk indicators for a specific diagnostic group (12,19,24-26). Studies of only psychotic bipolar disorder patients are uncommon (12), however, so the uniqueness of specific P300 abnormalities to a particular psychotic group is unknown. P300 abnormalities quantified using traditional ERP latency and peak amplitude measurements have been described for multiple behavioral deviations (27,28). The frequent association of traditional P300 measurements with diverse patho-physiological conditions may indicate the importance of developing alternative quantification schemes that capture this etio-pathological diversity.
A complement to traditional ERP voltage measurements is quantification of frequency variations in neural oscillations as a function of time. ERP peak voltage measurements may not capture the complexity of neural responding evident in time-frequency representations (29). Such an integrated analysis approach allows quantification of both evoked and oscillatory components of neural responses to stimulus events (30), perhaps not obvious in traditional ERP waveforms, to be evaluated simultaneously. Studying neural responses during auditory oddball processing using both voltage and frequency domain approaches may help elucidate differences between nosologically similar diagnostic groups. The current study provides direct comparison of SZ and BPP on common ERP voltage and time-frequency measurements of the same responses, and may help illuminate the most useful approach for discriminating between diagnostic groups and identifying endophenotypes for these disorders.
Frequency domain measures have shown usefulness for clarifying psychosis-related neural deviations during auditory processing (31-33). Hamm et al. (32) found an accentuation of late (250 ms post-stimulus) beta-range activity peculiar to BPP among psychosis patients. Increased frontoparietal beta band activity has been reported in BP patients as compared to unipolar patients during an implicit emotion task (34). Ozerdem et al. (35) described early stimulus-processing-related beta activity increases in manic BP as compared to healthy subjects during a visual oddball task. These effects hint at differences in stimulus processing between SZ and BPP that could reveal critical variations in neuropathology. Synchronized beta-range activity is required for distant cortico-cortical communication (36), and is supported by the very cortical circuitry that is purportedly hypo- functional in SZ (37). This difference may also reveal a neurophysiological correlate of the enhanced sustained attention/vigilance that at least partially characterizes BPP (38).
The present investigation of the Bipolar and Schizophrenia Network on Intermediate Phenotypes (B-SNIP) (22) sample compared ERP amplitude measurements and event-related oscillations across a broad frequency range during an auditory oddball task. Neural activations from whole-head EEG recordings during stimulus processing were compared between healthy (H), schizophrenia (SZ), and bipolar disorder with psychosis (BPP) participants, using a comprehensive analysis approach to describe their shared and unique neural auditory processing characteristics. The aim of this study was to utilize large matched samples of SZ and BPP with an analysis approach that can identify neural processing deviations either shared between or unique to each psychotic disorder. It was predicted that [1] time-frequency measures would provide useful information beyond that of traditional ERP measures, [2] the P300 response would not discriminate psychosis groups, and [3] increased late beta activity would characterize BPP.
Methods and Materials
Participants
As part of the large, multi-site data collection project (B-SNIP), subjects were recruited, interviewed, and tested at four sites: University of Illinois-Chicago, Yale University/IOL (Hartford, CT), University of Texas Southwestern (Dallas, TX), and University of Maryland (Baltimore, MD). Clinically stable participants absent of symptomatic evidence of acute illness were recruited via community advertisements, linked community facilities and programs, and local NAMI-type organizations. All subjects provided written informed consent prior to participation. This study was approved by IRBs at each data collection and analysis site.
Three age- and gender-matched groups were constructed blind to brain activity measurements: 60 SZ (30 female; mean age = 36.3; range 19-55); 60 BPP (30 female; mean age = 36.3; range 18-53); and 60 healthy persons (30 female; mean age = 36.2; range 18-54; recruited via random digit dialing or community advertisements). Groups were also matched on proportion of subjects from each data collection site (Table 1). Data from a different paradigm for these same participants were also used in a previous manuscript (32). All but five SZ and nine BPP were taking psychotropic medications (Table S1 in the Supplement).
Table 1.
Demographic and clinical statistics.
| Bipolar I Psychosis | Schizophrenia | Healthy | Significance Test | |
|---|---|---|---|---|
| Subjects | 60 | 60 | 60 | |
| From each site | ||||
| UICa | 22 | 21 | 21 | X2(6)=1.28 |
| YU | 16 | 14 | 17 | |
| UTS | 12 | 13 | 14 | |
| UM | 10 | 12 | 8 | |
| Percent female | 50% | 50% | 50% | |
| Age | 36.3 (18-53) | 36.6 (19-55) | 36.2 (18-54) | F(2,177)=.026 |
| Trials accepted | ||||
| Standards | 525 (374-567) | 530 (406-567) | 540 (342-567) | F(2,177)=1.53 |
| Targets | 93 (69-100) | 94 (79-100) | 96 (64-100) | F(2,177)=2.26 |
| Percent Correct | 93.7% (SD=10.8) | 87.6% (SD=19.2) | 93.6% (SD=11.9) | F(2,138)=2.71 |
| Response Latency (ms) | 423.4 (SD=80.5) | 437.8 (SD=89.4) | 418.1 (SD=83.2) | F(2,138)=.681 |
| Clinical Scales | ||||
| GAF | 59.1 (30-82) | 47.8 (30-90) | – | t(112)=4.73*** |
| PANSS-pos | 13.6 (7-24) | 17.4 (8-29) | – | t(112)=4.47*** |
| PANSS-neg | 11.9 (7-30) | 17.2 (7-32) | – | t(112)=5.45*** |
| PANSS-gen | 29.1 (18-56) | 33.3 (17-52) | – | t(112)=2.65** |
| MADRS | 11.3 (0-43) | 10.7 (0-30) | – | t(112)=.327 |
| YMS | 5.15 (0-21) | 4.47 (0-19) | – | t(112)=.714 |
UIC, University of Illinois, Chicago; YU, Yale University/IOL; UTS, University of Texas Southwestern; UM, University of Maryland; GAF, Global Assessment of Functioning; PANSS, Positive and Negative Symptom Scale (positive, negative, general) ; MADRS, Montgomery-Åsberg Depression Rating Scale; YMS, Young Mania Scale
*p<.05
p<.01
p<.001
Medical history, structured clinical interview for DSM-IV diagnosis (SCID patient or nonpatient version as appropriate) (39), Positive and Negative Symptom Scale (PANSS) (40), Young Mania Rating Scale (40), Montgomery-Asberg Depression Rating Scale (MADRS) (41), and Global Assessment of Functioning scale (GAF; axis V of Diagnostic and Statistical Manual of Mental Disorders IV [DSM-IV]) were acquired by trained Masters or Doctoral-level clinicians. Presence of serious medical, neuro-opthalmological, or neurological illness (e.g., cancer, seizure disorders, coarse brain-disease), mental retardation, head trauma with >30 minutes unconsciousness, current substance use ascertained by history as well as urine drug screens on the day of testing (8 panel screen for amphetamines, barbiturates, cocaine, methadone, opiates, cannabinoids, propoxyphene and TCAs), abuse in the past three months, and dependence within 6 months or extensive history of drug dependence (DSM-IV) were criteria for exclusion. Healthy persons were free of any lifetime psychotic or mood disorder and a family history of psychotic or BP disorders in first-degree relatives according to Family History Research Diagnostic Criteria (42). All clinical information and diagnoses for each subject were reviewed and confirmed in a best estimate diagnostic meeting including a senior psychiatrist/psychologist and the clinician who conducted the structured interviews.
Stimuli and Procedures
Recording conditions were equivalent and stimulus presentation and recording equipment identical across sites. Seated in a sound and electrically shielded booth (ambient sound = 61-63 dB; luminance = 0.11-0.12 foot-candles) subjects listened to tones delivered by two 8-ohm speakers located 50 cm in front of them. Stimuli were 567 standard (1500Hz) and 100 target (1000Hz) tones presented in pseudorandom order (1300 ms inter-trial interval). Subjects were asked to press a button when a target was detected. Subjects refrained from smoking 1 hour prior to testing.
EEG recording
EEG was continuously recorded from 64 Ag/AgCl sensors (impedance <5 KΩ; Quik-Cap, Compumedrics Neuroscan, El Paso, TX), positioned according to the standard 10-10 EEG system plus mastoids and CP1/2 locations to provide greater sampling below the cantho-meatal line, with nose reference and forehead ground. Recordings were amplified (12,500x) and digitized (1000Hz) using Neuroscan Acquire and Synamps2 recording systems (Compumedrics Neuroscan, El Paso, TX).
EEG processing
Raw EEG data were inspected for bad sensors and artifacts. Bad sensors were interpolated (<5% for any subject) using spherical spline interpolation (BESA 5.3; MEGIS Software, Grafelfing, Germany). Blink and cardiac artifacts were removed using Independent Components Analysis (EEGLAB 6.0) (43). Data were segmented into 1000 ms epochs from 250 ms before to 750 ms after stimulus onset and digitally bandpass filtered from 0.5 – 55 Hz (zero-phase filter; rolloff: 6 and 48 dB/octave, respectively). The 250 ms pre-stimulus period was used for baseline adjustment. Epochs containing activity greater than 75 μV at any sensor were not included; at least 60% of trials were accepted for all subjects.
PCA data reduction
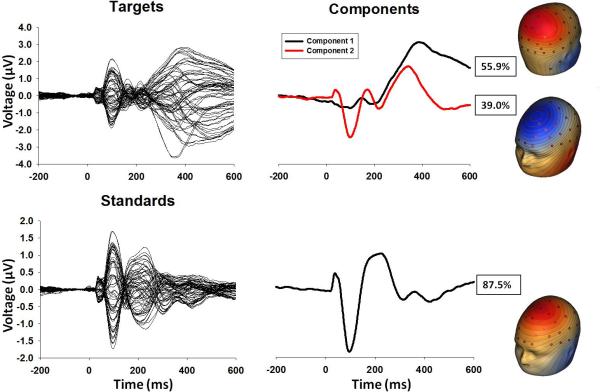
To use EEG data recorded from every sensor and, thus, to most accurately and comprehensively capture the spatial topography of evoked brain responses across time, spatial principal components analysis (PCA) on grand average data (44-45) was implemented using BESA (MEGIS Software, Grafelfing, Germany) and Matlab (The Mathworks, Matick, MA) to identify spatial patterns in the EEG topography. Target and standard conditions were averaged separately across groups. For each condition, PCA with promax (oblique) vector rotation and Kaiser normalization (46) was calculated on a 64×64 sensor covariance matrix (1000 time-points as observations). Scree tests (47) identified 2 components for the target condition (accounting for 55.9 and 39.0 percent of the variance) and 1 component (87.5 percent of the variance) for the standard condition (Figure 1). Spatial distributions of these components and amount of variance accounted for in the grand average were nearly identical across groups (Figure S1 in the Supplement, all correlations between groups greater than r=.975). Each set of component weights was multiplied by each subject's grand average data, summed across sensors, and divided by the plus sum of the component weights, reducing waveforms from one for each sensor to one waveform per component for each subject for targets and standards (Figure 2).
Figure 1.
Grand averaged butterfly plots and PCA factor waveforms and topographies for target and standard conditions.
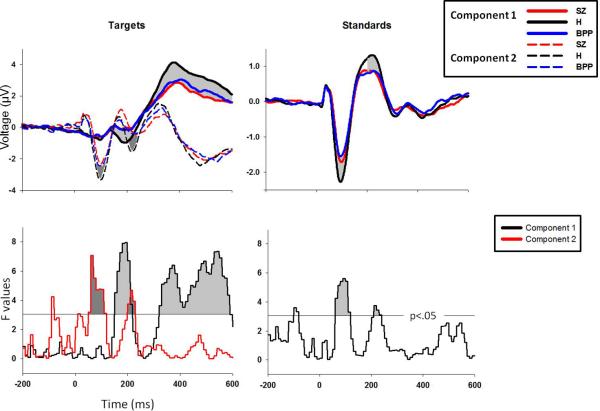
Figure 2.
PCA weighted waveforms for each group for targets and standards. The lower plots display F values for one-way ANOVAs for each 10ms time-bin. Time-bin clusters were significant at p<.01 if three consecutive time-bins were significant at p<.05 (F=3.05, indicated by the horizontal gray line).
ERP time-course analyses
For each condition and subject, component waveforms from -200 ms to 600 ms were grouped into 80 separate 10 ms bins and averaged within each bin. For each bin, a one-way ANOVA was calculated to determine group differences in waveform amplitude. To control for family-wise error due to multiple comparisons, a clustering method was implemented using Monte Carlo simulations calculated across time-bins using AlphaSim (48). In order to maintain a family-wise alpha of .01, three sequential time-bins were required to be significant at alpha<.05 (Figure 2).
Time-frequency analyses
For each subject, separately for each component waveform, oscillatory power for frequencies from 3-52 Hz was calculated with 1 Hz frequency resolution using a modified Morlet wavelet transformation every 4 ms on the grand averaged ERPs. In order to balance the tradeoff between temporal resolution at low frequencies and stability of measurement at higher frequencies (49), wavelet length increased linearly from 1 cycle at 3 Hz to 8 cycles at 52 Hz (Figure 3). Group comparisons for each condition and component were calculated using point-by-point one-way ANOVAs across the time-frequency matrix. Again, a clustering method was implemented to control for family-wise error. Utilizing the same procedure as for the ERP analyses but also accounting for addition of frequency information to the number of comparisons, family-wise alpha at .01 was maintained with clusters of 5 or more sequential time-points significant at p<.01. To control for aberrant significant effects due to a small number of large power values, F-value distributions were created using a bootstrap procedure. For each condition and factor, the same one-way ANOVAs were run 5000 times with group membership randomly shuffled at each step (sampling with replacement). Probability estimates of observed F-values were calculated as the proportion of randomly generated F-values greater than the actual estimate. Possible site effects were examined by adding site as a variable to the group comparisons; there were no significant site by group interactions on any of the variables significantly distinguishing groups (Figures S2 and S3 in the Supplement; Table 1 for number of subjects per site).
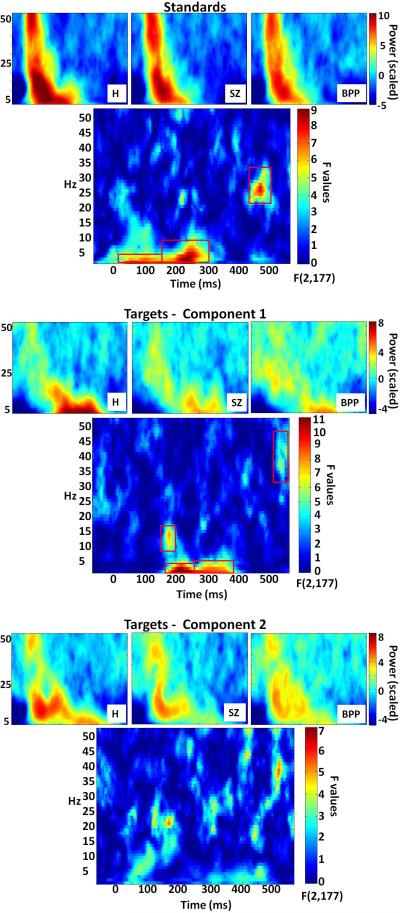
Figure 3.
Time-frequency plots by group and point-by-point F value plots with significant clusters (indicated by red boxes) for each factor and condition. For display purposes, group time-frequency plots are rescaled by each frequency's mean across all groups and time-points
Post-hoc discriminant analyses
To efficiently summarize variables uniquely differentiating groups, values from significant time-bin and time-frequency clusters were averaged within adjacent clusters for each subject and submitted to a linear discriminant analysis with group as the dependent variable (H, SZ, BPP). Variables that minimized the overall Wilks’ lambda and had individual multiple F-statistics significant at p<.05 were entered in a stepwise fashion (50), leaving a parsimonious selection of neurophysiological measures. This statistical analysis was included specifically to describe the variables most important to group separations rather than as an attempt to classify observations. The latter approach will be more appropriate when we can add additional tasks and observations to our data collection efforts.
Results
Behavioral
For the UTS site response data was not available, so behavioral information was calculated on the remaining 80% of subjects. There were no differences between groups on either response latency or percent correct (Table 1).
ERP time-bins
One-way ANOVAs for each condition and spatial factor revealed 7 clusters that differentiated groups (Figure 2). Given a bimodal F-value distribution for the P300 cluster (Figure 2), it was split into early and late sections at the point of rarity (410ms). For the following comparisons, patient groups did not significantly differ from each other.
For targets component 1, from 150 to 210 ms (early N2 time range), peaking at 190 ms, F(2,177)=7.95, p<.001, BPP showed significantly less voltage change from baseline than H, t(118)=2.09, p<.05.
For targets component 1, from 320 to 400 ms (early P3b time range), peaking at 370 ms, F(2,177)=6.39, p=.002, both BPP, t(118)=2.23, p=.03, and SZ, t(118)=2.77, p=.006, showed less change from baseline than H.
For targets component 1, from 410 to 580 ms (late P3b time range), peaking at 540 ms, F(2,177)=7.34, p<.001, both BPP, t(118)=2.19, p=.03, and SZ, t(118)=3.17, p=.002, showed less change from baseline than H.
For targets component 2, from 50 to 110 ms (N1 time range), peaking at 60 ms, F(2,177)=7.08, p=.001, both BPP, t(118)=2.43, p=.02, and SZ, t(118)=3.95, p<.001, showed less change from baseline than H.
For targets component 2, from 200 to 220 ms (late N2 time range), peaking at 210 ms, F(2,177)=4.69, p=.01, both BPP, t(118)=2.89, p<.01, and SZ, t(118)=3.5, p<.001, showed less change from baseline than H.
For standards from 60 to 110 ms (N1 time range), peaking at 100 ms, F(2,177)=5.61, p=.004, both BPP, t(118)=3.14, p=.002, and SZ, t(118)=2.78, p=.006, showed less change from baseline than H.
For standards from 210 to 230 ms (P2 time range), peaking at 210 ms F(2,177)=3.73, p<.05, both BPP, t(118)=2.27, p=.03, and SZ, t(118)=2.39, p=.02, showed less change from baseline than H.
Time-frequency
Point-by-point one-way ANOVAs on averaged time-frequency plots revealed 7 clusters which significantly differentiated groups (red outlines in Figure 3). For the purposes of statistical and post-hoc analyses, clusters that spanned large amounts of time and showed evidence for multiple centers of convergence were split at the time-point with the lowest F-value.
For targets component 1, from 165 to 195 ms, peaking at 180 ms and 15 Hz (early beta), F(2,177)=7.76, p<.001, both SZ, t(118)=3.68, p<.001, and BPP, t(118)=2.89, p=.005, showed less power than H.
For targets component 1, from 165 to 265 ms, peaking at 220 ms and 3 Hz (early theta/alpha), F(2,177)=12.34, p<.001, both SZ, t(118)=3.83, p<.001, and BPP, t(118)=3.82, p<.001, showed less power than H.
For targets component 1, from 265 to 400 ms, peaking at 275 ms and 3 Hz (mid-range theta/alpha), F(2,177)=9.41, p<.001, both SZ, t(118)=3.95, p<.001, and BPP, t(118)=3.27, p=.001, showed less power than H.
For targets component 1, from 530-560 ms, peaking at 535 ms and 37 Hz (late beta/gamma), F(2,177)=5.84, p=.003, BPP showed more power than both SZ, t(118)=2.72, p<.01, and H, t(118)=3.73, p<.001.
For standards, from 20 to 150 ms, peaking at 60 ms and 3 Hz (early theta), F(2,177)=7.79, p<.001, both SZ, t(118)=2.98, p=.004, and BPP, t(118)=3.51, p<.001, showed less power than H.
For standards, from 150 to 305 ms, peaking at 230 ms and 4 Hz (mid-range theta/alpha), F(2,177)=9.18, p<.001, both SZ, t(118) =3.58, p<.001, and BPP, t(118)=3.74, p<.001, showed less power than H.
For standards, from 440 to 490 ms, peaking at 470 ms and 27 Hz (late beta), F(2,177)=7.78, p<.001, BPP showed more power than both SZ, t(118)=3.45, p<.001, and H, t(118)=3.51, p<.001.
Discriminant Analyses
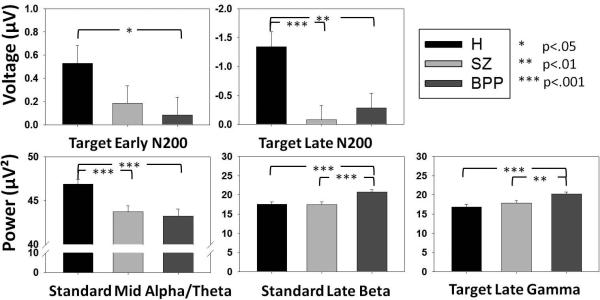
All 14 variables that differentiated groups were used in a linear discriminant analysis, resulting in five variables that best discriminated groups, 2 ERP time-bins and 3 time-frequency clusters (Table 2):
The target late N2 (component 2) significantly discriminated patient groups from H.
The target early N2 (component 1) significantly discriminated BPP, but not SZ, from H.
Late beta power to standards significantly discriminated BPP from both SZ and H.
Late beta-gamma power to targets significantly discriminated BPP from both SZ and H.
Mid-range theta-alpha power to standards significantly discriminated patient groups from H.
Table 2.
Results from linear discriminant analysis
| Measure | Wilk's Lambda | F-value* | H vs. SZ | H vs. BPP | SZ vs. BPP |
|---|---|---|---|---|---|
| Standard Late Beta | 0.821 | 9.13 | t(118)=.08 p=.94 |
t(118)=3.51
p<.001 |
t(118)=3.45
p<.001 |
| Target Late Gamma | 0.775 | 7.94 | t(118)=1.14 p=.26 |
t(118)=3.73
p<.001 |
t(118)=2.72
p<.01 |
| Standard Mid Alpha/Theta | 0.741 | 7.03 |
t(118)=3.58
p<.001 |
t(118)=3.74
p<.001 |
t(118)=.47 p=.63 |
| Target Late N2 | 0.704 | 6.63 |
t(118)=3.50
p<.001 |
t(118)=2.89
p<.01 |
t(118)=.59 p=.56 |
| Target Early N2 | 0.667 | 6.43 | t(118)=1.62 p=.11 |
t(118)=2.09
p<.05 |
t(118)=.47 p=.64 |
All F values are significant at p<.001
Clinical Correlations
Separately for both patient groups, all available clinical variables (Table 1) were submitted to Pearson correlations with the 5 discriminating EEG variables. The only significant correlation was late beta/gamma power to targets with MADRS (Montgomery-Asberg Depression Rating Scale) scores for the BPP group (r=.276, p<.05).
Discussion
The purpose of this study was to use voltage and time-frequency quantifications of auditory oddball processing to characterize neurophysiologically distinct measures of similarities and differences among SZ and BPP. Out of 14 variables showing between-groups effects, five efficiently summarized group discrimination variance, with all of these measures providing important information for understanding the neurophysiological correlates of psychosis generally and (perhaps) BPP specifically. Variables best differentiating psychosis from healthy subjects were mid-latency theta/alpha activity to standards and the target-specific late N2 ERP. Late beta/gamma activity to standard and target stimuli best differentiated BPP from SZ and H. The target-specific early N2 ERP, with a topography co-varying with the P3b, discriminated BPP from H. No variable specifically discriminated SZ from the other two groups.
Theta/alpha band activity to standards and the late N2 to targets differentiated psychosis groups from healthy subjects. Theta/alpha activity is associated with selective attention and working memory processes among healthy persons (51). Reduced low-frequency power to both targets and standards has been shown for SZ (52-55); this effect had not been previously described for BPP, perhaps because large samples of bipolar patients with psychosis are uncommonly studied. Abnormalities in low-frequency response may serve as a good indicator of psychosis; indeed, reduced delta and theta synchrony may be a better discriminator of SZ and H than P3b amplitude (31). Task-related theta and alpha abnormalities have been reported for SZ and their first-degree relatives, suggesting that low-frequency processing deficits are indicative of genetic risk for psychosis (56).
Amplitude reductions of the target-related N2 ERP, associated with initial stimulus categorization primarily for deviant or target stimuli (57-58), have been shown for SZ (59-60), but have not been extensively studied in BPP. Using a passive oddball paradigm, Kaur et al. (61) reported amplitude reductions of N2 and P3 among both BPP and SZ, but no difference between patient groups. Distinct early and late components in the N2 range were found in the present study (see Patel and Azzam, 2005 (58) for a discussion of N2 subcomponents in oddball tasks), with the earlier N2 (differentiating BPP and H) topographically associated with P3b and the later N2 (differentiating psychosis from H) topographically associated with N1 and P3a (Figure 2). Similar to findings of Kaur and colleagues (61), neither of these N2s discriminated between SZ and BPP, suggesting that neurophysiological processing deviations captured by the target-specific N2 (61,62) are shared between psychotic disorders.
Augmented late responses in higher frequencies (beta and gamma) discriminated BPP from both SZ and H. In healthy subjects, beta- and gamma-band oscillations are associated with salience processing and perceptual encoding, respectively (63-64). Increased beta-band response in a visual oddball task, an abnormality somewhat normalized by valproate (35), is associated with bipolar I disorder. Hamm et al. (32) reported this same increased beta-band response following auditory paired stimuli processing in this sample. While the paired stimuli paradigm requires no response selection, it does rely on top-down processing for contextual stimulus salience evaluation (65). Beta oscillations are critical for long-range cortico-cortical communication (36) and rely on GABAergic NMDA networks (66-68). Widespread disruption of NMDA receptor function has been associated with both SZ and bipolar disorder (69), particularly affecting excitatory signaling of GABAergic interneurons (51). GABAergic activity is modulated by dopamine, and increased dopamine activity is associated with both psychosis in general and with mania (70-72), which may facilitate cyclical down-regulation of dopamine receptors during depressive phases (73). Perhaps interactions between cyclic dopamine levels and disrupted GABAergic neurotransmission lead to heightened beta- and gamma-band responses when an interaction between long-range communication and local processing is required. It is possible that the increased late beta response in BPP indicates a heightened salience imparted to the normally low salience standard stimuli and that increased beta/gamma response to targets indicates abnormally prolonged stimulus evaluation after response selection has occurred. In the current study, accentuated late beta/gamma activity correlated with MADRS scores, indicating that this response may be linked to the emotion regulation abnormalities more endemic to BPP. Due to its uniqueness to BPP, augmented beta/gamma-band response may afford an interesting avenue as a potential endophenotype for BPP, although further work incorporating familial risk factors will be necessary to support this assertion.
Consistent with previous work (12,19,24-26), the P3b did not differentiate SZ and BPP. As indicated by target waveforms (Figure 2), earlier target-related ERPs (early and late N2s) share the same component topographies and may capture some of the variance associated with the P3b and P3a, respectively; both of these ERPs were smaller in amplitude among SZ and BP. Indeed, SZ and BPP had similar decreases in P3b amplitude (Figure 2), suggesting that this target-specific response captured psychosis variance (24). A recent meta-analysis of structural MRI studies on SZ and bipolar I disorder found overlapping areas of grey-matter volume loss for both groups in the cingulate cortex, a structure likely contributing nontrivial variance to P3 generation (1,74). However, P3b amplitude reductions are associated with multiple other pathologies (27-28,75), so this ERP measure's utility for capturing disease-specific variance is at present uncertain.
A genetic basis for reduced P3 amplitude as an endophenotype for psychosis risk is supported by shared variation between P3 abnormalities and DISC1, a gene associated with psychosis risk (both SZ and bipolar I disorder) and memory impairments (76-78). DISC1 is translated into a protein primarily localizing to mitochondria in neurons and glial cells (78) and has functional consequences for glutamatergic activity (79), neurite outgrowth (80), and early development of the major dopamine systems associated with both schizophrenia and P3 generation(81-82).
There were a few limitations to this study. All but 14 psychosis patients were taking psychotropic medications, so possible effects of antipsychotics on neurophysiological measures were not straightforwardly addressable in this sample. Antipsychotic medication may affect group differences (83-84). In most cases, however, these medications alleviate abnormal responses in psychosis patients, suggesting that the group differences observed here are robust to medication status and more inherent to underlying etiological differences between groups. Duration of illness also was not equated between-groups, so it is unclear whether the observed effects would change over the course of illness. Repetition of this study in first-episode, unmedicated psychotic patients would be a useful addition to the literature.
This study differs in a number of ways from previous literature on auditory oddball response in clinical populations. Rather than selecting a single sensor as representative of the ERP response, we used spatial PCA to derive a data-driven integrated neural response incorporating information from all sensors across the head. Additionally, rather than comparing data only at waveform peaks or time-frequency intensities, a binning and clustering approach was used to identify areas of maximal variance across the entire time range of neural response generation to stimulus events. The results suggest that time-frequency measures complement ERP voltages for characterizing neural abnormalities in psychosis groups. Incorporating information from the entire neural processing range can illuminate deviations not readily apparent from studying specific, predefined frequency bands or time ranges. The utility of the auditory oddball task for identifying BPP-specific neural processing deviations (late beta/gamma response) in particular is a significant new revelation for this research domain. Our more comprehensive analysis strategy was critical for yielding a series of new discoveries. Future work implementing this approach to investigate neural correlates and genetic/familial risk factors associated with auditory processing deviations in psychosis will open particularly exciting new avenues for research.
Supplementary Material
Figure 4.
Group averages and standard errors for five main group discriminators determined in the linear discriminant analysis. P-values are based on two-tailed independent samples t-tests (df=118).
Acknowledgements
Funding for this study was provided by NIH Grants MH077945, MH077862, MH077851, MH078113, and MH085485.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
Dr. Sweeney reports consulting for Takeda, Pfizer, and Lilly, and receiving a research grant from Janssen. Dr. Tamminga discloses the following financial interests and associations: Ad Hoc Consultant to Acadia Pharmaceuticals, Astellas, Eli Lilly Pharmaceuticals, Lundbeck, Inc, PureTech Ventures, Sunovion, Merck; Deputy Editor for American Psychiatric Association; Expert Witness for Finnegan Henderson Farabow Garrett & Dunner, LLP; Council Member and Unpaid Volunteer for The Brain & Behavior Foundation, the Institute of Medicine, and the National Alliance on Mental Illness; Organizer and Unpaid Volunteer for International Congress on Schizophrenia Research; Council Member of National Institute of Mental Health; and, Advisory Board, Drug Development for Intra-cellular Therapies (ITI, Inc.). The remaining authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Linden DE. The p300: where in the brain is it produced and what does it tell us? Neuroscientist. 2005;11(6):563–576. doi: 10.1177/1073858405280524. [DOI] [PubMed] [Google Scholar]
- 2.Roth WT, Cannon EH. Some features of the auditory evoked response in schizophrenics. Arch Gen Psychiatry. 1972;27(4):466–471. doi: 10.1001/archpsyc.1972.01750280034007. [DOI] [PubMed] [Google Scholar]
- 3.Bramon E, Rabe-Hesketh S, Sham P, Murray RM, Frangou S. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophr Res. 2004;70(2-3):315–329. doi: 10.1016/j.schres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Ford JM, White P, Lim KO, Pfefferbaum A. Schizophrenics have fewer and smaller P300s: a single-trial analysis. Biol Psychiatry. 1994;35(2):96–103. doi: 10.1016/0006-3223(94)91198-3. [DOI] [PubMed] [Google Scholar]
- 5.McCarley RW, Shenton ME, O'Donnell BF, Faux SF, Kikinis R, Nestor PG, et al. Auditory P300 abnormalities and left posterior superior temporal gyrus volume reduction in schizophrenia. Arch Gen Psychiatry. 1993;50(3):190–197. doi: 10.1001/archpsyc.1993.01820150036003. [DOI] [PubMed] [Google Scholar]
- 6.O'Donnell BF, Vohs JL, Hetrick WP, Carroll CA, Shekhar A. Auditory event-related potential abnormalities in bipolar disorder and schizophrenia. Int J Psychophysiol. 2004;53(1):45–55. doi: 10.1016/j.ijpsycho.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Salisbury DF, Shenton ME, Sherwood AR, Fischer IA, Yurgelun-Todd DA, Tohen M, et al. First-episode schizophrenic psychosis differs from first-episode affective psychosis and controls in P300 amplitude over left temporal lobe. Arch Gen Psychiatry. 1998;55(2):173–180. doi: 10.1001/archpsyc.55.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathalon DH, Hoffman RE, Watson TD, Miller RM, Roach BJ, Ford JM. Neurophysiological Distinction between Schizophrenia and Schizoaffective Disorder. Front Hum Neurosci. 2010;3:70. doi: 10.3389/neuro.09.070.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fridberg DJ, Hetrick WP, Brenner CA, Shekhar A, Steffen AN, Malloy FW, et al. Relationships between auditory event-related potentials and mood state, medication, and comorbid psychiatric illness in patients with bipolar disorder. Bipolar Disord. 2009;11(8):857–866. doi: 10.1111/j.1399-5618.2009.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muir WJ, St Clair DM, Blackwood DH. Long-latency auditory event-related potentials in schizophrenia and in bipolar and unipolar affective disorder. Psychol Med. 1991;21(4):867–879. doi: 10.1017/s003329170002986x. [DOI] [PubMed] [Google Scholar]
- 11.Salisbury DF, Shenton ME, McCarley RW. P300 topography differs in schizophrenia and manic psychosis. Biol Psychiatry. 1999;45(1):98–106. doi: 10.1016/s0006-3223(98)00208-x. [DOI] [PubMed] [Google Scholar]
- 12.Hall MH, Schulze K, Rijsdijk F, Kalidindi S, McDonald C, Bramon E, et al. Are auditory P300 and duration MMN heritable and putative endophenotypes of psychotic bipolar disorder? A Maudsley Bipolar Twin and Family Study. Psychol Med. 2009;39(8):1277–1287. doi: 10.1017/S0033291709005261. [DOI] [PubMed] [Google Scholar]
- 13.Frangou S, Sharma T, Alarcon G, Sigmudsson T, Takei N, Binnie C, et al. The Maudsley Family Study, II: Endogenous event-related potentials in familial schizophrenia. Schizophr Res. 1997;23(1):45–53. doi: 10.1016/S0920-9964(96)00089-8. [DOI] [PubMed] [Google Scholar]
- 14.Karoumi B, Laurent A, Rosenfeld F, Rochet T, Brunon AM, Dalery J, et al. Alteration of event related potentials in siblings discordant for schizophrenia. Schizophr Res. 2000;41(2):325–334. doi: 10.1016/s0920-9964(99)00062-6. [DOI] [PubMed] [Google Scholar]
- 15.Kidogami Y, Yoneda H, Asaba H, Sakai T. P300 in first degree relatives of schizophrenics. Schizophr Res. 1991;6(1):9–13. doi: 10.1016/0920-9964(91)90015-j. [DOI] [PubMed] [Google Scholar]
- 16.Roxborough H, Muir WJ, Blackwood DH, Walker MT, Blackburn IM. Neuropsychological and P300 abnormalities in schizophrenics and their relatives. Psychol Med. 1993;23(2):305–314. doi: 10.1017/s0033291700028385. [DOI] [PubMed] [Google Scholar]
- 17.Turetsky BI, Cannon TD, Gur RE. P300 subcomponent abnormalities in schizophrenia: III. Deficits In unaffected siblings of schizophrenic probands. Biol Psychiatry. 2000;47(5):380–390. doi: 10.1016/s0006-3223(99)00290-5. [DOI] [PubMed] [Google Scholar]
- 18.Winterer G, Egan MF, Raedler T, Sanchez C, Jones DW, Coppola R, Weinberger DR. P300 and genetic risk for schizophrenia. Arch Gen Psychiatry. 2003;60(11):1158–67. doi: 10.1001/archpsyc.60.11.1158. [DOI] [PubMed] [Google Scholar]
- 19.Schulze KK, Hall MH, McDonald C, Marshall N, Walshe M, Murray RM, et al. Auditory P300 in patients with bipolar disorder and their unaffected relatives. Bipolar Disord. 2008;10(3):377–386. doi: 10.1111/j.1399-5618.2007.00527.x. [DOI] [PubMed] [Google Scholar]
- 20.van Beijsterveldt CE, van Baal GC, Molenaar PC, Boomsma DI, de Geus EJ. Stability of genetic and environmental influences on P300 amplitude: a longitudinal study in adolescent twins. Behav Genet. 2001;31(6):533–543. doi: 10.1023/a:1013389226795. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Kiehl KA, Pearlson G, Perrone-Bizzozero NI, Eichele T, Calhoun VD. Genetic determinants of target and novelty-related event-related potentials in the auditory oddball response. Neuroimage. 2009;46(3):809–816. doi: 10.1016/j.neuroimage.2009.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thaker GK. Neurophysiological endophenotypes across bipolar and schizophrenia psychosis. Schizophr Bull. 2008;34(4):760–773. doi: 10.1093/schbul/sbn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 24.Bestelmeyer PE, Phillips LH, Crombie C, Benson P, St Clair D. The P300 as a possible endophenotype for schizophrenia and bipolar disorder: Evidence from twin and patient studies. Psychiatry Res. 2009;169(3):212–219. doi: 10.1016/j.psychres.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 25.Picton TW. The P300 wave of the human event-related potential. J Clin Neurophysiol. 1992;9(4):456–479. doi: 10.1097/00004691-199210000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Simons CJ, Sambeth A, Krabbendam L, Pfeifer S, van Os J, Riedel WJ. Auditory P300 and N100 components as intermediate phenotypes for psychotic disorder: Familial liability and reliability. Clin Neurophysiol. 2011;122(10):1984–1990. doi: 10.1016/j.clinph.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 27.Carlson SR, Iacono WG, McGue M. P300 amplitude in nonalcoholic adolescent twin pairs who become discordant for alcoholism as adults. Psychophysiology. 2004;41(6):841–844. doi: 10.1111/j.1469-8986.2004.00238.x. [DOI] [PubMed] [Google Scholar]
- 28.Carlson SR, McLarnon ME, Iacono WG. P300 amplitude, externalizing psychopathology, and earlier- versus later-onset substance-use disorder. J Abnorm Psychol. 2007;116(3):565–577. doi: 10.1037/0021-843X.116.3.565. [DOI] [PubMed] [Google Scholar]
- 29.Privman E, Fisch L, Neufeld MY, Kramer U, Kipervasser S, Andelman F, et al. Antagonistic relationship between gamma power and visual evoked potentials revealed in human visual cortex. Cereb Cortex. 2011;21(3):616–624. doi: 10.1093/cercor/bhq128. [DOI] [PubMed] [Google Scholar]
- 30.Lakatos P, O'Connell MN, Barczak A, Mills A, Javitt DC, Schroeder CE. The leading sense: supramodal control of neurophysiological context by attention. Neuron. 2009;64(3):419–430. doi: 10.1016/j.neuron.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ford JM, Roach BJ, Hoffman RS, Mathalon DH. The dependence of P300 amplitude on gamma synchrony breaks down in schizophrenia. Brain Res. 2008;1235:133–142. doi: 10.1016/j.brainres.2008.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamm JP, Ethridge LE, Shapiro JR, Stevens MC, Boutros NN, Summerfelt AT, et al. Spatio-temporal and frequency domain analysis of auditory paired stimuli processing in schizophrenia and psychotic bipolar disorder. Psychophys. 2012 doi: 10.1111/j.1469-8986.2011.01327.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lenz D, Fischer S, Schadow J, Bogerts B, Herrmann CS. Altered evoked gamma-band responses as a neurophysiological marker of schizophrenia? Int J Psychophysiol. 2011;79(1):25–31. doi: 10.1016/j.ijpsycho.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Lee PS, Chen YS, Hsieh JC, Su TP, Chen LF. Distinct neuronal oscillatory responses between patients with bipolar and unipolar disorders: a magnetoencephalographic study. J Affect Disord. 2010;123(1-3):270–275. doi: 10.1016/j.jad.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 35.Ozerdem A, Guntekin B, Tunca Z, Basar E. Brain oscillatory responses in patients with bipolar disorder manic episode before and after valproate treatment. Brain Res. 2008;1235:98–108. doi: 10.1016/j.brainres.2008.06.101. [DOI] [PubMed] [Google Scholar]
- 36.Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci U S A. 2000;97(4):1867–1872. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vierling-Claassen D, Siekmeier P, Stufflebeam S, Kopell N. Modeling GABA alterations in schizophrenia: a link between impaired inhibition and altered gamma and beta range auditory entrainment. J Neurophysiol. 2008;99(5):2656–2671. doi: 10.1152/jn.00870.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braboszcz C, Delorme A. Lost in thoughts: neural markers of low alertness during mind wandering. Neuroimage. 2011;54(4):3040–3047. doi: 10.1016/j.neuroimage.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 39.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV--clinical version (SCID-CV) American Psychiatric Press, Inc.; Washington, D.C.: 1997. [Google Scholar]
- 40.Lancon C, Auquier P, Nayt G, Reine G. Stability of the five-factor structure of the Positive and Negative Syndrome Scale (PANSS). Schizophr Res. 2000;42(3):231–239. doi: 10.1016/s0920-9964(99)00129-2. [DOI] [PubMed] [Google Scholar]
- 41.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 42.Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria. Reliability and validity. Arch Gen Psychiatry. 1977;34(10):1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- 43.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 44.Clementz BA, Blumenfeld LD. Multichannel electroencephalographic assessment of auditory evoked response suppression in schizophrenia. Exp Brain Res. 2001;139(4):377–390. doi: 10.1007/s002210100744. [DOI] [PubMed] [Google Scholar]
- 45.Carroll CA, Kieffaber PD, Vohs JL, O'Donnell BF, Shekhar A, Hetrick WP. Contributions of spectral frequency analyses to the study of P50 ERP amplitude and suppression in bipolar disorder with or without a history of psychosis. Bipolar Disord. 2008;10(7):776–787. doi: 10.1111/j.1399-5618.2008.00622.x. [DOI] [PubMed] [Google Scholar]
- 46.Dien J, Khoe W, Mangun GR. Evaluation of PCA and ICA of simulated ERPs: Promax vs. Infomax rotations. Hum Brain Mapp. 2007;28(8):742–763. doi: 10.1002/hbm.20304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cattell RB. Evaluating therapy as total personality change: theory and available instruments. Am J Psychother. 1966;20(1):69–88. doi: 10.1176/appi.psychotherapy.1966.20.1.69. [DOI] [PubMed] [Google Scholar]
- 48.Cox LA., Jr. Reassessing benzene risks using internal doses and Monte-Carlo uncertainty analysis. Environ Health Perspect. 1996;104(Suppl 6):1413–1429. doi: 10.1289/ehp.961041413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Busch NA, VanRullen R. Spontaneous EEG oscillations reveal periodic sampling of visual attention. Proc Natl Acad Sci U S A. 2010;107(37):16048–16053. doi: 10.1073/pnas.1004801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mardia KV, Kent JT, Bibby JM. Multivariate Analysis. Academic Press; 1979. [Google Scholar]
- 51.Uhlhaas PJ, Haenschel C, Nikolic D, Singer W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr Bull. 2008;34(5):927–943. doi: 10.1093/schbul/sbn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doege K, Bates AT, White TP, Das D, Boks MP, Liddle PF. Reduced event-related low frequency EEG activity in schizophrenia during an auditory oddball task. Psychophysiology. 2009;46(3):566–577. doi: 10.1111/j.1469-8986.2009.00785.x. [DOI] [PubMed] [Google Scholar]
- 53.Koh Y, Shin KS, Kim JS, Choi JS, Kang DH, Jang JH, et al. An MEG study of alpha modulation in patients with schizophrenia and in subjects at high risk of developing psychosis. Schizophr Res. 2011;126(1-3):36–42. doi: 10.1016/j.schres.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 54.Roschke J, Fell J. Spectral analysis of P300 generation in depression and schizophrenia. Neuropsychobiology. 1997;35(2):108–114. doi: 10.1159/000119400. [DOI] [PubMed] [Google Scholar]
- 55.Shin YW, Krishnan G, Hetrick WP, Brenner CA, Shekhar A, Malloy FW, et al. Increased temporal variability of auditory event-related potentials in schizophrenia and Schizotypal Personality Disorder. Schizophr Res. 2010;124(1-3):110–118. doi: 10.1016/j.schres.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hong LE, Summerfelt A, Mitchell BD, O'Donnell P, Thaker GK. A shared low-frequency oscillatory rhythm abnormality in resting and sensory gating in schizophrenia. Clin Neurophysiol. 2011 doi: 10.1016/j.clinph.2011.07.025. doi: 10.1016/j.clinph.2011.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Naatanen R, Picton TW. N2 and automatic versus controlled processes. Electroencephalogr Clin Neurophysiol Suppl. 1986;38:169–186. [PubMed] [Google Scholar]
- 58.Patel SH, Azzam PN. Characterization of N200 and P300: selected studies of the Event-Related Potential. Int J Med Sci. 2005;2(4):147–154. doi: 10.7150/ijms.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Calhoun V, Wu L, Kiehl K, Eichele T, Pearlson G. Aberrant Processing of Deviant Stimuli in Schizophrenia Revealed by Fusion of FMRI and EEG Data. Acta Neuropsychiatr. 2010;22(3):127–138. doi: 10.1111/j.1601-5215.2010.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O'Donnell BF, Shenton ME, McCarley RW, Faux SF, Smith RS, Salisbury DF, et al. The auditory N2 component in schizophrenia: relationship to MRI temporal lobe gray matter and to other ERP abnormalities. Biol Psychiatry. 1993;34(1-2):26–40. doi: 10.1016/0006-3223(93)90253-a. [DOI] [PubMed] [Google Scholar]
- 61.Kaur M, Battisti RA, Ward PB, Ahmed A, Hickie IB, Hermens DF. MMN/P3a deficits in first episode psychosis: comparing schizophrenia-spectrum and affective-spectrum subgroups. Schizophr Res. 2011;130(1-3):203–209. doi: 10.1016/j.schres.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 62.Naatanen R. The role of attention in auditory information processing as revealed by event-related potentials and other brain measures of cognitive function. Behav. Brain Sciences. 1990;13:201–288. [Google Scholar]
- 63.Kisley MA, Cornwell ZM. Gamma and beta neural activity evoked during a sensory gating paradigm: effects of auditory, somatosensory and cross-modal stimulation. Clin Neurophysiol. 2006;117(11):2549–2563. doi: 10.1016/j.clinph.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rieder MK, Rahm B, Williams JD, Kaiser J. Human gamma-band activity and behavior. Int J Psychophysiol. 2011;79(1):39–48. doi: 10.1016/j.ijpsycho.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 65.Nuechterlein KH, Dawson ME, Green MF. Information-processing abnormalities as neuropsychological vulnerability indicators for schizophrenia. Acta Psychiatr Scand Suppl. 1994;384:71–79. doi: 10.1111/j.1600-0447.1994.tb05894.x. [DOI] [PubMed] [Google Scholar]
- 66.Arai J, Natsume K. The properties of carbachol-induced beta oscillation in rat hippocampal slices. Neurosci Res. 2006;54(2):95–103. doi: 10.1016/j.neures.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 67.Traub RD, Bibbig A, LeBeau FE, Buhl EH, Whittington MA. Cellular mechanisms of neuronal population oscillations in the hippocampus in vitro. Annu Rev Neurosci. 2004;27:247–278. doi: 10.1146/annurev.neuro.27.070203.144303. [DOI] [PubMed] [Google Scholar]
- 68.Yamawaki N, Stanford IM, Hall SD, Woodhall GL. Pharmacologically induced and stimulus evoked rhythmic neuronal oscillatory activity in the primary motor cortex in vitro. Neuroscience. 2008;151(2):386–395. doi: 10.1016/j.neuroscience.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 69.Farber NB. The NMDA receptor hypofunction model of psychosis. Ann N Y Acad Sci. 2003;1003:119–130. doi: 10.1196/annals.1300.008. [DOI] [PubMed] [Google Scholar]
- 70.Pearlson GD, Wong DF, Tune LE, Ross CA, Chase GA, Links JM, Dannals RF, Wilson AA, Ravert HT, Wagner HN, Jr, et al. In vivo D2 dopamine receptor density in psychotic and nonpsychotic patients with bipolar disorder. Arch Gen Psychiatry. 1995 Jun;52(6):471–7. doi: 10.1001/archpsyc.1995.03950180057008. [DOI] [PubMed] [Google Scholar]
- 71.Wong DF, Pearlson GD, Tune LE, Young LT, Meltzer CC, Dannals RF, Ravert HT, Reith J, Kuhar MJ, Gjedde A. Quantification of neuroreceptors in the living human brain: IV. Effect of aging and elevations of D2-like receptors in schizophrenia and bipolar illness. J Cereb Blood Flow Metab. 1997 Mar;17(3):331–42. doi: 10.1097/00004647-199703000-00010. [DOI] [PubMed] [Google Scholar]
- 72.Yatham LN, Liddle PF, Lam RW, Shiah IS, Lane C, Stoessl AJ, et al. PET study of the effects of valproate on dopamine D(2) receptors in neuroleptic- and mood-stabilizer-naive patients with nonpsychotic mania. Am J Psychiatry. 2002;159(10):1718–1723. doi: 10.1176/appi.ajp.159.10.1718. [DOI] [PubMed] [Google Scholar]
- 73.Berk M, Dodd S, Kauer-Sant'anna M, Malhi GS, Bourin M, Kapczinski F, et al. Dopamine dysregulation syndrome: implications for a dopamine hypothesis of bipolar disorder. Acta Psychiatr Scand Suppl. 2007;(434):41–49. doi: 10.1111/j.1600-0447.2007.01058.x. [DOI] [PubMed] [Google Scholar]
- 74.Yu C, Tu S, Wang T, Qiu J. The neural basis of self-evaluation processing in social judgment. Neuroreport. 2010;21(7):497–501. doi: 10.1097/WNR.0b013e3283383449. [DOI] [PubMed] [Google Scholar]
- 75.Singh SM, Basu D. The P300 event-related potential and its possible role as an endophenotype for studying substance use disorders: a review. Addict Biol. 2009;14(3):298–309. doi: 10.1111/j.1369-1600.2008.00124.x. [DOI] [PubMed] [Google Scholar]
- 76.Shaikh M, Hall MH, Schulze K, Dutt A, Li K, Williams I, et al. Effect of DISC1 on the P300 Waveform in Psychosis. Schizophr Bull. 2011 doi: 10.1093/schbul/sbr101. doi: 10.1093/schbul/sbr101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blackwood DH, Muir WJ. Clinical phenotypes associated with DISC1, a candidate gene for schizophrenia. Neurotox Res. 2004;6(1):35–41. doi: 10.1007/BF03033294. [DOI] [PubMed] [Google Scholar]
- 78.James R, Adams RR, Christie S, Buchanan SR, Porteous DJ, Millar JK. Disrupted in Schizophrenia 1 (DISC1) is a multicompartmentalized protein that predominantly localizes to mitochondria. Mol Cell Neurosci. 2004;26(1):112–122. doi: 10.1016/j.mcn.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 79.Hayashi-Takagi A, Takaki M, Graziane N, Seshadri S, Murdoch H, Dunlop AJ, et al. Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat Neurosci. 2010;13(3):327–332. doi: 10.1038/nn.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miyoshi K, Honda A, Baba K, Taniguchi M, Oono K, Fujita T, et al. Disrupted-In-Schizophrenia 1, a candidate gene for schizophrenia, participates in neurite outgrowth. Mol Psychiatry. 2003;8(7):685–694. doi: 10.1038/sj.mp.4001352. [DOI] [PubMed] [Google Scholar]
- 81.Niwa M, Kamiya A, Murai R, Kubo K, Gruber AJ, Tomita K, et al. Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron. 2010;65(4):480–489. doi: 10.1016/j.neuron.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ayhan Y, Abazyan B, Nomura J, Kim R, Ladenheim B, Krasnova IN, et al. Differential effects of prenatal and postnatal expressions of mutant human DISC1 on neurobehavioral phenotypes in transgenic mice: evidence for neurodevelopmental origin of major psychiatric disorders. Mol Psychiatry. 2011;16(3):293–306. doi: 10.1038/mp.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Coburn KL, Shillcutt SD, Tucker KA, Estes KM, Brin FB, Merai P, et al. P300 delay and attenuation in schizophrenia: reversal by neuroleptic medication. Biol Psychiatry. 1998;44(6):466–474. doi: 10.1016/s0006-3223(97)00402-2. [DOI] [PubMed] [Google Scholar]
- 84.Hirayasu Y, Asato N, Ohta H, Hokama H, Arakaki H, Ogura C. Abnormalities of auditory event-related potentials in schizophrenia prior to treatment. Biol Psychiatry. 1998;43(4):244–253. doi: 10.1016/S0006-3223(97)00275-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.