Abstract
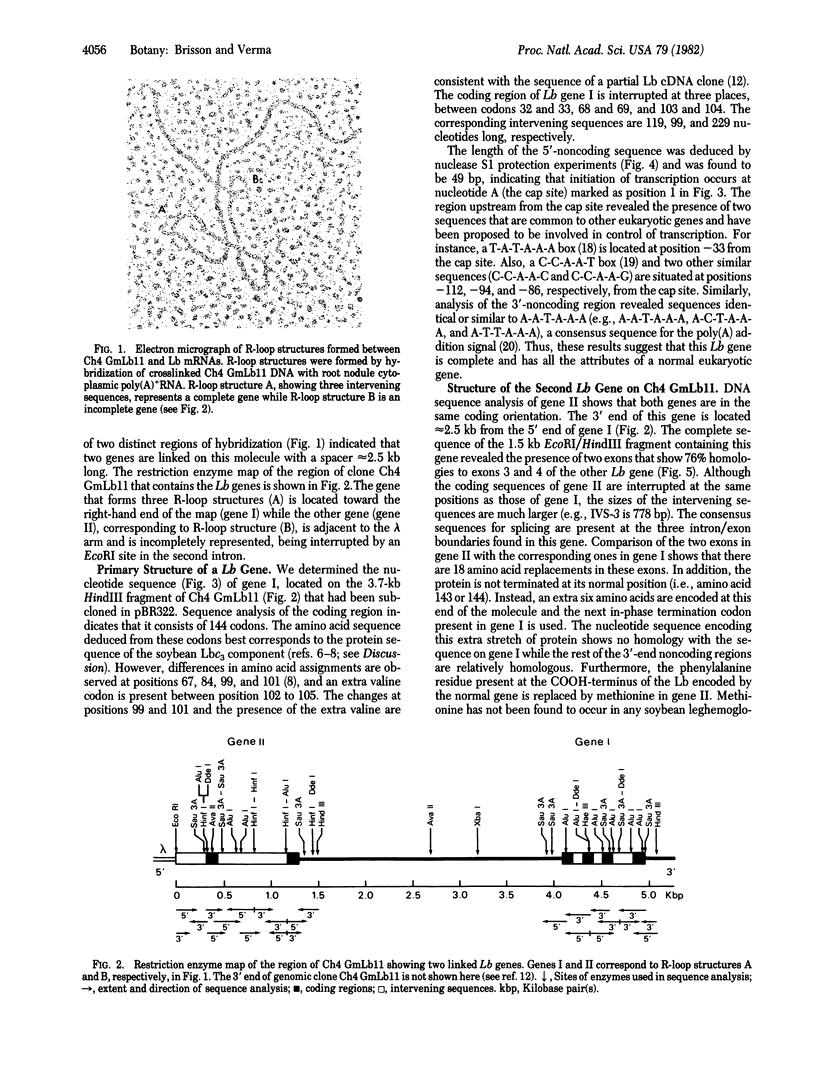
Leghemoglobin (Lb) genes in soybean represent a small family of closely related genes. Three Lb sequences isolated from a genomic library were analyzed at the nucleotide sequence level. A Lb gene present on an 11.5-kilobase (kb) EcoRI genomic fragment spans approximately 1,200 nucleotides and is interrupted at amino acid positions 32 to 33, 68 to 69, and 103 to 104. The intervening sequences, as well as the 5' and 3' flanking regions of this gene, contain the consensus sequences found in other eukaryotic genes. The length of the 5'-untranslated region is 49 bases as determined by nuclease S1 mapping. R-loop analysis of the DNA from the recombinant phage containing the 11.5-kb EcoRI genomic fragment showed that another Lb gene is located 2.5 kb away. The nucleotide sequence of the second gene showed that this gene is incomplete, containing only exons 3 and 4. The deduced amino acid sequence of this gene, although showing 76% homology with the corresponding region of the other Lb gene, is not represented in any of the known Lb proteins. Both genes are oriented in the same direction with respect to the coding strand. Analysis of the sequence present on a second genomic clone containing a 4.2-kb EcoRI fragment revealed a truncated Lb gene showing homology with the last exon and the noncoding region at the 3' end of the two other Lb genes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleby C. A., Nicola N. A., Hurrell J. G., Leach S. J. Characterization and improved separation of soybean leghemoglobins. Biochemistry. 1975 Oct 7;14(20):4444–4450. doi: 10.1021/bi00691a016. [DOI] [PubMed] [Google Scholar]
- Baulcombe D., Verma D. P. Preparation of a complementary DNA for leghaemoglobin and direct demonstration that leghaemoglobin is encoded by the soybean genome. Nucleic Acids Res. 1978 Nov;5(11):4141–4155. doi: 10.1093/nar/5.11.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Corden J., Wasylyk B., Buchwalder A., Sassone-Corsi P., Kedinger C., Chambon P. Promoter sequences of eukaryotic protein-coding genes. Science. 1980 Sep 19;209(4463):1406–1414. doi: 10.1126/science.6251548. [DOI] [PubMed] [Google Scholar]
- Edlund T., Normark S. Recombination between short DNA homologies causes tandem duplication. Nature. 1981 Jul 16;292(5820):269–271. doi: 10.1038/292269a0. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Farabaugh P. J., Schmeissner U., Hofer M., Miller J. H. Genetic studies of the lac repressor. VII. On the molecular nature of spontaneous hotspots in the lacI gene of Escherichia coli. J Mol Biol. 1978 Dec 25;126(4):847–857. doi: 10.1016/0022-2836(78)90023-2. [DOI] [PubMed] [Google Scholar]
- Fuchsman W. H., Appleby C. A. Separation and determination of the relative concentrations of the homogeneous components of soybean leghemoglobin by isoelectric focusing. Biochim Biophys Acta. 1979 Aug 28;579(2):314–324. doi: 10.1016/0005-2795(79)90059-x. [DOI] [PubMed] [Google Scholar]
- Go M. Correlation of DNA exonic regions with protein structural units in haemoglobin. Nature. 1981 May 7;291(5810):90–92. doi: 10.1038/291090a0. [DOI] [PubMed] [Google Scholar]
- Hurrell J. G., Leach S. J. The amino acid sequence of soybean leghaemoglobin c2. FEBS Lett. 1977 Aug 1;80(1):23–26. doi: 10.1016/0014-5793(77)80398-0. [DOI] [PubMed] [Google Scholar]
- Hyldig-Nielsen J. J., Jensen E. O., Paludan K., Wiborg O., Garrett R., Jørgensen P., Marcker K. A. The primary structures of two leghemoglobin genes from soybean. Nucleic Acids Res. 1982 Jan 22;10(2):689–701. doi: 10.1093/nar/10.2.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback D. B., Angerer L. M., Davidson N. Improved methods for the formation and stabilization of R-loops. Nucleic Acids Res. 1979 Jun 11;6(7):2499–2317. doi: 10.1093/nar/6.7.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legocki R. P., Verma D. P. Identification of "nodule-specific" host proteins (nodoulins) involved in the development of rhizobium-legume symbiosis. Cell. 1980 May;20(1):153–163. doi: 10.1016/0092-8674(80)90243-3. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Maier R. J., Brill W. J. Ineffective and non-nodulating mutant strains of Rhizobium japonicum. J Bacteriol. 1976 Aug;127(2):763–769. doi: 10.1128/jb.127.2.763-769.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nishioka Y., Leder A., Leder P. Unusual alpha-globin-like gene that has cleanly lost both globin intervening sequences. Proc Natl Acad Sci U S A. 1980 May;77(5):2806–2809. doi: 10.1073/pnas.77.5.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgard M. V., Emigholz K., Monahan J. J. Increased amplification of pBR322 plasmid deoxyribonucleic acid in Escherichia coli K-12 strains RR1 and chi1776 grown in the presence of high concentrations of nucleoside. J Bacteriol. 1979 Apr;138(1):270–272. doi: 10.1128/jb.138.1.270-272.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Verma D. P., Ball S., Guérin C., Wanamaker L. Leghemoglobin biosynthesis in soybean root nodules. Characterization of the nascent and released peptides and the relative rate of synthesis of the major leghemoglobins. Biochemistry. 1979 Feb 6;18(3):476–483. doi: 10.1021/bi00570a016. [DOI] [PubMed] [Google Scholar]
- Verma D. P., Haugland R., Brisson N., Legocki R. P., Lacroix L. Regulation of the expression of leghaemoglobin genes in effective and ineffective root nodules of soybean. Biochim Biophys Acta. 1981 Mar 26;653(1):98–107. doi: 10.1016/0005-2787(81)90108-8. [DOI] [PubMed] [Google Scholar]
- Verma D. P., Nash D. T., Schulman H. M. Isolation and in vitro translation of soybean leghaemoglobin mRNA. Nature. 1974 Sep 6;251(5470):74–77. doi: 10.1038/251074a0. [DOI] [PubMed] [Google Scholar]




