Abstract
This study used two mouse models with genetic manipulation of the melanocortin system to investigate prolactin regulation. Mice with overexpression of the melanocortin receptor (MC-R) agonist, α-melanocyte-stimulating hormone (Tg-MSH) or deletion of the MC-R antagonist agouti-related protein (AgRP KO) were studied. Male Tg-MSH mice had lower blood prolactin levels at baseline (2.9±0.3 vs 4.7±0.7 ng/ml) and after restraint stress(68 ±6.5 vs 117±22 ng/ml) versus WT (p<0.05); however, pituitary prolactin content was not different. Blood prolactin was also decreased in male AgRP KO mice at baseline (4.2±0.5 vs 7.6±1.3 ng/ml) and after stress (60±4.5 vs 86.1±5.7 ng/ml) vs WT (p <0.001). Pituitary prolactin content was lower in male AgRP KO mice (4.3±0.3 vs 6.7±0.5 μg/pituitary, p <0.001) versus WT. No differences in blood or pituitary prolactin levels were observed in female AgRP KO mice versus WT. Hypothalamic dopamine activity was assessed as the potential mechanism responsible for changes in prolactin levels. Hypothalamic tyrosine hydroxylase mRNA was measured in both genetic models versus WT mice and hypothalamic dopamine and 3,4-dihydroxyphenylacetic acid (DOPAC) content were measured in male AgRP KO and WT mice but neither were significantly different. However, these results do not preclude changes in dopamine activity as dopamine turnover was not directly investigated. This is the first study to show that baseline and stress-induced prolactin release and pituitary prolactin content are reduced in mice with genetic alterations of the melanocortin system and suggests that changes in hypothalamic melanocortin activity may be reflected in measurements of serum prolactin levels.
Keywords: melanocortin, α-MSH, AgRP, prolactin
1. Introduction
Prolactin is a lactogenic hormone secreted by the anterior pituitary that normally increases during pregnancy and lactation [8]. Although a number of different factors have been shown to modulate prolactin release, the most critical factor is dopamine produced by tuberoinfundibular dopamine (TIDA) neurons in the arcuate nucleus of the hypothalamus [1]. These neurons project to the median eminence where dopamine is released into hypophyseal portal blood and subsequently binds to dopamine-type 2 (D2) receptors in lactotrophs and inhibits prolactin synthesis and release [1]. Although prolactin releasing factors do exist, prolactin secretion is primarily under tonic inhibitory dopamine control such that antagonism of D2 receptors causes a robust stimulation of prolactin release [1]. Prolactin is also regulated by a short-loop feedback system, in which prolactin inhibits its own release by binding to prolactin receptors on TIDA neurons [17, 22]. Many factors can regulate prolactin release via modulation of the TIDA system. The hypothalamic neuropeptides α-MSH and AgRP are part of the brain melanocortin system and have been shown to inhibit and stimulate prolactin release respectively when injected icv in rodents and primates [13–15, 27, 38, 40, 47]. These peptides are produced by POMC and AgRP neurons in the arcuate nucleus and play a critical role in regulating energy balance and metabolism [21]. Proopiomelanocortin (POMC) is post-translationally processed to α-MSH which is the agonist for MC3-R and MC4-Rs in the brain and promotes negative energy balance, while AgRP is the antagonist for these receptors and promotes positive energy balance [21]. The melanocortin system also has a number of neuroendocrine effects on the thyroid, gonadal and adrenal axes [11, 19, 37]. With respect to prolactin secretion, acute central injection of α-MSH suppresses basal blood prolactin levels as well as blunts increases in prolactin due to stress, interleukin 1-α administration, estrogen treatment, or the surge on the day of proestrus [15, 27, 38, 40]. Conversely, AgRP has been shown to increase prolactin release and AgRP-induced increases in prolactin are blocked by α-MSH [47]. The effects of α-MSH on prolactin release have been shown to be mediated by dopamine as α-MSH cannot block dopamine-receptor antagonist induced increases in prolactin levels [15, 38]. Furthermore, icv α-MSH increases DOPAC and 3,4-dihydroxyphenylalanine (DOPA) content specifically in the median eminence [23].
There is evidence that endogenous α-MSH may play a physiological role in prolactin regulation as icv injection of an α-MSH antiserum has been shown to enhance basal and stress-induced prolactin release in the rat [13]. There is also evidence of an anatomical connection between POMC and TIDA neurons as revealed by electron microscopy showing synaptic connections between POMC immunoreactive axon terminals and tyrosine hydroxylase-immunopositive cell bodies and dendrites [10]. However, in contrast at the pituitary level, there are some reports of stimulatory effects of α-MSH on prolactin including stimulation from cultured mouse pituitary cells [25, 45, 46].
It was unknown how chronic changes in hypothalamic melanocortin activity would affect pituitary prolactin content and release. Therefore, in this study, genetic models of increased melanocortin signaling were employed to determine if chronic activation of the melanocortin system by either increasing α-MSH or eliminating AgRP signaling would suppresses prolactin levels. We used mice with overexpression of an N-terminal POMC transgene (Tg-MSH) that includes α- and γ3-MSH (but not ACTH or β-EP) which were previously reported to possess a leaner phenotype, and mice with selective AgRP deletion, which were reported to possess a metabolic phenotype similar to WT counterparts [20, 31, 36]. Although the metabolic phenotypes have been carefully described, the effects on prolactin regulation are unknown. In both mouse models, we investigated blood prolactin levels under both unstressed and stressed conditions and measured pituitary prolactin content. Male mice were used in most experiments but these parameters were investigated in female AgRP KO mice as well. For comparison, basal and stress-induced corticosterone levels were measured in parallel in several of these experiments. To investigate the mechanism responsible for alterations in prolactin levels we measured mediobasal hypothalamic (MBH) tyrosine hydroxylase activity and dopamine and DOPAC content, as well as the effects of functional dopamine receptor antagonism.
2. Materials and Methods
2.1 Animals and treatment protocols
All animals were housed under barrier conditions with a 12-h light, 12-h dark cycle. Animals had ad libitum access to water and rodent chow. Mice were handled regularly to limit any stress while procedures were performed. All protocols were approved by the Columbia University Institutional Animal Care and Use Committee and were conducted in accordance with the NIH guide for the care and use of laboratory animals.
Tg-MSH
Transgenic mice were generated as described previously to overexpress NH2-terminal POMC under the control of the cytomegalovirus (CMV) promoter [36]. The transgene contained part of the 5′UTR, the signal sequence, the sorting sequence, γ3-MSH, the joining peptide, and α-MSH, including the COOH-terminal glycine necessary for amidation. The transgene was expressed in multiple tissues, including the hypothalamus and pituitary. Mice were backcrossed to a coisogenic C57BL/6J line, C57BL/6J-AwJstrain for six generations, and used for the current studies. This is a white-bellied agouti-colored line, which was used instead of black mice to visualize the darkening effect of MSH on coat color. Studies were performed in transgenic homozygous mice with control mice being wild-type (WT) animals generated from the backcross at the N6 generation.
AgRP KO
The AgRP KO mouse line was obtained from Dr. Van der Ploeg [31]. Mice were backcrossed to a coisogenic C57BL/6J line, C57BL/6J-AwJ strain for seven generations. AgRP KO and WT mice were generated from homozygous matings and mice were used in the experiments as indicated. Mice were genotyped using the following primers: F1: 5′GCTTCTTCAATGCCTTTTGC3′, F2: 5′GCCAGAGGCCACTTGTGTAG 3′, R: 5′GTTTCGGAGCCAAATGGTTA3′.
Stress-experiments
Animals were placed under light restraint stress for 5 or 15 minutes using TV-150 (Braintree Scientific, Braintree, MA) and 25 ul of blood was obtained after stress.
2.2 Experimental procedures
2.2.1 Experiment 1
Effects of transgenic MSH overexpression on blood and pituitary prolactin levels. Experiment 1a: A trunk blood sample was obtained at sacrifice from non-stressed male Tg-MSH and WT mice (n=40 or 43/group). Experiment 1b: A trunk blood sample was obtained at sacrifice in male Tg-MSH and WT mice (n=9–10/group) after 5 minutes of restraint stress. Experiment 1c: At sacrifice, pituitary was collected from male Tg-MSH and WT mice (n=8 or 10/group).
2.2.2 Experiment 2
Effects of AgRP deletion on blood and pituitary prolactin levels. Experiment 2a: A submandibular blood sample was obtained from non-stressed male AgRP KO and WT mice (n=17 or 20/group) acclimated to handling. Experiment 2b: Trunk blood was obtained at sacrifice from non-stressed male AgRP KO and WT mice (n=8 or 11/group). Experiment 2c: Mice from Experiment 2arecovered and were subjected to restraint stress. Measurements were obtained 5 and 15 minutes after stress (n=17–18/group). Experiment 2d: Male AgRP KO and WT mice (n=16/group) were sacrificed and the pituitary was collected. Experiment 2e: Female AgRP KO and WT mice (n=9–10/group) were utilized in this experiment. The stage of the estrus cycle was monitored by daily vaginal smear. Blood samples were obtained on metestrus to minimize cyclic fluctuations in prolactin [2]. After 5 and 15 minutes of restraint stress, a blood sample was obtained. Mice were sacrificed approximately 1.5 hours later and the pituitary was collected.
2.2.3 Experiment 3
Effects of transgenic MSH overexpression or AgRP deletion on MBH tyrosine hydroxylase mRNA levels. Experiment 3a: Male Tg-MSH and WT mice (n=8/group) were sacrificed and an MBH dissection was obtained for RNA analyses. Experiment 3b: Male AgRP KO and WT mice (n=7–8/group) were sacrificed and an MBH dissection was obtained for RNA analyses.
2.2.4 Experiment 4
Effects of AgRP deletion on MBH dopamine and DOPAC content. Male AgRP KO and WT mice (n=7/group) were sacrificed and an MBH dissection (~10 mg tissue) was collected for high-performance liquid chromatography (HPLC) analyses. Samples were homogenized in 0.1 N perchloric acid (PCA) with 50 ng/mL of 3,4 dihydroxybenzylamine (DHBA), which was utilized as an internal standard. Dopamine and DOPAC content were determined by HPLC-EC and normalized to protein content in each sample as previously described [26].
2.2.5 Experiment 5
Effects of AgRP deletion on serum prolactin levels after dopamine receptor antagonism. Male AgRP KO and WT mice (n=9 or 11/group) were utilized in this experiment. A submandibular blood sample was obtained from non-stressed mice. After two weeks, mice received a subcutaneous metoclopramide injection (2 μg/g body weight), and a blood sample was obtained after 30 minutes.
2.3 RNA and hormone measurements
2.3.1 Measurement of hypothalamic RNA levels
RNA isolation was performed using the RNeasy Lipid Tissue Mini Kit (Qiagen USA, Valencia, CA) in conjunction with the RNase-Free DNase set (Qiagen USA) under the manufacturer’s instructions and total RNA was quantified using spectrophotometry. cDNA was synthesized using the Superscript III First-Strand cDNA Synthesis Kit (Life Technologies Corporation/Invitrogen, Grand Island, NY) and was analyzed using quantitative RT- PCR performed with Lightcycler 480 SYBR Green I Master (Roche Applied Science, Indianapolis, IN) in the Lightcycler 480 Real-Time PCR system (Roche Applied Science). Samples were normalized to β-actin. Primer sequence: Tyrosine Hydroxylase: F5′CGAGGTGCCCAGTGGCGACC 3′ R 5′GGTCCAGGTCCAGGTCAGGG 3′.
2.3.2 Blood and pituitary hormone measurements
Blood was collected as plasma or serum and stored at −80° C. The pituitary was homogenized in 0.1 N HCl and used for analyses. Prolactin levels were measured by a double antibody RIA with an antiserum to mouse prolactin and reference preparations provided by the National Hormone and Pituitary Program. Purified mouse prolactin was iodinated with I-125 by the lactoperoxidase method for use as tracer. Corticosterone levels were measured using a sensitive double antibody RIA from MP Biomedicals (Irvine, CA USA) [30].
2.4 Statistical Analysis
Statistical analysis
Statistical analysis was performed with Student’s t test. P < 0.05 was considered statistically significant. Results are reported as mean values ± SEM.
3. Results
3.1 Experiment 1: Effects of transgenic MSH overexpression on blood and pituitary prolactin levels
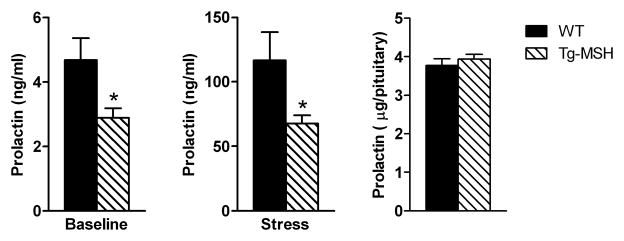
Three different groups of mice were used in this experiment. In the first group, male Tg-MSH mice were found to have significantly lower baseline plasma prolactin levels at sacrifice compared to WT mice (p<0.05, Fig 1A). A second set of mice were exposed to restraint stress for 5 minutes and then sacrificed; plasma prolactin levels were also found to be lower in these Tg-MSH mice versus WT mice (p<0.05, Fig 1B). Notably, plasma corticosterone levels were not different in these mice (WT 22.8±2.4 vs Tg-MSH 20.7±1.0 ug/dl, p=0.40). In another cohort of mice, pituitary prolactin content was found to be similar between Tg-MSH and WT mice (Fig 1C).
Figure 1. Effects of transgenic MSH overexpression on blood and pituitary prolactin levels.
(A) Baseline plasma prolactin levels were significantly lower in Tg-MSH mice vs WT mice. (B) Prolactin levels were significantly lower in Tg-MSH mice vs WT mice after 5 minutes of restraint stress. (C) Pituitary prolactin content was similar between Tg-MSH and WT mice. *p<0.05
3.2 Experiment 2: Effects of AgRP deletion on blood and pituitary prolactin levels
3.2.1 Experiments 2a,b
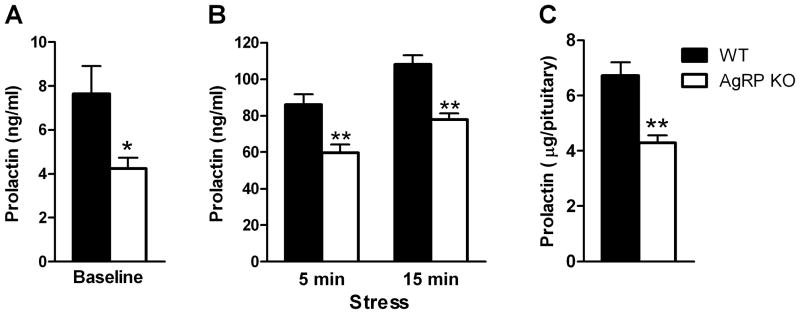
Baseline serum prolactin levels obtained from a submandibular bleed were significantly lower in male AgRP KO mice (p<0.05, Fig 2A). Serum corticosterone levels were also measured in these mice and were found to be similar between groups (WT 1.9±0.3 vs AgRP KO 1.6±0.3 ug/dl, p=0.64). In a separate cohort of AgRP KO mice, plasma prolactin levels at sacrifice were also significantly lower (KO 3.4±0.5 vs WT 7.9±1.6 ng/ml, p<0.05).
Figure 2. Effects of AgRP deletion on blood and pituitary prolactin levels in male mice.
(A) Baseline prolactin levels were significantly lower in male AgRP KO vs WT mice. (B) Prolactin levels were significantly reduced after 5 and 15 minutes of restraint stress in male AgRP KO vs WT mice. (C) Pituitary prolactin content was significantly lower in male AgRP KO vs WT mice. *p<0.05, **p<0.001
3.2.2 Experiment 2c
Mice from Experiment 2a were subjected to restraint stress which increased serum prolactin levels; however stress-induced prolactin levels were still significantly lower in AgRP KO versus WT mice at both the 5 and 15 minute timepoints (p<0.001, Fig 2B). Serum corticosterone levels were analyzed in a subset of these mice after stress (n=9 or 11/group) and it was found that stress-induced corticosterone levels were not significantly lower in AgRP KO versus WT mice at either the 5 or 15 minute timepoints (data not shown).
3.2.3 Experiment 2d
In another cohort of male mice, pituitary prolactin content was found to be significantly lower in AgRP KO vs WT mice (p<0.001, Fig 2C).
3.2.4 Experiment 2e
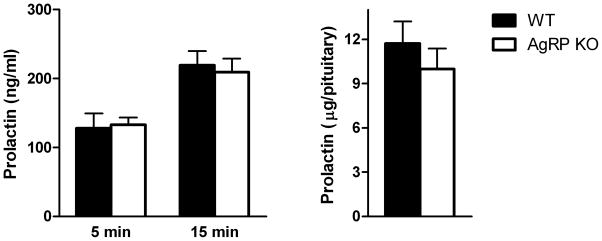
The estrous cycle of female AgRP KO and WT mice was monitored daily and measurements were obtained on metestrus. Stress-induced serum prolactin levels were not different between AgRP KO and WT mice after either 5 or 15 minutes of restraint stress. Pituitary prolactin content tended to be lower in AgRP KO versus WT mice; however this did not reach significance (p=0.23) (Fig 3A,B).
Figure 3. Effects of AgRP deletion on blood and pituitary prolactin levels in female mice.
(A) Prolactin levels were similar in female AgRP KO mice compared to WT mice after both 5 and 15 minutes of restraint stress. (B) Pituitary prolactin content was not different between female AgRP KO and WT mice (p=.23).
3.3 Experiment 3: Effects of transgenic MSH overexpression or AgRP deletion on MBH tyrosine hydroxylase mRNA levels
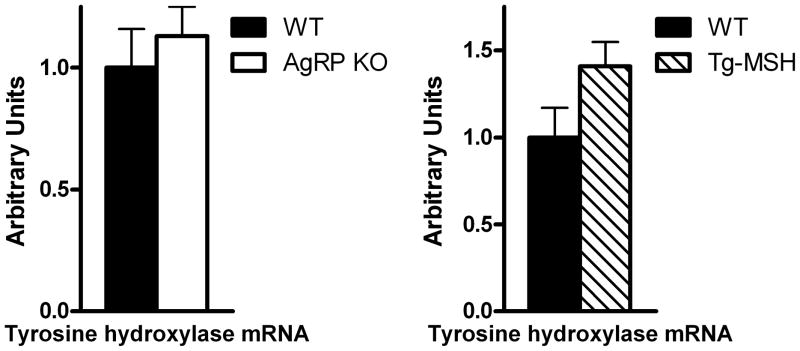
As tyrosine hydroxylase is the rate-limiting step in dopamine synthesis [5], we sought to investigate mRNA levels in the MBH of AgRP KO and WT mice and Tg-MSH and WT mice. Both the AgRP KO (p=0.29) and Tg-MSH (p=0.08) mice tended to have higher tyrosine hydroxylase mRNA expression compared to their respective WT counterparts; however, this did not reach significance (Fig 4A,B).
Figure 4. Effects of transgenic MSH overexpression or AgRP deletion on MBH tyrosine hydroxylase mRNA expression.
(A) No significant difference in tyrosine hydroxylase mRNA was detected between AgRP KO and WT mice (p=0.29). (B) Tyrosine hydroxylase mRNA was not significantly different between Tg-MSH vs WT mice, however expression tended to be higher in Tg-MSH mice (p=0.08).
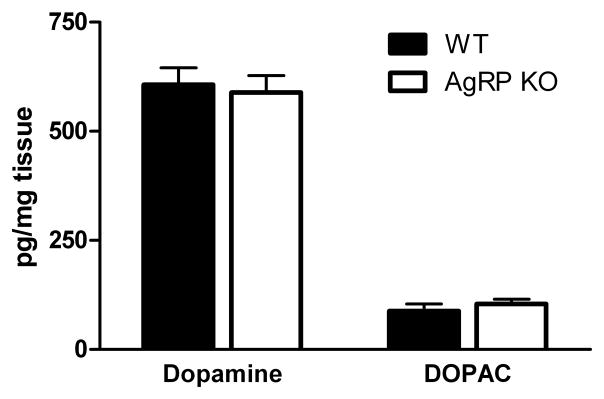
3.4 Experiment 4: Effects of AgRP deletion on MBH dopamine and DOPAC content
MBH dopamine and DOPAC content were measured by HPLC from AgRP KO and WT mice. Analyses revealed that dopamine and DOPAC content were not different between groups (Fig 5).
Figure 5. Effects of AgRP deletion on mediobasal hypothalamic dopamine and DOPAC levels.
Dopamine and DOPAC levels were measured in AgRP KO and WT mice and were found to be similar between groups.
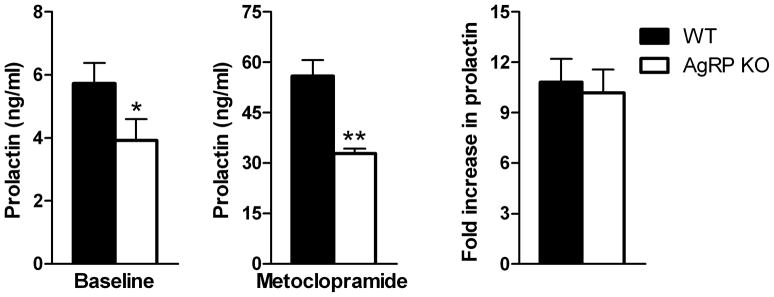
3.5 Experiment 5: Effects of AgRP deletion on serum prolactin levels after dopamine receptor antagonism
Baseline serum prolactin levels were significantly lower in AgRP KO vs WT mice (p<0.05, Fig 6A). Mice were injected subcutaneously with metoclopramide and prolactin levels were measured 30 minutes later. Serum prolactin levels increased with metoclopramide treatment; however, these levels were still significantly lower in AgRP KO mice (p<0.001, Fig 6B). The fold-increase in prolactin levels from baseline was also similar between groups (Fig 6C).
Figure 6. Effects of AgRP deletion on serum prolactin levels after dopamine receptor antagonism.
Serum prolactin measurements were obtained from AgRP KO and WT mice before and after metoclopramide treatment. (A) Baseline prolactin levels were lower in AgRP KO vs WT mice. (B) Thirty minutes after metoclopramide injection prolactin levels were still lower in AgRP KO vs WT mice. (C) The fold-increase in prolactin levels after metoclopramide treatment was similar between groups.*p<0.05, **p<0.001
4. Discussion
This study demonstrates that prolactin levels are lower with two different genetic manipulations of the melanocortin system resulting in either overexpression of the MC-R agonist α-MSH, or deletion of the antagonist AgRP. In male Tg-MSH and AgRP KO mice, both baseline and stress-induced blood prolactin levels were significantly lower compared to WT mice, and further, pituitary prolactin content was lower in male AgRP KO mice. However, no differences in serum or pituitary prolactin levels were detected in female AgRP KO mice. As dopamine is well recognized to inhibit prolactin secretion [1], dopamine metabolism was also investigated. Tyrosine hydroxylase mRNA, the rate-limiting step in dopamine synthesis [5], was measured in the MBH of both mouse models, and although a trend for increased levels was evident, it did not reach significance in either group. Hypothalamic dopamine and DOPAC content were also examined in male AgRP KO and WT mice; however, no differences were detected. Finally, we used the D2 receptor antagonist metoclopramide to investigate functional dopamine receptor antagonism and found that prolactin levels were similarly increased in both groups after treatment.
Previous studies have shown that the melanocortin system can acutely regulate prolactin secretion. Central injections of α-MSH can suppress prolactin levels [15, 27, 38]. Oppositely icv AgRP increases prolactin levels and these increases can be blocked by α-MSH [47]. Furthermore, the mu-opioid receptor agonist β-EP, which is not part of the melanocortin system per se, is also derived from POMC and interacts with α-MSH to regulate prolactin secretion by modulating hypothalamic dopamine turnover [6, 9, 44]. There is evidence that endogenous α-MSH may play a physiological role in prolactin regulation as icv injection of an α-MSH antiserum has been shown to enhance basal and stress-induced prolactin release in the rat [14, 15]. In contrast, there are several reports demonstrating stimulatory effects of α-MSH on prolactin. α-MSH has been shown to stimulate prolactin release and DNA replication in cultured mouse lactotrophs and to sensitize these cells to other secretagogues [25, 29]. This appears to be via stimulation of the MC3-R that is expressed by lactotrophs [25]. There is also a report that an α-MSH antiserum attenuates stimulation of prolactin by leptin [45]. Thus α-MSH may modulate prolactin release at both the pituitary and hypothalamic level. Most studies have only investigated the relatively acute effects of α-MSH on prolactin release. Our study is the first to demonstrate that chronic altered melanocortin signaling can affect prolactin levels.
Several lines of evidence support a role for hypothalamic dopamine in mediating the effects of α-MSH on prolactin secretion. For example, icv α-MSH can no longer inhibit prolactin secretion in the presence of a dopamine-receptor antagonist [15, 38]. Anatomic studies have also shown that POMC-immunoreactive terminals contact tyrosine hydroxylase-positive cell bodies and dendrites in the hypothalamus of rats [10], showing a physical interaction between these neurons. Although the MC3-R is highly expressed in the arcuate where TIDA neurons are localized, it is at present unclear which MC-R mediates effects of α-MSH on TIDA activity. In this study, we were unable to show that increased dopaminergic activity was responsible for the suppression of prolactin levels in AgRP KO mice. We investigated tyrosine hydroxylase gene expression and dopamine and DOPAC content in the MBH of AgRP KO and WT mice. Although we did not detect a change in tyrosine hydroxylase mRNA levels, this does not preclude a change in enzyme activity as tyrosine hydroxylase activity was not measured. Furthermore, it should be noted that the failure to detect changes in static measurements of dopamine and DOPAC content does not preclude a role for dopamine in mediating the observed effects on prolactin, as dopamine turnover was not directly assessed. We also used a D2R antagonist metoclopramide to investigate if blocking this receptor would yield a greater fold increase in prolactin release in AgRP KO mice compared to WT mice. However, we found that prolactin levels were elevated to a similar degree after D2R antagonism in AgRP KO and WT mice. It is possible that the chronically lower pituitary prolactin content in these mice affected their response to metoclopramide.
It is of interest that despite similarly lower blood prolactin levels in male AgRP KO and Tg-MSH mice, pituitary prolactin content was only lower in the AgRP KO mice. It should be noted that Tg-MSH mice express the transgene in the pituitary as well and have higher circulating levels of α-MSH [36]. It is possible that α-MSH acting at the pituitary level prevented the fall in pituitary prolactin content in the Tg-MSH mice. This would be consistent with the stimulatory effect of α-MSH on prolactin release reported in vitro in cultured lactotrophs[45]. However, given that basal and stress-induced prolactin release is suppressed in Tg-MSH mice, the effects of α-MSH on the dopaminergic regulation of prolactin appears to predominate.
In several experiments, we observed that while prolactin levels were suppressed at either baseline or after stress in AgRP KO or MSH-Tg mice, corticosterone measured in the same mice were not different between groups. This is important as it shows that the mechanisms responsible for stress-induced prolactin and corticosterone release in these models are distinct and only prolactin release was affected by these genetic manipulations. A sexually dimorphic effect of AgRP deletion on prolactin levels was also noted in this study. Although reproductive function was not formally assessed, AgRP KO and WT mice appeared to reproduce with equal frequency and yield similar litter sizes. As prolactin levels are critical for reproduction and nursing including maternal behavior, it is possible that there was developmental compensation in females and hence lower prolactin levels were not evident in female mice as they were in male mice [8]. Indeed, there is evidence for developmental compensation in AgRP KO mice with respect to energy balance [31]. In this study, only stress-induced prolactin release and pituitary content were measured in female AgRP KO mice at one stage of the estrus cycle; however, there could be changes under other conditions such as pregnancy or suckling that were not examined.
In addition to the data showing that α-MSH may regulate dopamine turnover in the hypothalamus, there is evidence that dopamine may regulate POMC in the hypothalamus [24, 33, 43]. Furthermore, a positive correlation was reported between Pomc mRNA and tyrosine hydroxylase mRNA in the MBH of the rat suggesting coregulation or functional interaction between these two neuronal systems [32]. Thus there is evidence for bidirectional interactions between the hypothalamic dopamine system and melanocortin pathways that play critical roles in maintaining energy homeostasis [4, 12, 18, 39, 42].
This study provides additional evidence to support functional interactions between the hypothalamic melanocortin and dopaminergic systems that may be reflected by plasma prolactin levels. Prolactin is known to affect energy balance and fuel metabolism and has also been shown to stimulate AgRP [3, 7, 28, 35, 41]. There is some evidence that prolactin is elevated in human obesity and it has been postulated that the elevations in prolactin may result from decreased inhibitory input from hypothalamic dopaminergic neurons [16, 34]. It may thus be that elevated prolactin levels are a marker for decreased brain dopaminergic activity which in turn reflects a decrease in hypothalamic melanocortin activity. The current study shows that prolactin levels are reduced in genetic models of increased melanocortin signaling and suggests that melanocortin induced changes in dopamine turnover may be reflected in measurements of serum prolactin levels. The hypothesis that circulating prolactin levels may serve as a biomarker of central melanocortinergic and dopaminergic tone deserves further study.
Highlights.
Prolactin was studied in mice with genetic manipulation of the melanocortin system
Mice with MSH overexpression (Tg-MSH) and AgRP deletion (AgRP KO) were utilized
Baseline and stress-induced blood prolactin levels were reduced in Tg-MSH vs WT
Blood and pituitary prolactin levels were reduced in AgRP KO vs WT
Differences inhypothalamic tyrosine hydroxylase and dopamine were not detected
Acknowledgments
This work was supported by NIH Grant DK08003 (S.L.W.).
We would like to thank Kana Meece and Shveta Dighe for their technical assistance.
Abbreviations
- ACTH
Adrenocorticotropin
- AgRP
Agouti-Related Protein
- β-EP
β-Endorphin
- CMV
Cytomegalovirus
- D2R
Dopamine Type-2 Receptor
- DHBA
3,4 dihydroxybenzylamine
- DOPA
3,4-dihydroxyphenylalanine
- DOPAC
3,4-dihydroxyphenylacetic acid
- MBH
mediobasal hypothalamus
- MC-R
melanocortin receptor
- α-MSH
α-melanocyte-stimulating hormone
- PCA
perchloric acid
- POMC
proopiomelanocortin
- Tg-MSH
MSH-overexpressing mice
- TIDA
tuberoinfundibular dopaminergic
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ben-Jonathan N. Dopamine: a prolactin-inhibiting hormone. Endocr Rev. 1985;6:564–89. doi: 10.1210/edrv-6-4-564. [DOI] [PubMed] [Google Scholar]
- 2.Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–8. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- 3.Byatt JC, Staten NR, Salsgiver WJ, Kostelc JG, Collier RJ. Stimulation of food intake and weight gain in mature female rats by bovine prolactin and bovine growth hormone. Am J Physiol. 1993;264:E986–92. doi: 10.1152/ajpendo.1993.264.6.E986. [DOI] [PubMed] [Google Scholar]
- 4.Cincotta AH, Tozzo E, Scislowski PW. Bromocriptine/SKF38393 treatment ameliorates obesity and associated metabolic dysfunctions in obese (ob/ob) mice. Life Sci. 1997;61:951–6. doi: 10.1016/s0024-3205(97)00599-7. [DOI] [PubMed] [Google Scholar]
- 5.Daubner SC, Le T, Wang S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys. 2011;508:1–12. doi: 10.1016/j.abb.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deyo SN, Swift RM, Miller RJ. Morphine and endorphins modulate dopamine turnover in rat median eminence. Proc Natl Acad Sci U S A. 1979;76:3006–9. doi: 10.1073/pnas.76.6.3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freemark M, Fleenor D, Driscoll P, Binart N, Kelly P. Body weight and fat deposition in prolactin receptor-deficient mice. Endocrinology. 2001;142:532–7. doi: 10.1210/endo.142.2.7979. [DOI] [PubMed] [Google Scholar]
- 8.Grattan DR, Kokay IC. Prolactin: a pleiotropic neuroendocrine hormone. J Neuroendocrinol. 2008;20:752–63. doi: 10.1111/j.1365-2826.2008.01736.x. [DOI] [PubMed] [Google Scholar]
- 9.Gudelsky GA, Porter JC. Morphine- and opioid peptide-induced inhibition of the release of dopamine from tuberoinfundibular neurons. Life Sci. 1979;25:1697–702. doi: 10.1016/0024-3205(79)90411-9. [DOI] [PubMed] [Google Scholar]
- 10.Horvath TL, Naftolin F, Leranth C. Beta-endorphin innervation of dopamine neurons in the rat hypothalamus: a light and electron microscopic double immunostaining study. Endocrinology. 1992;131:1547–55. doi: 10.1210/endo.131.3.1354605. [DOI] [PubMed] [Google Scholar]
- 11.Israel DD, Sheffer-Babila S, de Luca C, Jo YH, Liu SM, Xia Q, et al. Effects of Leptin and Melanocortin Signaling Interactions on Pubertal Development and Reproduction. Endocrinology. 2012;153:2408–19. doi: 10.1210/en.2011-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones B, Basson BR, Walker DJ, Crawford AM, Kinon BJ. Weight change and atypical antipsychotic treatment in patients with schizophrenia. J Clin Psychiatry. 2001;62 (Suppl 2):41–4. [PubMed] [Google Scholar]
- 13.Khorram O, Bedran de Castro JC, McCann SM. Physiological role of alpha-melanocyte-stimulating hormone in modulating the secretion of prolactin and luteinizing hormone in the female rat. Proc Natl Acad Sci U S A. 1984;81:8004–8. doi: 10.1073/pnas.81.24.8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khorram O, Bedran de Castro JC, McCann SM. Stress-induced secretion of alpha-melanocyte-stimulating hormone and its physiological role in modulating the secretion of prolactin and luteinizing hormone in the female rat. Endocrinology. 1985;117:2483–9. doi: 10.1210/endo-117-6-2483. [DOI] [PubMed] [Google Scholar]
- 15.Khorram O, Mizunuma H, McCann SM. Effect of alpha-melanocyte-stimulating hormone on basal and stimulated release of prolactin: evidence for dopaminergic mediation. Neuroendocrinology. 1982;34:433–7. doi: 10.1159/000123341. [DOI] [PubMed] [Google Scholar]
- 16.Kok P, Roelfsema F, Frolich M, Meinders AE, Pijl H. Prolactin release is enhanced in proportion to excess visceral fat in obese women. J Clin Endocrinol Metab. 2004;89:4445–9. doi: 10.1210/jc.2003-032184. [DOI] [PubMed] [Google Scholar]
- 17.Kokay IC, Grattan DR. Expression of mRNA for prolactin receptor (long form) in dopamine and pro-opiomelanocortin neurones in the arcuate nucleus of non-pregnant and lactating rats. J Neuroendocrinol. 2005;17:827–35. doi: 10.1111/j.1365-2826.2005.01374.x. [DOI] [PubMed] [Google Scholar]
- 18.Lacruz A, Baptista T, de Mendoza S, Mendoza-Guillen JM, Hernandez L. Antipsychotic drug-induced obesity in rats: correlation between leptin, insulin and body weight during sulpiride treatment. Mol Psychiatry. 2000;5:70–6. doi: 10.1038/sj.mp.4000566. [DOI] [PubMed] [Google Scholar]
- 19.Lechan RM, Fekete C. The TRH neuron: a hypothalamic integrator of energy metabolism. Prog Brain Res. 2006;153:209–35. doi: 10.1016/S0079-6123(06)53012-2. [DOI] [PubMed] [Google Scholar]
- 20.Lee M, Kim A, Chua SC, Jr, Obici S, Wardlaw SL. Transgenic MSH overexpression attenuates the metabolic effects of a high-fat diet. Am J Physiol Endocrinol Metab. 2007;293:E121–31. doi: 10.1152/ajpendo.00555.2006. [DOI] [PubMed] [Google Scholar]
- 21.Lee M, Wardlaw SL. The central melanocortin system and the regulation of energy balance. Front Biosci. 2007;12:3994–4010. doi: 10.2741/2366. [DOI] [PubMed] [Google Scholar]
- 22.Lerant A, Freeman ME. Ovarian steroids differentially regulate the expression of PRL-R in neuroendocrine dopaminergic neuron populations: a double label confocal microscopic study. Brain Res. 1998;802:141–54. doi: 10.1016/s0006-8993(98)00583-6. [DOI] [PubMed] [Google Scholar]
- 23.Lindley SE, Lookingland KJ, Moore KE. Activation of tuberoinfundibular but not tuberohypophysial dopaminergic neurons following intracerebroventricular administration of alpha-melanocyte-stimulating hormone. Neuroendocrinology. 1990;51:394–9. doi: 10.1159/000125381. [DOI] [PubMed] [Google Scholar]
- 24.Matera C, Wardlaw SL. Dopamine and sex steroid regulation of POMC gene expression in the hypothalamus. Neuroendocrinology. 1993;58:493–500. doi: 10.1159/000126582. [DOI] [PubMed] [Google Scholar]
- 25.Matsumura R, Takagi C, Kakeya T, Okuda K, Takeuchi S, Takahashi S. Alpha-melanocyte-stimulating hormone stimulates prolactin secretion through melanocortin-3 receptors expressed in mammotropes in the mouse pituitary. Neuroendocrinology. 2003;78:96–104. doi: 10.1159/000071965. [DOI] [PubMed] [Google Scholar]
- 26.Mosharov EV, Larsen KE, Kanter E, Phillips KA, Wilson K, Schmitz Y, et al. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron. 2009;62:218–29. doi: 10.1016/j.neuron.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newman CB, Wardlaw SL, Frantz AG. Suppression of basal and stress-induced prolactin release and stimulation of luteinizing hormone secretion by alpha-melanocyte-stimulating hormone. Life Sci. 1985;36:1661–8. doi: 10.1016/0024-3205(85)90369-8. [DOI] [PubMed] [Google Scholar]
- 28.Noel MB, Woodside B. Effects of systemic and central prolactin injections on food intake, weight gain, and estrous cyclicity in female rats. Physiol Behav. 1993;54:151–4. doi: 10.1016/0031-9384(93)90057-m. [DOI] [PubMed] [Google Scholar]
- 29.Nunez L, Frawley LS. alpha-MSH potentiates the responsiveness of mammotropes by increasing Ca2+ entry. Am J Physiol. 1998;274:E971–7. doi: 10.1152/ajpendo.1998.274.6.E971. [DOI] [PubMed] [Google Scholar]
- 30.Papadopoulos AD, Wardlaw SL. Endogenous alpha-MSH modulates the hypothalamic-pituitary-adrenal response to the cytokine interleukin-1beta. J Neuroendocrinol. 1999;11:315–9. doi: 10.1046/j.1365-2826.1999.00327.x. [DOI] [PubMed] [Google Scholar]
- 31.Qian S, Chen H, Weingarth D, Trumbauer ME, Novi DE, Guan X, et al. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Mol Cell Biol. 2002;22:5027–35. doi: 10.1128/MCB.22.14.5027-5035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rasmussen DD, Jakubowski M, Allen DL, Roberts JL. Positive correlation between proopiomelanocortin and tyrosine hydroxylase mRNA levels in the mediobasohypothalamus of ovariectomized rats: response to estradiol replacement and withdrawal. Neuroendocrinology. 1992;56:285–94. doi: 10.1159/000126241. [DOI] [PubMed] [Google Scholar]
- 33.Rasmussen DD, Liu JH, Wolf PL, Yen SS. Neurosecretion of human hypothalamic immunoreactive beta-endorphin: in vitro regulation by dopamine. Neuroendocrinology. 1987;45:197–200. doi: 10.1159/000124725. [DOI] [PubMed] [Google Scholar]
- 34.Rojdmark S, Rossner S. Decreased dopaminergic control of prolactin secretion in male obesity: normalization by fasting. Metabolism. 1991;40:191–5. doi: 10.1016/0026-0495(91)90173-t. [DOI] [PubMed] [Google Scholar]
- 35.Sauve D, Woodside B. The effect of central administration of prolactin on food intake in virgin female rats is dose-dependent, occurs in the absence of ovarian hormones and the latency to onset varies with feeding regimen. Brain Res. 1996;729:75–81. [PubMed] [Google Scholar]
- 36.Savontaus E, Breen TL, Kim A, Yang LM, Chua SC, Jr, Wardlaw SL. Metabolic effects of transgenic melanocyte-stimulating hormone overexpression in lean and obese mice. Endocrinology. 2004;145:3881–91. doi: 10.1210/en.2004-0263. [DOI] [PubMed] [Google Scholar]
- 37.Savontaus E, Conwell IM, Wardlaw SL. Effects of adrenalectomy on AGRP, POMC, NPY and CART gene expression in the basal hypothalamus of fed and fasted rats. Brain Res. 2002;958:130–8. doi: 10.1016/s0006-8993(02)03674-0. [DOI] [PubMed] [Google Scholar]
- 38.Scimonelli T, Celis ME. A central action of alpha-melanocyte-stimulating hormone on serum levels of LH and prolactin in rats. J Endocrinol. 1990;124:127–32. doi: 10.1677/joe.0.1240127. [DOI] [PubMed] [Google Scholar]
- 39.Scislowski PW, Tozzo E, Zhang Y, Phaneuf S, Prevelige R, Cincotta AH. Biochemical mechanisms responsible for the attenuation of diabetic and obese conditions in ob/ob mice treated with dopaminergic agonists. Int J Obes Relat Metab Disord. 1999;23:425–31. doi: 10.1038/sj.ijo.0800893. [DOI] [PubMed] [Google Scholar]
- 40.Shalts E, Feng YJ, Ferin M, Wardlaw SL. Alpha-melanocyte-stimulating hormone antagonizes the neuroendocrine effects of corticotropin-releasing factor and interleukin-1 alpha in the primate. Endocrinology. 1992;131:132–8. doi: 10.1210/endo.131.1.1319315. [DOI] [PubMed] [Google Scholar]
- 41.Strader AD, Buntin JD. Changes in agouti-related peptide during the ring dove breeding cycle in relation to prolactin and parental hyperphagia. J Neuroendocrinol. 2003;15:1046–53. doi: 10.1046/j.1365-2826.2003.01092.x. [DOI] [PubMed] [Google Scholar]
- 42.Szczypka MS, Rainey MA, Kim DS, Alaynick WA, Marck BT, Matsumoto AM, et al. Feeding behavior in dopamine-deficient mice. Proc Natl Acad Sci U S A. 1999;96:12138–43. doi: 10.1073/pnas.96.21.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tong Y, Pelletier G. Role of dopamine in the regulation of proopiomelanocortin (POMC) mRNA levels in the arcuate nucleus and pituitary gland of the female rat as studied by in situ hybridization. Brain Res Mol Brain Res. 1992;15:27–32. doi: 10.1016/0169-328x(92)90147-4. [DOI] [PubMed] [Google Scholar]
- 44.Van Vugt DA, Bruni JF, Sylvester PW, Chen HT, Ieiri T, Meites J. Interaction between opiates and hypothalamic dopamine on prolactin release. Life Sci. 1979;24:2361–7. doi: 10.1016/0024-3205(79)90534-4. [DOI] [PubMed] [Google Scholar]
- 45.Watanobe H, Schioth HB, Izumi J. Pivotal roles of alpha-melanocyte-stimulating hormone and the melanocortin 4 receptor in leptin stimulation of prolactin secretion in rats. J Neurochem. 2003;85:338–47. doi: 10.1046/j.1471-4159.2003.01683.x. [DOI] [PubMed] [Google Scholar]
- 46.Watanobe H, Schioth HB, Wikberg JE, Suda T. The melanocortin 4 receptor mediates leptin stimulation of luteinizing hormone and prolactin surges in steroid-primed ovariectomized rats. Biochem Biophys Res Commun. 1999;257:860–4. doi: 10.1006/bbrc.1999.0547. [DOI] [PubMed] [Google Scholar]
- 47.Xiao E, Xia-Zhang L, Vulliemoz NR, Ferin M, Wardlaw SL. Agouti-related protein stimulates the hypothalamic-pituitary-adrenal (HPA) axis and enhances the HPA response to interleukin-1 in the primate. Endocrinology. 2003;144:1736–41. doi: 10.1210/en.2002-220013. [DOI] [PubMed] [Google Scholar]