Abstract
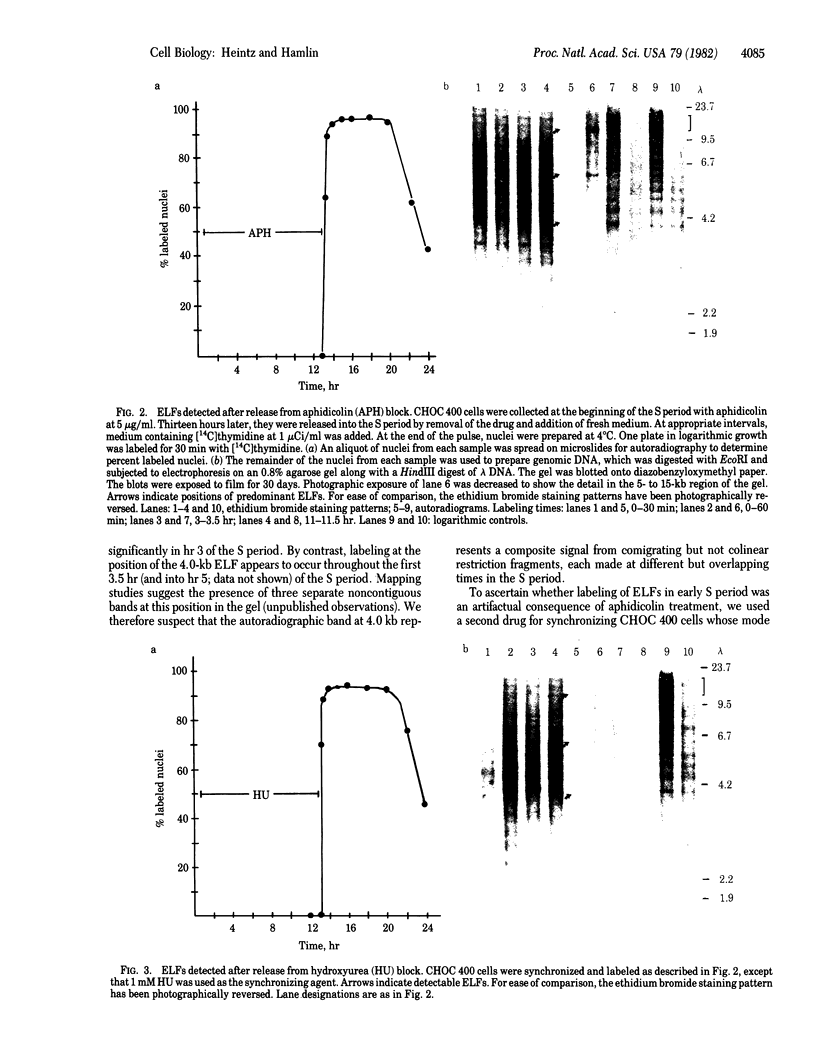
We have developed a methotrexate-resistant Chinese hamster ovary cell line (CHOC 400) containing a 500-fold amplification of a 135-kilobase chromosomal DNA sequence. This sequence includes the gene for dihydrofolate reductase (tetrahydrofolate dehydrogenase, 5,6,7,8-tetrahydrofolate: NADP+ oxidoreductase, EC 1.5.1.3). The high copy number of the amplified sequence permits it to be visualized as a distinct series of restriction fragments in genomic digests separated on ethidium bromide-stained agarose gels. Initiation of DNA replication in the amplified sequence was studied by radiolabeling DNA synthesized during the onset of S phase in synchronized CHOC 400 cells. Autoradiography of Southern blots of labeled genomic digests shows that DNA synthesis initiates in a small subset of the EcoRI fragments derived from the amplified units. These early labeled fragments are not synthesized at later times during S phase, when different subsets of fragments are synthesized. Regardless of the drug used to collect cells at the beginning of S phase, the replication pattern observed remains the same. These data suggest that replication of the amplified sequence initiates at specific sites within each repeated unit and proceeds in nonrandom order throughout the remainder of the sequence--i.e., that initiation of DNA synthesis in the chromosomes of mammalian cells is sequence specific.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biedler J. L., Spengler B. A. A novel chromosome abnormality in human neuroblastoma and antifolate-resistant Chinese hamster cell lives in culture. J Natl Cancer Inst. 1976 Sep;57(3):683–695. doi: 10.1093/jnci/57.3.683. [DOI] [PubMed] [Google Scholar]
- Bostock C. J., Clark E. M. Satellite DNA in large marker chromosomes of methotrexate-resistant mouse cells. Cell. 1980 Mar;19(3):709–715. doi: 10.1016/s0092-8674(80)80047-x. [DOI] [PubMed] [Google Scholar]
- Bozzoni I., Baldari C. T., Amaldi F., Buongiorno-Nardelli M. Replication of ribosomal DNA in Xenopus laevis. Eur J Biochem. 1981 Sep 1;118(3):585–590. doi: 10.1111/j.1432-1033.1981.tb05559.x. [DOI] [PubMed] [Google Scholar]
- Courtenay V. D., Robins A. B. Loss of resistance to methotrexate in L5178Y mouse leukemia grown in vitro. J Natl Cancer Inst. 1972 Jul;49(1):45–53. [PubMed] [Google Scholar]
- Denniston-Thompson K., Moore D. D., Kruger K. E., Furth M. E., Blattner F. R. Physical structure of the replication origin of bacteriophage lambda. Science. 1977 Dec 9;198(4321):1051–1056. doi: 10.1126/science.929187. [DOI] [PubMed] [Google Scholar]
- Dhar R., Lai C. J., Khoury G. Nucleotide sequence of the DNA replication origin for human papovavirus BKV: sequence and structural homology with SV40. Cell. 1978 Feb;13(2):345–358. doi: 10.1016/0092-8674(78)90203-9. [DOI] [PubMed] [Google Scholar]
- Edenberg H. J., Huberman J. A. Eukaryotic chromosome replication. Annu Rev Genet. 1975;9:245–284. doi: 10.1146/annurev.ge.09.120175.001333. [DOI] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Grosschedl R., Hobom G. DNA sequences and structural homologies of the replication origins of lambdoid bacteriophages. Nature. 1979 Feb 22;277(5698):621–627. doi: 10.1038/277621a0. [DOI] [PubMed] [Google Scholar]
- Hamlin J. L., Biedler J. L. Replication pattern of a large homogenously staining chromosome region in antifolate-resistant Chinese hamster cell lines. J Cell Physiol. 1981 Apr;107(1):101–114. doi: 10.1002/jcp.1041070112. [DOI] [PubMed] [Google Scholar]
- Hamlin J. L., Pardee A. B. S phase synchrony in monolayer CHO cultures. Exp Cell Res. 1976 Jul;100(2):265–275. doi: 10.1016/0014-4827(76)90147-6. [DOI] [PubMed] [Google Scholar]
- Hand R. Eucaryotic DNA: organization of the genome for replication. Cell. 1978 Oct;15(2):317–325. doi: 10.1016/0092-8674(78)90001-6. [DOI] [PubMed] [Google Scholar]
- Harland R. M., Laskey R. A. Regulated replication of DNA microinjected into eggs of Xenopus laevis. Cell. 1980 Oct;21(3):761–771. doi: 10.1016/0092-8674(80)90439-0. [DOI] [PubMed] [Google Scholar]
- Huberman J. A. New views of the biochemistry of eucaryotic DNA replication revealed by aphidicolin, an unusual inhibitor of DNA polymerase alpha. Cell. 1981 Mar;23(3):647–648. doi: 10.1016/0092-8674(81)90426-8. [DOI] [PubMed] [Google Scholar]
- Huberman J. A., Riggs A. D. On the mechanism of DNA replication in mammalian chromosomes. J Mol Biol. 1968 Mar 14;32(2):327–341. doi: 10.1016/0022-2836(68)90013-2. [DOI] [PubMed] [Google Scholar]
- Kaguni L. S., Clayton D. A. Template-directed pausing in in vitro DNA synthesis by DNA polymerase a from Drosophila melanogaster embryos. Proc Natl Acad Sci U S A. 1982 Feb;79(4):983–987. doi: 10.1073/pnas.79.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J., Brown P. C., Schimke R. T. Amplified dihydrofolate reductase genes in unstably methotrexate-resistant cells are associated with double minute chromosomes. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5669–5673. doi: 10.1073/pnas.76.11.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Langeveld S. A., van Mansfeld A. D., Baas P. D., Jansz H. S., van Arkel G. A., Weisbeek P. J. Nucleotide sequence of the origin of replication in bacteriophage phiX174 RF DNA. Nature. 1978 Feb 2;271(5644):417–420. doi: 10.1038/271417a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Harland R. M. Replication origins in the eucaryotic chromosome. Cell. 1981 May;24(2):283–284. doi: 10.1016/0092-8674(81)90316-0. [DOI] [PubMed] [Google Scholar]
- McCutchan T. F., Singer M. F. DNA sequences similar to those around the simian virus 40 origin of replication are present in the monkey genome. Proc Natl Acad Sci U S A. 1981 Jan;78(1):95–99. doi: 10.1073/pnas.78.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Bustin M., Miller O. L., Jr Electron microscopic analysis of chromosome metabolism in the Drosophila melanogaster embryo. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):741–754. doi: 10.1101/sqb.1978.042.01.075. [DOI] [PubMed] [Google Scholar]
- Milbrandt J. D., Heintz N. H., White W. C., Rothman S. M., Hamlin J. L. Methotrexate-resistant Chinese hamster ovary cells have amplified a 135-kilobase-pair region that includes the dihydrofolate reductase gene. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6043–6047. doi: 10.1073/pnas.78.10.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans D. Restriction endonucleases, simian virus 40, and the new genetics. Science. 1979 Nov 23;206(4421):903–909. doi: 10.1126/science.228393. [DOI] [PubMed] [Google Scholar]
- Shlomai J., Kornberg A. An Escherichia coli replication protein that recognizes a unique sequence within a hairpin region in phi X174 DNA. Proc Natl Acad Sci U S A. 1980 Feb;77(2):799–803. doi: 10.1073/pnas.77.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims J., Benz E. W., Jr Initiation of DNA replication by the Escherichia coli dnaG protein: evidence that tertiary structure is involved. Proc Natl Acad Sci U S A. 1980 Feb;77(2):900–904. doi: 10.1073/pnas.77.2.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog L., Nordenskjöld B. Effects of hydroxyurea and 1-beta-D-arabinofuranosyl-cytosine on deoxyribonucleotide pools in mouse embryo cells. Eur J Biochem. 1971 Mar 1;19(1):81–89. doi: 10.1111/j.1432-1033.1971.tb01290.x. [DOI] [PubMed] [Google Scholar]
- Soeda E., Arrand J. R., Smolar N., Griffin B. E. Sequence from early region of polyoma virus DNA containing viral replication origin and encoding small, middle and (part of) large T antigens. Cell. 1979 Jun;17(2):357–370. doi: 10.1016/0092-8674(79)90162-4. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda S., Hirota Y. Cloning and mapping of the replication origin of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5458–5462. doi: 10.1073/pnas.74.12.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyskind J. W., Deen L. T., Smith D. W. Isolation and mapping of plasmids containing the Salmonella typhimurium origin of DNA replication. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3097–3101. doi: 10.1073/pnas.76.7.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]