Abstract
Background
Controversy exists over the optimal management of thyroid cancer. The proportion of patients with low-risk thyroid cancer treated with radioactive iodine (RAI) increased over the last 20 years and little is known about the role of clinicians in hospital-level RAI use for low-risk disease.
Methods
Thyroid surgeons affiliated with 368 hospitals with Commission on Cancer-accredited cancer programs were surveyed. Survey data were linked to data reported to the National Cancer Database (NCDB). A multivariable analysis was used to assess the relationship between the clinician decision maker and hospital-level RAI use after total thyroidectomy in Stage I well-differentiated thyroid cancer.
Results
The survey response rate was 70% (560/804). The surgeon was identified as the primary decision maker by 16% of the surgeons, endocrinologist by 69%, and nuclear medicine, radiologist, or other physician by 15%. In a multivariable analysis controlling for hospital case volume and hospital type, when the primary decision maker was in a specialty other than endocrinology or surgery, there was greater use of RAI at the hospital (P<0.001). A greater number of providers at the hospital administering RAI and access to a tumor board were also associated with increased use of RAI (P<0.001, P=0.006).
Conclusion
The specialty of the primary decision maker, number of providers administering RAI, and access to a tumor board are significantly associated with use of RAI for Stage I thyroid cancer. The findings have implications for addressing non-clinical variation between hospitals, with the marked heterogeneity in decision-making suggesting standardization of care will be challenging.
Keywords: thyroid cancer, radioactive iodine, physician, decision making
Introduction
Thyroid cancer is a common malignancy with a persistently rising incidence rate.1, 2 In contrast to many other common malignancies, there is great controversy over standard of care.3, 4 In the setting of this ongoing dispute, over the last 20 years there has been an increase in the use of radioactive iodine (RAI) after total thyroidectomy as treatment for low-risk thyroid cancer.5, 6 The benefit of RAI use in low risk disease is unclear7–10 and this rise in RAI use has potential implications for patient health and health care costs. Treatment with RAI is associated with increased risk of second primary malignancy and damage to salivary glands and lacrimal ducts.6, 11, 12 In addition, there are clear cost-saving benefits when RAI is not administered to patients with low-risk disease.13
Although it is known that there is marked hospital-level variation in the use of RAI, with the most variation seen in low-risk patients,5 the role of surgeons, endocrinologists, and nuclear medicine physicians in RAI use and in the inter-hospital variation in its use is unknown. It is not clear whether the number and specialty of the providers involved in the decision-making process influence use of RAI at the hospital.
By linking surgeon surveys to data from the National Cancer Database (NCDB) we could obtain details on providers and RAI use that would not otherwise be available. We aimed to assess the role of clinicians in hospital-level use of RAI for AJCC Stage I well-differentiated thyroid cancer.
Methods
Data Source and Study Population
We selected the 1159 hospitals with Commission on Cancer-accredited cancer programs who reported having treated thyroid cancer to the National Cancer Database (NCDB), a joint project of the American College of Surgeons’ and the American Cancer Society, in at least four of the five years between 2004–2008. We excluded the 235 hospitals that treated fewer than six thyroid cancer patients a year. We divided the remaining hospitals by quartiles of hospital case volume and then quartiles of RAI use. We randomly sampled 589 hospitals across these quartiles. We then contacted the hospital registrars and searched hospital websites to identify the surgeons who performed the majority of thyroid surgeries at each hospital. We identified 850 thyroid surgeons.
We used a modified Dillman method of survey administration14 when surveying the 850 surgeons. This method consists of three waves of mailings with a gift included with the first mailing.
Survey data were de-identified, scanned and confirmed. The surgeon survey responses were linked to details on hospital case volume and hospital use of RAI from the NCDB. The NCDB captures close to 85% of all thyroid cancer cases in the United States.15 When treatment post-surgery does not occur at the specified hospital, the hospital registrar is responsible for documenting the remainder of the patients’ disease course and treatment.16 Since RAI is not typically used after thyroid lobectomy and is not recommended in the treatment of medullary or anaplastic cancer, we selected hospital treatment with RAI in patients who underwent total thyroidectomy and had AJCC Stage I well-differentiated thyroid cancer (papillary, follicular, and Hurthle cell types).
As described above, all surveys were de-identified and data were analyzed in summary form only. Exemption was granted by the University of Michigan Institutional Review Board.
Measures
The survey was designed to collect key information about thyroid cancer management through use of clinical vignettes and survey questions (including five and six-point Likert scales). Prior to survey administration, we piloted our survey instrument in a diverse group of surgeons.
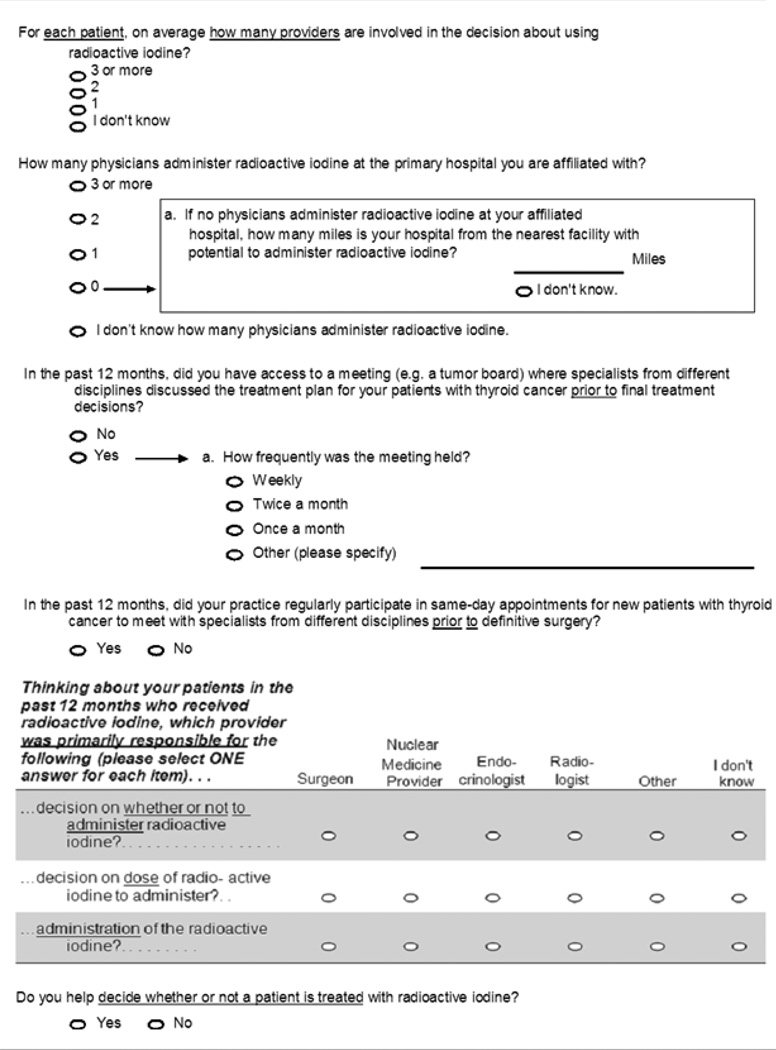
The dependent variable, use of RAI in Stage I thyroid cancer, and one independent variable, hospital case volume, were obtained from the NCDB. There were four categories for hospital case volume: low (7–11 thyroid cancer cases/year), low-moderate (12–19 thyroid cancer cases/year), moderate (20–34 thyroid cancer cases/year), and high (≥ 35 thyroid cancer cases/year). The data from the NCDB were then linked to the de-identified surgeon surveys affiliated with the specified hospitals. As shown in Table 1, the remaining independent variables (number of providers involved in decision-making, number of providers administering RAI, access to a tumor board, frequency of tumor board meetings, same day visits with other providers, primary decision maker on RAI receipt, primary decision maker on RAI dose, primary provider to administer RAI, surgeon involvement in decision-making) were obtained from surgeon surveys. Because surgeons could choose more than one practice setting, when more than one practice setting was selected we applied an algorithm previously described by Alderman et al.17 If they selected an academic tertiary care center (including when they also selected community affiliate or private practice) the assigned practice setting was academic. If they chose both community-based academic affiliate and private practice, the assigned practice setting was community.
Table 1.
Survey Items
Statistical analyses
When more than one surgeon responded from the same hospital, the surgeon responses were weighted by reported case volume. Surgeon case volume was categorized as 1, 5, 25, 50, 100 using the lower limits of the corresponding response intervals to a survey item that specifically asks how many patients with thyroid cancer the surgeon operates on in one year (for the 0–4 interval, the surgeon was assigned a value of 1).
We evaluated the hospital-level use of RAI across all independent variables. We then included the decision-making variables that were significant on univariate analysis in a multivariable regression model adjusted for hospital case volume and surgeon-reported practice setting (academic tertiary care, community-based academic affiliate and private practice). Two-way interactions were evaluated.
We also determined the distribution of patients by tumor size (≤ 1 cm, 1.1–2 cm, 2.1–4 cm, > 4 cm) and lymph node status (N0, N1, NX) within the hospitals based on the three categories of physician decision makers: surgeon, endocrinologist, and nuclear medicine/radiology/other, access to a tumor board, and number of providers administering RAI.
All statistical tests were performed using SAS 9.2 (SAS Institute Inc., Cary, North Carolina). Two-sided tests were used with P <0.05 considered statistically significant.
Results
Sample Characteristics
As shown in Figure 1, 46 of the 850 surgeons were ineligible for the study. Of the 804 response eligible surgeons, 560 (70%) completed the survey. The majority (90%) of respondents were male and they had an average of 19 ± 10 years in practice. Otolaryngologists (44%) were the largest surgical specialists represented, followed by general surgeons (39%), then endocrine surgeons (9%) and other surgeon specialists (8%). Most (61%) of the surgeons were in private practice, but 23% worked in an academic setting and 16% were in a community-based academic affiliate.
Figure 1.
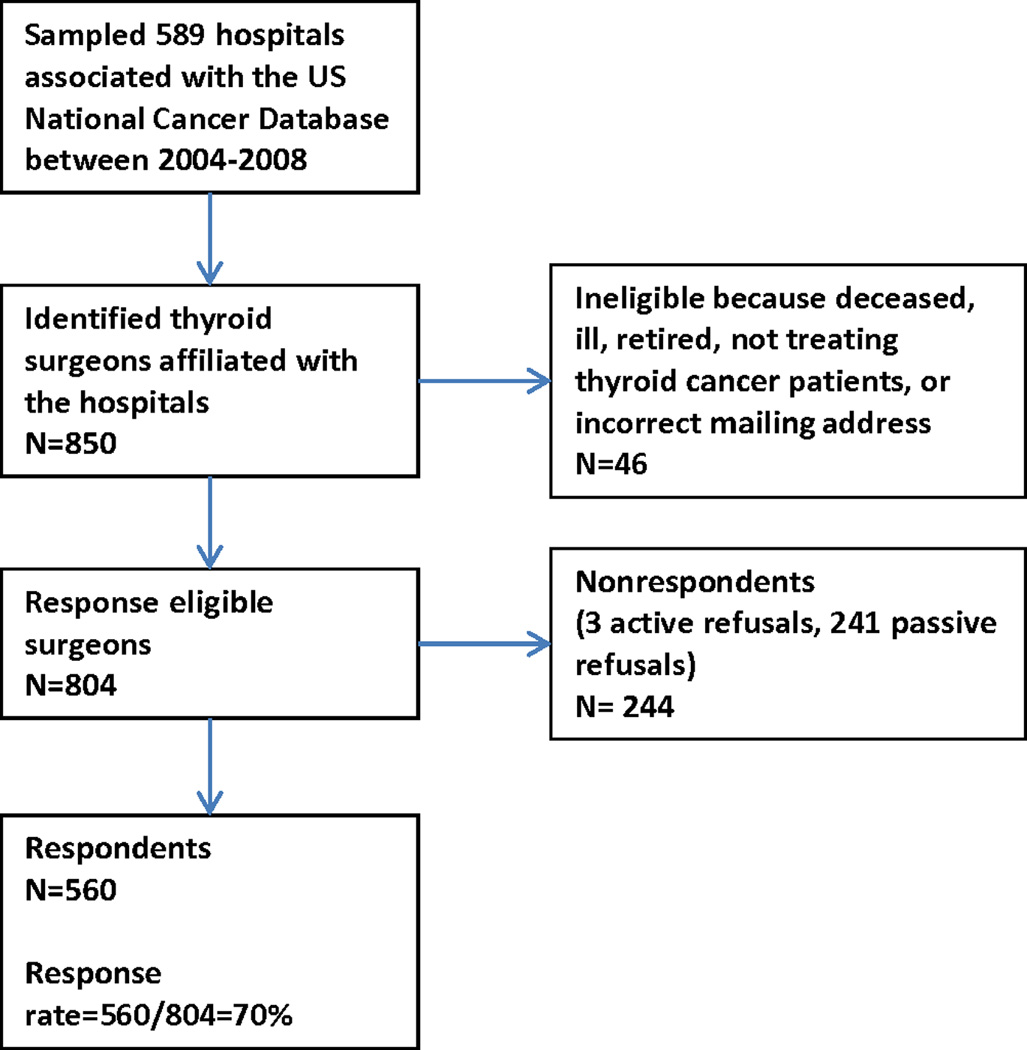
Sampling method and subject flow
Impact of Providers on Use of RAI
Univariate analyses
As shown in Table 2, the majority of surgeons (63%) reported that two providers were involved in the RAI decision-making process with 74% of surgeons reporting personal involvement. Three or more providers administered RAI at 46% of the affiliated hospitals. In univariate analysis there was a strong association between number of providers administering RAI and the likelihood of a patient with Stage I thyroid cancer receiving RAI at the hospital-level (P<0.001). Access to a tumor board was also associated with greater likelihood of receiving RAI but the frequency of the tumor board meeting or same-day appointments with specialists from different disciplines did not impact receipt.
Table 2.
Univariate Analysis of Clinician Decision-Making and RAI Use
| Number (%) | Proportion treated with RAI at hospital (mean % ± S.D.) |
P Value | ||
|---|---|---|---|---|
| Number of provider involved in decision making about use of RAI | 0.201 | |||
| 3 or more | 105 (20%) | 47.88 ± 19.00 | ||
| 2 | 332 (63%) | 45.86 ± 21.35 | ||
| 1 | 90 (17%) | 42.48 ± 22.52 | ||
| Number of providers administering RAI | <0.001 | |||
| 3 or more | 240 (46%) | 47.47 ± 19.21 | ||
| 2 | 143 (27%) | 48.76 ± 19.46 | ||
| 1 | 102 (20%) | 44.55 ± 25.01 | ||
| 0 | 36 (7%) | 29.28 ± 22.63 | ||
| Access to tumor board | 0.011 | |||
| Yes | 395 (71%) | 47.54 ± 20.50 | ||
| No | 158 (29%) | 42.55 ± 22.04 | ||
| Frequency of tumor board | 0.188 | |||
| Weekly | 166 (42%) | 49.42 ± 19.61 | ||
| Twice a month | 73 (19%) | 48.15 ± 19.14 | ||
| Once a month | 134 (34%) | 44.31 ± 22.57 | ||
| Other | 20 (5%) | 45.91 ± 20.22 | ||
| Same day visits with other providers | 0.791 | |||
| Yes | 110 (20%) | 45.46 ± 19.66 | ||
| No | 446 (80%) | 46.06 ± 21.55 | ||
| Primary decision maker on whether or not to administer RAI | 0.004 | |||
| Surgeon | 87 (16%) | 42.29 ± 21.57 | ||
| Endocrinologist | 374 (69%) | 45.42 ± 20.37 | ||
| NM/Radiol/Other | 83 (15%) | 52.50 ± 23.05 | ||
| Primary decision maker on dose of RAI | 0.894 | |||
| Surgeon | 4 (1%) | 41.22 ± 18.63 | ||
| Endocrinologist | 232 (45%) | 46.27 ± 20.26 | ||
| NM/Radiol/Other | 282 (54%) | 46.14 ± 21.91 | ||
| Primarily administers RAI | 0.192 | |||
| Surgeon | 0 (0%) | |||
| Endocrinologist | 165 (31%) | 44.18 ± 21.72 | ||
| NM/Radiol/Other | 361 (69%) | 46.80 ± 21.22 | ||
| Surgeon involved in RAI decision making | 0.591 | |||
| Yes | 414 (74%) | 45.73 ± 21.20 | ||
| No | 143 (26%) | 46.83 ± 21.07 | ||
RAI = radioactive iodine, NM = nuclear medicine provider
Endocrinologists were more often the primary decision maker on whether or not to administer RAI (69%) but nuclear medicine/radiology/other were more frequently the primary decision makers regarding dose of RAI (54%) and were most often the providers responsible for administering RAI (69%). Specialty of the primary decision maker on whether or not to administer RAI was associated with increased likelihood of a Stage I thyroid cancer patient receiving RAI (P=0.004). If the surgeon was primary decision maker, the mean proportion of patients receiving RAI use for Stage I disease was 42% and if the endocrinologist was the primary decision maker the mean proportion receiving RAI was 45%. When the nuclear medicine provider/radiologist/other was the primary decision maker the proportion of patients receiving RAI was higher at 52%. There was no significant association between proportion of patients receiving RAI at the hospital and specialty of primary decision maker on dose or specialty of the administering provider.
The proportion of patients within each tumor size category (≤ 1 cm, 1.1–2 cm, 2.1–4 cm, > 4 cm) was the same when the surgeon versus endocrinologist versus nuclear medicine/radiology/other physician is the primary decision maker. Similarly, 88% of the patients affiliated with hospitals where the nuclear medicine/radiologist/other physicians are the primary decision maker are without lymph node metastases versus 87% of those affiliated with hospitals where the surgeons or endocrinologists are the primary decision makers. The distribution by both tumor size and lymph node status were almost identical in hospitals with access to a tumor board versus no access. Similarly, there were very similar distributions by tumor size and lymph node status in hospitals with one, two, and three physicians administering RAI. However, not having a provider administer RAI at the affiliated hospital was associated with a lower proportion of tumors ≤ 1 cm (20% versus 24%) and a higher proportion of N0 cancers (89% versus 86%) compared to hospitals with three or more providers administering RAI.
Multivariable analysis
In multivariable analysis, high case volume, private practice, and access to a tumor board were associated with a statistically greater likelihood of a Stage I thyroid cancer patient receiving RAI (Table 3). There was also a significant difference in the proportion of patients treated with RAI if no provider (P<0.001) or one provider (P=0.010) at the hospital administered RAI versus if three or more providers administered RAI. There was no statistically significant difference in hospital-level RAI use when two providers versus three administer RAI. However, when nuclear medicine/radiology/other providers were the primary decision makers on whether or not to administer RAI there was a significantly higher proportion of Stage I thyroid cancer patients receiving RAI at the hospital than if the decision maker was a surgeon (P<0.001) or endocrinologist (P<0.001) (Table 3 and Figure 2). There was a statistically significant interaction between the primary decision maker and number of providers administering RAI (P=0.020). Having just one provider administering RAI at the hospital was associated with the nuclear medicine/radiologist/other not being the primary decision maker. There was no interaction with access to a tumor board.
Table 3.
Multivariable Analysis of RAI Use for Stage I Thyroid Cancer
| Proportion treated with RAI at hospital (Mean % ± SD) |
Multivariable P value | ||
|---|---|---|---|
| Hospital Characteristics | |||
| Case Volume | |||
| Low | 40.13 ± 25.66 | 0.002 | |
| Low-Mod | 48.63 ± 21.97 | 0.518 | |
| Moderate | 44.29 ± 20.14 | 0.075 | |
| High | 48.48 ± 17.75 | Ref. | |
| Practice Setting | |||
| Academic | 44.91 ± 17.34 | Ref. | |
| Community based academic affiliate | 44.09 ± 19.52 | 0.715 | |
| Private | 46.48 ± 22.92 | 0.036 | |
| Decision-making process | |||
| Access to tumor board | |||
| Yes | 47.57 ± 20.50 | 0.006 | |
| No | 42.55 ± 22.04 | Ref. | |
| No. of providers administering RAI | |||
| 3 or more | 47.47 ± 19.21 | Ref. | |
| 2 | 48.76 ± 19.46 | 0.694 | |
| 1 | 44.55 ± 25.01 | 0.010 | |
| 0 | 29.28 ± 22.63 | <0.001 | |
| Primary decision maker on whether or not to administer RAI | |||
| Surgeon | 42.29 ± 21.57 | <0.001 | |
| Endocrinologist | 45.42 ± 20.37 | <0.001 | |
| NM/Radiol/Other | 52.50 ± 23.05 | Ref. | |
RAI = radioactive iodine, NM = nuclear medicine provider
Figure 2.
When the primary decision maker on whether or not to administer radioactive iodine is nuclear medicine/radiology/other provider there is a greater proportion of AJCC Stage I thyroid cancer patients receiving radioactive iodine at the hospital than if the primary decision maker is a surgeon (P<0.001) or endocrinologist (P<0.001).
Discussion
This study demonstrates heterogeneous treatment processes in the care of patients with well-differentiated thyroid cancer. The number and type of providers involved in decision-making vary by hospital. Controlling for hospital case volume and hospital type, we found that the specialty of the primary physician decision maker, the number of providers administering RAI, and access to a tumor board influence use of RAI for Stage I thyroid cancer.
Similar to studies in other disease states illustrating the relationship between access to care and treatment,18–21 if there are more providers administering RAI or access to a tumor board then the likelihood of treatment with RAI increases. This difference is most marked when there is not a provider at the affiliated hospital that administers RAI (29% versus 47% of patients receiving RAI, p<0.001) but is also seen when there is just one administering provider instead of three or more. Thus, both lack of access and supply-demand may influence receipt of RAI.
In the treatment of other malignancies, it is known that cancer specialists are more likely to recommend the treatments their specialty provides.22, 23 Although this study is novel since the focus is thyroid cancer management and the details provided by surgeon surveys are linked to hospital-level use, some of the findings parallel what has been seen in other malignancies.22, 23 The majority of surgeons (69%) report that nuclear medicine/radiology/other providers administer RAI. When these specialists act as the primary decision maker with respect to use of RAI, there is greater hospital-level use of RAI in Stage I disease (52% versus 42–45% of patients receiving RAI, p<0.001). Specialty differences in administration rate may be related to differences in training, variable adherence to clinical guidelines, varying views on risks-benefits, or the influence of financial incentives. Previous studies have demonstrated the role of financial incentive in influencing cancer care.24, 25
Although regional differences in physician opinion about RAI use post total thyroidectomy have previously been evaluated26 and variation in interspecialty opinion on post-operative management noted,27 as far as we know this is the first study to evaluate characteristics of decision-making, including specialty of the primary decision maker, with respect to hospital-level RAI use.
Strengths of this study include a large sample size, a high response rate, an exhaustive set of independent variables, and reliable information on use of RAI at the hospital-level.
Despite the strengths of this study, there are limitations. First, similar to other survey studies, there is a risk of non-response selection bias. Second, several of the independent variables are based on surgeon report. Surgeon report has, however, been demonstrated to be accurate in terms of surgical volume,17 and surgeon self-report is commonly used to report other cancer care processes.17,23, 28, 29 Finally, we cannot control for selection of patients to hospitals. In attempt to assess the influence of patient selection to hospitals, we determined the distribution of patients by tumor size and lymph node status. However, these measures are imperfect as hospital use of imaging studies may impact the distribution of cancers based on size and hospital use of prophylactic lymph node dissections may affect the proportion of patients with known lymph node metastases.
In conclusion, although we do not specifically evaluate appropriateness of use, this study sheds light on the role of clinicians in the wide variation in RAI use for low-risk thyroid cancer. In addition to previously described patient and hospital characteristics,5 it appears that providers influence RAI use. This study also illustrates the heterogeneity in clinician decision making in thyroid cancer management, which reflects the complexity of multidisciplinary care. This heterogeneity suggests that standardization of thyroid cancer care will be challenging. These findings have implications for targeted clinical guideline dissemination, future studies on thyroid cancer management, and most important, patient care.
Acknowledgements
The authors would like to thank Brittany Gay, Barbara Salem, and Ashley Gay for their work in data collection and processing. Cornell University Survey Research Institute scanned the surveys for data file. This study was funded by 1K07CA154595-01 to Dr. Haymart from the National Institutes of Health, the University of Michigan Comprehensive Cancer Center Idea Award, the Cancer Surveillance and Outcomes Research Team (CanSORT) Pilot of Feasibility Fund, and the Elizabeth Caroline Crosby Fund.
Footnotes
The authors have no conflict of interest to disclose.
References
- 1.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295(18):2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 2.Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer. 2009;115(16):3801–3807. doi: 10.1002/cncr.24416. [DOI] [PubMed] [Google Scholar]
- 3.Hay ID. Managing patients with a preoperative diagnosis of AJCC/UICC stage I (T1N0M0) papillary thyroid carcinoma: East versus West, whose policy is best? World J Surg. 2010;34(6):1291–1293. doi: 10.1007/s00268-010-0469-5. [DOI] [PubMed] [Google Scholar]
- 4.Mazzaferri EL. What is the optimal initial treatment of low-risk papillary thyroid cancer (and why is it controversial)? Oncology (Williston Park) 2009;23(7):579–588. [PubMed] [Google Scholar]
- 5.Haymart MR, Banerjee M, Stewart AK, Koenig RJ, Birkmeyer JD, Griggs JJ. Use of radioactive iodine for thyroid cancer. JAMA. 2011;306(7):721–728. doi: 10.1001/jama.2011.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iyer NG, Morris LG, Tuttle RM, Shaha AR, Ganly I. Rising incidence of second cancers in patients with low-risk (T1N0) thyroid cancer who receive radioactive iodine therapy. Cancer. 2011;117(19):4439–4446. doi: 10.1002/cncr.26070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jonklaas J, Sarlis NJ, Litofsky D, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid. 2006;16(12):1229–1242. doi: 10.1089/thy.2006.16.1229. [DOI] [PubMed] [Google Scholar]
- 8.Podnos YD, Smith DD, Wagman LD, Ellenhorn JD. Survival in patients with papillary thyroid cancer is not affected by the use of radioactive isotope. J Surg Oncol. 2007;96(1):3–7. doi: 10.1002/jso.20656. [DOI] [PubMed] [Google Scholar]
- 9.Ito Y, Masuoka H, Fukushima M, et al. Excellent prognosis of patients with solitary T1N0M0 papillary thyroid carcinoma who underwent thyroidectomy and elective lymph node dissection without radioiodine therapy. World J Surg. 2010;34(6):1285–1290. doi: 10.1007/s00268-009-0356-0. [DOI] [PubMed] [Google Scholar]
- 10.Hay ID, McConahey WM, Goellner JR. Managing patients with papillary thyroid carcinoma: insights gained from the Mayo Clinic's experience of treating 2,512 consecutive patients during 1940 through 2000. Trans Am Clin Climatol Assoc. 2002;113:241–260. [PMC free article] [PubMed] [Google Scholar]
- 11.Brown AP, Chen J, Hitchcock YJ, Szabo A, Shrieve DC, Tward JD. The risk of second primary malignancies up to three decades after the treatment of differentiated thyroid cancer. J Clin Endocrinol Metab. 2008;93(2):504–515. doi: 10.1210/jc.2007-1154. [DOI] [PubMed] [Google Scholar]
- 12.Solans R, Bosch JA, Galofre P, et al. Salivary and lacrimal gland dysfunction (sicca syndrome) after radioiodine therapy. J Nucl Med. 2001;42(5):738–743. [PubMed] [Google Scholar]
- 13.Pace-Asciak PZ, Payne RJ, Eski SJ, Walfish P, Damani M, Freeman JL. Cost savings of patients with a MACIS score lower than 6 when radioactive iodine is not given. Arch Otolaryngol Head Neck Surg. 2007;133(9):870–873. doi: 10.1001/archotol.133.9.870. [DOI] [PubMed] [Google Scholar]
- 14.Dillman DA. Mail and Internet Surveys: The Tailored Design Method (ed Second) New York, New York: Wiley; 2007. [Google Scholar]
- 15.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683–690. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raval MV, Bilimoria KY, Stewart AK, Bentrem DJ, Ko CY. Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. J Surg Oncol. 2009;99(8):488–490. doi: 10.1002/jso.21173. [DOI] [PubMed] [Google Scholar]
- 17.Alderman AK, Hawley ST, Waljee J, Morrow M, Katz SJ. Correlates of referral practices of general surgeons to plastic surgeons for mastectomy reconstruction. Cancer. 2007;109(9):1715–1720. doi: 10.1002/cncr.22598. [DOI] [PubMed] [Google Scholar]
- 18.Goodman DC, Fisher ES, Little GA, Stukel TA, Chang CH, Schoendorf KS. The relation between the availability of neonatal intensive care and neonatal mortality. N Engl J Med. 2002;346(20):1538–1544. doi: 10.1056/NEJMoa011921. [DOI] [PubMed] [Google Scholar]
- 19.Nallamothu BK, Rogers MA, Chernew ME, Krumholz HM, Eagle KA, Birkmeyer JD. Opening of specialty cardiac hospitals and use of coronary revascularization in medicare beneficiaries. JAMA. 2007;297(9):962–968. doi: 10.1001/jama.297.9.962. [DOI] [PubMed] [Google Scholar]
- 20.Wennberg JE, Barnes BA, Zubkoff M. Professional uncertainty and the problem of supplier-induced demand. Soc Sci Med. 1982;16(7):811–824. doi: 10.1016/0277-9536(82)90234-9. [DOI] [PubMed] [Google Scholar]
- 21.Laycock WS, Siewers AE, Birkmeyer CM, Wennberg DE, Birkmeyer JD. Variation in the use of laparoscopic cholecystectomy for elderly patients with acute cholecystitis. Arch Surg. 2000;135(4):457–462. doi: 10.1001/archsurg.135.4.457. [DOI] [PubMed] [Google Scholar]
- 22.Fowler FJ, Jr, McNaughton Collins M, Albertsen PC, Zietman A, Elliott DB, Barry MJ. Comparison of recommendations by urologists and radiation oncologists for treatment of clinically localized prostate cancer. JAMA. 2000;283(24):3217–3222. doi: 10.1001/jama.283.24.3217. [DOI] [PubMed] [Google Scholar]
- 23.Jagsi R, Abrahamse P, Morrow M, Hamilton AS, Graff JJ, Katz SJ. Coordination of Breast Cancer Care Between Radiation Oncologists and Surgeons: A Survey Study. Int J Radiat Oncol Biol Phys. 2012;82(5):2072–2078. doi: 10.1016/j.ijrobp.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shahinian VB, Kuo YF, Gilbert SM. Reimbursement policy and androgen-deprivation therapy for prostate cancer. N Engl J Med. 2010;363(19):1822–1832. doi: 10.1056/NEJMsa0910784. [DOI] [PubMed] [Google Scholar]
- 25.Gross CP, Andersen MS, Krumholz HM, McAvay GJ, Proctor D, Tinetti ME. Relation between Medicare screening reimbursement and stage at diagnosis for older patients with colon cancer. JAMA. 2006;296(23):2815–2822. doi: 10.1001/jama.296.23.2815. [DOI] [PubMed] [Google Scholar]
- 26.Sawka AM, Rotstein L, Brierley JD, et al. Regional differences in opinions on adjuvant radioactive iodine treatment of thyroid carcinoma within Canada and the United States. Thyroid. 2007;17(12):1235–1242. doi: 10.1089/thy.2007.0077. [DOI] [PubMed] [Google Scholar]
- 27.Clark JR, Freeman JL. Interspecialty and intraspecialty differences in the management of thyroid nodular disease and cancer. Head Neck. 2005;27(6):513–523. doi: 10.1002/hed.20187. discussion 34–4. [DOI] [PubMed] [Google Scholar]
- 28.Katz SJ, Hawley ST, Abrahamse P, et al. Does it matter where you go for breast surgery?: attending surgeon's influence on variation in receipt of mastectomy for breast cancer. Med Care. 2010;48(10):892–899. doi: 10.1097/MLR.0b013e3181ef97df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friese CR, Hawley ST, Griggs JJ, et al. Employment of nurse practitioners and physician assistants in breast cancer care. J Oncol Pract. 2010;6(6):312–316. doi: 10.1200/JOP.2010.000039. [DOI] [PMC free article] [PubMed] [Google Scholar]