Abstract
Adipose tissue inflammation is associated with insulin resistance and increased cardiovascular disease risk in obesity. We previously showed that addition of cholesterol to a diet rich in saturated fat and refined carbohydrate significantly worsens dyslipidemia, insulin resistance, adipose tissue macrophage accumulation, systemic inflammation, and atherosclerosis in LDL receptor-deficient (Ldlr−/−) mice. To test whether inhibition of intestinal cholesterol absorption would improve metabolic abnormalities and adipose tissue inflammation in obesity, we administered ezetimibe, a dietary and endogenous cholesterol absorption inhibitor, to Ldlr−/− mice fed chow or high-fat, high-sucrose (HFHS) diets without or with 0.15% cholesterol (HFHS+C). Ezetimibe blunted weight gain and markedly reduced plasma lipids in the HFHS+C group. Ezetimibe had no effect on glucose homeostasis or visceral adipose tissue macrophage gene expression in the HFHS+C fed mice, although circulating inflammatory markers serum amyloid A (SSA) and serum amyloid P (SSP) levels decreased. Nevertheless, ezetimibe treatment led to a striking (>85%) reduction in atherosclerotic lesion area with reduced lesion lipid and macrophage content in the HFHS+C group. Thus, in the presence of dietary cholesterol, ezetimibe did not improve adipose tissue inflammation in obese Ldlr−/− mice, but it led to a major reduction in atherosclerotic lesions associated with improved plasma lipids and lipoproteins.
Keywords: ezetimibe, insulin resistance, hepatic steatosis, macrophage
Intra-abdominal obesity occurs as part of the metabolic syndrome, which is associated with insulin resistance, type 2 diabetes and an increased risk of atherosclerosis (1). Intra-abdominal obesity also is associated with low-grade inflammation, including the accumulation of macrophages in adipose tissue (2) and an increase in levels of circulating inflammatory markers, such as C-reactive protein, serum amyloid A (SAA), and interleukin-6 (IL-6) in humans (3), and SAA and IL-6 in mouse models (4). Adipose tissue macrophages appear to play a role in the pathogenesis of insulin resistance (5) and systemic inflammation (6), both of which are associated with increased cardiovascular risk.
Dietary cholesterol also has long been thought to play a role in atherogenesis Although this might in part be related to the increase in plasma cholesterol that results from consumption of dietary cholesterol, a plasma cholesterol-independent effect of dietary cholesterol has been proposed (7, 8). However, until recently, it was not clear what such an effect might be. Studies from our laboratory suggest that dietary cholesterol might exacerbate systemic inflammation, which in turn is associated with increased atherosclerosis. The addition of a moderate amount of cholesterol to a Western-type diet fed to LDL receptor-deficient (Ldlr−/−) mice led to increased systemic inflammation that was independent of an effect on plasma lipids and lipoproteins. We subsequently confirmed our findings in Ldlr−/− mice fed a diet high in saturated fat and sucrose, which has many features of the metabolic syndrome (6). A surprising finding in that study was that the addition of 0.15% dietary cholesterol led to a dramatic increase in the accumulation of macrophages in adipose tissue. In this model, macrophages accumulate in visceral, but not subcutaneous, adipose tissue (6). Despite not gaining more weight, mice fed added cholesterol in the diets were more insulin resistant, had more systemic inflammation, and had greater atherosclerosis than mice fed the diet without added cholesterol (6). These findings could not be attributed to changes in plasma lipids and lipoproteins, and they suggest that dietary cholesterol increased macrophage accumulation in visceral adipose tissue and possibly led to the various downstream consequences of adipose tissue macrophage accumulation.
Inhibition of the intestinal sterol receptor Niemann-Pick C1 like 1 (NPC1L1) using the drug ezetimibe blocks absorption of both dietary and biliary cholesterol in the gut, resulting in reduced incorporation of cholesterol into chylomicrons, decreased delivery of cholesterol to the liver, and increased clearance of apoB-rich lipoproteins from plasma (9, 10). Ezetimibe is widely used for the treatment of hypercholesterolemia in humans, often in combination with a statin. Because it inhibits the absorption of both dietary and endogenous cholesterol, ezetimibe is an excellent tool with which to evaluate whether inhibition of cholesterol absorption will inhibit local inflammation (macrophage accumulation and the expression of several inflammatory genes in adipose tissue), insulin sensitivity, systemic inflammation, and atherosclerosis. We hypothesized that inhibition of cholesterol absorption would have beneficial effects on adipose tissue inflammation. Our findings indicate that although inhibition of intestinal cholesterol absorption using ezetimibe resulted in a marked and significant reduction in atherosclerosis, this effect was independent of a reduction in adipose tissue inflammation or insulin resistance.
METHODS
Animals and diets
Ten-week-old adult male Ldlr−/− mice on a C57BL/6J background were fed normal chow (4% calories from fat), a high-fat, high-sucrose diet (HFHS, 60% calories from fat, F1850, Bioserv, Frenchtown, NJ) or high-fat, high-sucrose diet with 0.15% added cholesterol (HFHS+C, F4997, Bioserv). Details of these diets have been published previously (6). The HFHS diet provides 20.5% protein, 36% fat (40% w/w saturated, 50% monounsaturated, and 10% polyunsaturated fats) and 36% carbohydrate. The energy content of the HFHS diet was 5.5 kcal/g. Mice received these diets with or without supplementation of ezetimibe 5 mg/kg (Merck Research, NJ), a dose that previously has been shown to be effective at lowering cholesterol levels in mice (11). Mice were maintained in a temperature and light-controlled facility in cages with micro-isolator filter tops, and they received the diets ad libitum for a total of 24 weeks. Body weights were measured weekly. Food intake was recorded after 6, 12, and 18 weeks of diet in individually housed mice and calculated as an average of three sequential days from a known amount of food given. The food was reweighed daily, and the amount of food consumed was calculated. The average energy intake of each mouse was estimated from the volume of food intake and its caloric content. At euthanasia, harvested tissues were snap-frozen in liquid nitrogen and stored at −70°C or were fixed with 10% neutral-buffered formalin and embedded in paraffin wax. All experimental procedures were undertaken with approval from the Institution Animal Care and Use Committee of the University of Washington.
Analytical procedures
Metabolic variables were measured in blood samples obtained from the retro-orbital sinus after a 4 h fast. Cholesterol and triglycerides in plasma and fast-phase liquid chromatography (FPLC) fractions were measured using colorimetric assay kits (12). Lipoproteins were separated from pooled plasma samples by FPLC. Plasma insulin, SAA, and serum amyloid P (SAP) levels were measured using ELISA as previously described (6). Alanine aminotransferase (ALT) was measured using an autoanalyzer through the Nutrition and Obesity Research Center at the University of Washington. Tissue and fecal lipids were extracted using the Folch technique (13, 14). Intra-peritoneal glucose and insulin tolerance tests were performed after a 4 h fast as described previously (6).
Real-time quantitative PCR
Total RNA was extracted from 50–100 mg of whole adipose or liver tissues using a commercially available RNA extraction kit according to the manufacturer's protocol (Agilent Technologies, Santa Clara, CA). After spectroscopic quantification, 2 µg of RNA was reverse-transcribed, and the cDNA obtained was analyzed by real-time quantitative PCR by standard protocols using an ABI 7900HT instrument. Primer and probe sets for individual genes were purchased from Applied Biosystems (Assay-on-Demand, Life Technologies, Carlsbad, CA). GAPDH was used as the housekeeping gene, levels of which did not change with the various diets. Relative amounts of the target gene were calculated using the ΔΔCt formula.
Histology, immunohistochemistry, and atherosclerosis quantification
The extent of atherosclerosis was measured in pinned aortas using the en face technique as previously described (4). Formalin-fixed, paraffin-embedded adipose tissue and frozen OCT-embedded aortic sinuses were sectioned and stained using Movat's pentachrome histochemical stain using standard protocols. Macrophages in adipose tissue and aortic root sections were detected using a rat monoclonal antibody (Mac2; titer 1:2500, Cedarlane Laboratories, Burlington, NC). Quantification of aortic root lesion area was performed as described previously (4). Oil Red-O staining of aortic root lesions was performed, photographed, and then extracted and stained with Movat's pentachrome. On an adjacent section, Mac2 staining of aortic root lesions was performed, photographed, and then extracted and stained with Movat's pentachrome. Area quantification for lesion area, Oil Red-O staining, and Mac2 staining was performed on digital images of immunostained tissue sections using image analysis software (Image Pro Plus software, Media Cybernetics, Bethesda, MD). Adipocyte cross-sectional area was measured by computer image analysis using techniques described previously (4).
Statistical analyses
Data were analyzed using GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA) and are represented as means and standard errors. Two-way ANOVA (ANOVA) was used to compare differences between mice receiving the diets with or without ezetimibe, and Bonferroni post-hoc testing was used to detect differences among mean values of the groups. A P value < 0.05 was considered statistically significant.
RESULTS
Ezetimibe blunts weight gain in obese Ldlr−/− mice fed added cholesterol
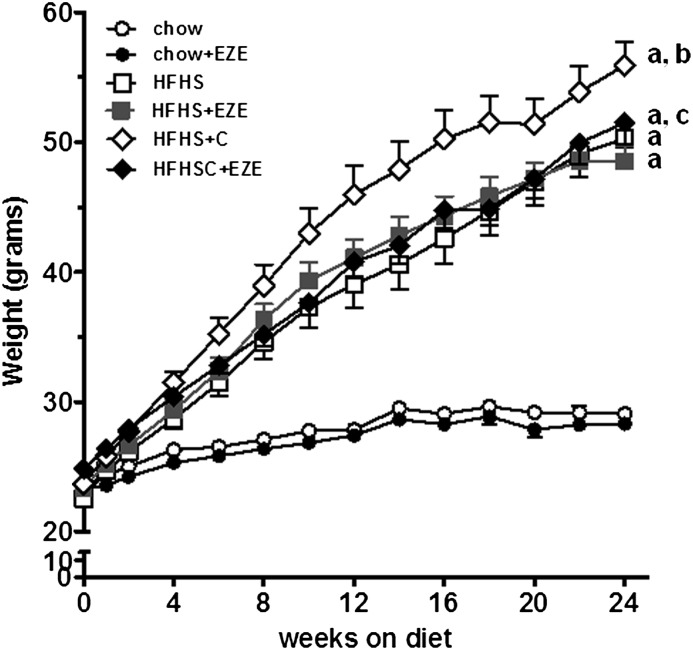
We previously have shown that Ldlr−/− mice fed a HFHS diet for 24 weeks developed obesity, hyperinsulinemia, and hyperlipidemia; addition of cholesterol to this diet (HFHS+C) worsened these findings (6). We confirmed these findings in the present study. Weight gain was greater in the mice that received HFHS+C compared with the HFHS group (Fig. 1). Ezetimibe supplementation did not affect weight gain in the chow or HFHS groups; however, ezetimibe added to HFHS+C significantly blunted weight gain (P < 0.01 versus HFHS+C, Fig. 1 and Table 1). Food intake was not different among the groups (Table 1). Thus, ezetimibe-supplemented HFHS+C mice gained less weight despite consuming equivalent calories compared with the other HFHS groups. Hyperglycemia developed in both HFHS and HFHS+C groups, and ezetimibe had no significant effect on glucose levels (Table 1). Hyperinsulinemia was improved by ezetimibe in the HFHS animals but not in the HFHS+C group (Table 1). Ezetimibe also had no beneficial effect on glucose homeostasis measured by glucose and insulin tolerance tests (data not shown).
Fig. 1.
Ezetimibe blunts body weight increase in the presence of dietary cholesterol. Data represent means ± SEM (n = 10–15 per group). Filled and open circles, chow ± ezetimibe (EZE); filled and open squares, HFHS ± EZE; filled and open diamonds, HFHS+C ± EZE. aP < 0.001 versus chow; bP < 0.001 versus HFHS; cP < 0.05 versus HFHS+C only, all by two-way ANOVA and post-hoc testing.
TABLE 1.
Characteristics of Ldlr mice in each group
| Variable | Initial Body Weight (g) | Final Body Weight (g) | Food Intake (g/day) | Energy Intake (kcal/day) | Liver Weight (g) | Perigonadal Adipose Weight (g) | Plasma Glucose (mg/dl) | Plasma Insulin (ng/ml) | HOMA-IR |
| Diet | |||||||||
| Chow | 23.3 ± 1 | 29.0 ± 2 | 3.8 ± 0.6a | 15.4 ± 2 | 1.3 ± 0.2 | 0.3 ± 0.1 | 199 ± 25 | 0.4 ± 0.2 | 3.6 ± 1.5 |
| Chow + EZE | 23.4 ± 0.9 | 28.2 ± 1 | 3.5 ± 0.4a | 14.1 ± 1 | 1.2 ± 0.1 | 0.4 ± 0.1 | 201 ± 26 | 0.4 ± 0.1 | 4.2 ± 1 |
| HFHS | 23.1 ± 1.9 | 50.4 ± 5b | 2.7 ± 0.6 | 14.7 ± 3 | 2.2 ± 0.6b | 2.0 ± 0.5b | 271 ± 80b | 3.8 ± 1.7b | 44.9 ± 21b |
| HFHS+EZE | 22.6 ± 2.1 | 48.6 ± 4.2b | 2.6 ± 0.3 | 14.1 ± 2 | 1.8 ± 0.5b | 2.2 ± 0.6b | 298 ± 45b | 1.6 ± 0.8bd | 22.1 ± 13bd |
| HFHS+C | 24.3 ± 1.9 | 55.9 ± 5.3bc | 3.2 ± 0.8 | 17.4 ± 4 | 3.2 ± 1.0b | 1.7 ± 0.3b | 272 ± 46b | 4.1 ± 2.3b | 44.7 ± 23.2b |
| HFHSC+EZE | 24.1 ± 1.5 | 50.1 ± 7.1bd | 2.9 ± 0.6 | 15.8 ± 3 | 2.2 ± 0.5bd | 2.4 ± 0.3bd | 339 ± 53bd | 3.5 ± 2.1b | 49.3 ± 24.5b |
| P value (diet) | 0.07 | <0.0001 | <0.0001 | 0.6776 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| P value (drug factor) | 0.09 | 0.0049 | 0.0249 | 0.5942 | 0.0007 | 0.0002 | 0.0291 | 0.0074 | 0.1222 |
| P value (diet × drug interaction) | 0.247 | 0.0833 | 0.6365 | 0.9818 | 0.0191 | 0.0056 | 0.0291 | 0.0221 | 0.0094 |
Values are expressed as means ± SEM.
EZE, ezetimibe, HOMA-IR, homeostasis model assessment of insulin resistance.
P < 0.05 versus HFHS and HFHS+C.
P < 0.001 versus chow.
P < 0.05 versus HFHS.
P < 0.05 versus no drug treatment.
Ezetimibe improves lipid abnormalities in obese Ldlr−/− mice
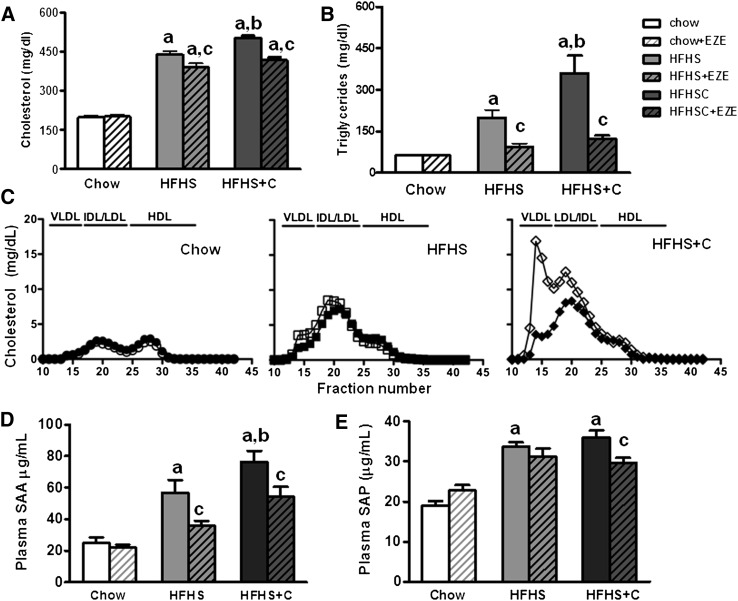
We determined the effects of ezetimibe on plasma lipids and lipoprotein abnormalities in obese Ldlr−/− mice. Hypercholesterolemia and hypertriglyceridemia developed in mice fed HFHS diets (P < 0.0001, two-way ANOVA). Ezetimibe improved plasma cholesterol levels in HFHS and HFHS+C mice (P < 0.05, Fig. 2A). Similarly, ezetimibe improved the elevation in triglyceride levels that occurred in both groups of obese animals, with a 50% reduction in levels in the HFHS group (P < 0.05 versus HFHS (Fig. 2B) and a 66% reduction in the HFHS+C group (P < 0.001 versus HFHS+C, Fig. 2B). Lipoprotein distribution profiles of animals in the HFHS+C group revealed increased VLDL/IDL particles, which were significantly improved in the mice that received ezetimibe (Fig. 2C). The drug had no effect on plasma lipids or lipoproteins in chow-fed animals.
Fig. 2.
Lipid, lipoprotein levels and circulating inflammatory markers in lean and obese Ldlr−/− mice fed high-fat diets without or with ezetimibe. (A) Plasma cholesterol and (B) triglycerides in mice fed diets. Open bars represent diets without ezetimibe (EZE), hatched bars represent diets with EZE. (C) Plasma lipoprotein distribution in the three groups of mice. (d) Plasma SAA and (E) SAP levels. n = 10–15 per group. aP < 0.001 versus chow; bP < 0.01 versus HFHS; cP < 0.05 versus HFHS+C by 2- way ANOVA and Bonferroni post-hoc test.
Ezetimibe decreases circulating plasma SAA and SAP levels in obese Ldlr−/− mice
To assess the presence of chronic systemic inflammation, we measured circulating levels of two inflammatory markers, SAA and SAP. In mice, these liver-derived molecules are modestly induced by low-grade chronic inflammation (15, 16). As expected, circulating SAA levels significantly increased in mice receiving HFHS and further increased in HFHS+C mice (P < 0.0001, two-way ANOVA, Fig. 2D). Ezetimibe significantly reduced circulating SAA levels in both groups of HFHS-fed animals (P < 0.05). This reduction was independent of the presence of dietary cholesterol. Similarly, plasma SAP levels increased in the obese mice (P < 0.001 versus chow), and ezetimibe improved circulating levels only in the animals fed HFHS+C (P < 0.05, Fig. 2E).
Ezetimibe does not affect adipose tissue inflammatory gene expression in obese mice
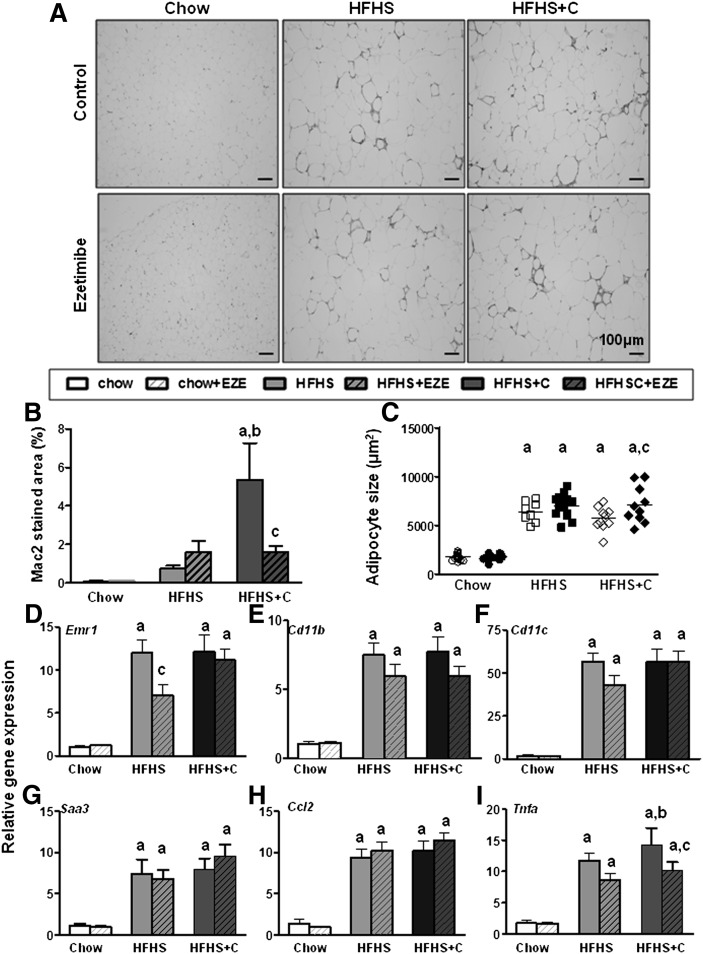
Visceral adipose tissue accumulation of macrophages is a key feature of obesity. We therefore determined whether inhibition of intestinal cholesterol absorption with ezetimibe would improve adipose tissue inflammation. Immunohistochemical analysis using an antibody to the pan-macrophage marker Mac2 revealed an increase in macrophage staining in perigonadal adipose tissue of HFHS+C-fed mice (P < 0.001, two-way ANOVA, Fig. 3A, B). Ezetimibe had no effect on macrophage accumulation in the HFHS or HFHS+C group (Fig. 3A, B). Adipocyte hypertrophy developed in both obese groups, and ezetimibe resulted in greater hypertrophy in the HFHS+C group (Fig. 3C). Analysis of macrophage-specific genes in whole perigonadal adipose tissue revealed increased expression of F4/80 (Emr1), CD11b, and CD11c in both HFHS±C groups, confirming increased adipose tissue macrophage content (P < 0.001 versus chow, Fig. 3D–F). Ezetimibe had no overall effect on macrophage gene expression in any of the obese groups, except for a reduction in F4/80 gene expression in the HFHS group. No differences in chemotactic factor genes (Mcp-1, Saa3) were observed among any of the obese groups (Fig. 3G, H), although expression of the pro-inflammatory cytokine Tnfα, was reduced by ezetimibe (Fig. 3I) in the HFHS+C group. These data suggest that ezetimibe had no major overall beneficial effects on adipose tissue inflammation.
Fig. 3.
Ezetimibe does not beneficially affect adipose tissue inflammatory gene expression in obese Ldlr−/− mice. (A) Adipose tissue sections stained with the macrophage-specific antibody Mac2 (red), 20× magnification, representative samples. (B) Quantification of macrophage Mac2 staining. (C) Perigonadal adipocyte size. Expression of genes for (D) F4/80 (Emr1), (E) CD11b, (F) CD11c, (G) SAA3, (H) MCP1 (Ccl2), and (I) TNFα in whole perigonadal adipose tissue. Open bars represent diets without ezetimibe (EZE); hatched bars represent diets admixed with EZE. n = 10–15 per group. aP < 0.001 versus chow; bP < 0.01 versus HFHS; cP < 0.05 within group by two-way ANOVA and Bonferroni post-hoc test.
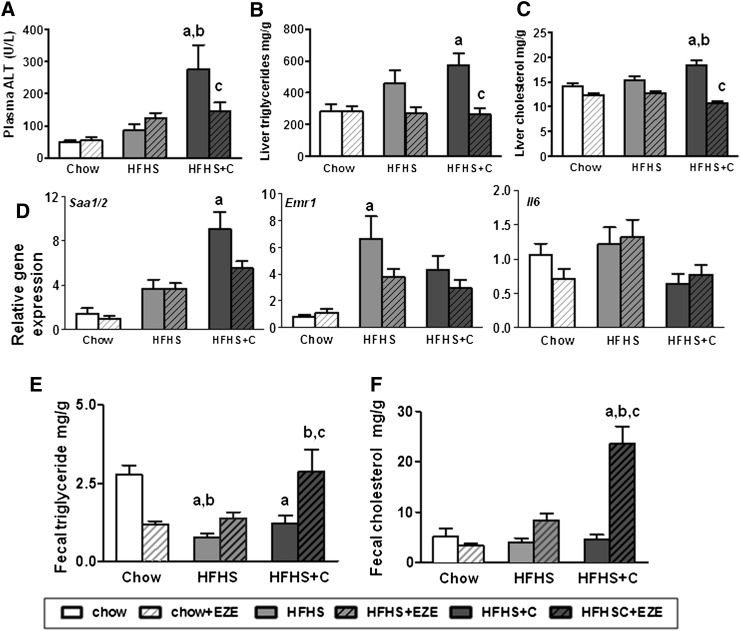
Ezetimibe decreases hepatic lipid content in obese Ldlr−/− mice fed added cholesterol
We next evaluated the effects of ezetimibe on liver fat accumulation in diet-induced obesity. Significant hepatomegaly developed in mice that were fed HFHS diets (Table 1). Ezetimibe decreased liver weights in the mice that received added cholesterol in the diet (Table 1). As shown earlier, ALT levels were increased significantly in mice fed HFHS+C, and ezetimibe resulted in a 50% reduction in levels (Fig. 4A). Similar effects were not observed in the HFHS-only group with ezetimibe. Hepatic triglyceride accumulation that occurred with both HFHS diets was normalized with ezetimibe in both HFHS diets, but this reduction was only significantly different in the group that received added cholesterol (P < 0.05, Fig. 4A). Hepatic cholesterol content also was decreased with ezetimibe administration in the HFHS+C group (P < 0.05, Fig. 4B). Ezetimibe reduced expression of the inflammatory gene SAA1/2 only in the HFHS+C animals (Fig. 4D). No changes were observed in macrophage or cytokine gene expression in any of the groups. Increased fecal excretion of cholesterol (P < 0.05 versus HFHS+C) and triglyceride (P < 0.05 versus HFHS+C) was observed only in obese mice that received ezetimibe and added dietary cholesterol (Fig. 4E, F).
Fig. 4.
Hepatic lipid content is decreased in cholesterol fed obese Ldlr−/− with ezetimibe. (A) Plasma alanine aminotransferase levels, (B) hepatic triglyceride content, and (C) hepatic cholesterol content in liver of mice fed HFHS diets. (D) Gene expression of SAA (Saa1/2), F4/80 (Emr1), and Il6 (Il6) in liver. (E) Fecal triglyceride and (F) fecal cholesterol excretion, expressed as mg lipid/g of feces excreted in 24 h. Open bars represent diets without ezetimibe (EZE); hatched bars represent diets admixed with ezetimibe. n = 10–15 per group. aP < 0.01 versus chow; bP < 0.01 versus HFHS; cP < 0.05 versus HFHS+C by two-way ANOVA and post-hoc Bonferroni test.
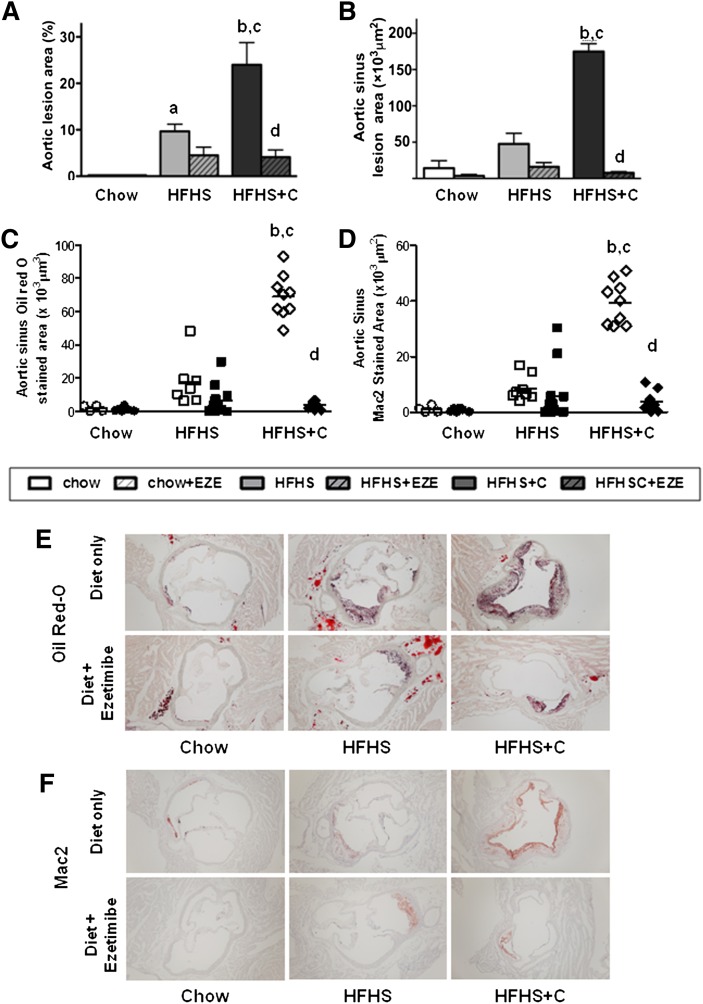
Ezetimibe dramatically reduces atherosclerosis in obese Ldlr−/− mice fed added cholesterol
To assess atherosclerosis, we first measured aortic atherosclerotic lesion area using the en face technique. As anticipated, a significant increase in atherosclerosis was observed on HFHS diet and was amplified in the HFHS+C group (P < 0.0001, two-way ANOVA). Ezetimibe reduced atherosclerosis by a striking 85% in the HFHS+C group only (P < 0.001 versus HFHS+C, Fig. 5A), suggesting that the drug was highly effective in reducing aortic atherosclerosis in the presence of dietary cholesterol. A similar pattern was also observed in the aortic root lesion area, which was significantly reduced in mice fed HFHS+C with ezetimibe (P < 0.001 versus HFHS+C, Fig. 5B). Aortic root Oil Red-O staining revealed relatively lipid-poor lesions with ezetimibe treatment only in the HFHS+C group (Fig. 5C, E). Immunohistochemistry of aortic root lesions showed increased macrophage Mac2-stained areas within lesions in the HFHS+C group with a marked 90% reduction in Mac2 staining in the HFHS+C group that received ezetimibe, suggesting reduced lesion macrophage content (Fig. 5D, E). Lesion area correlated strongly with plasma cholesterol (r = 0.58, P < 0.001) and triglyceride levels (r = 0.61, P < 0.001) in the obese animals fed high-fat diets with or without ezetimibe.
Fig. 5.
Aortic atherosclerosis is markedly reduced by ezetimibe in obese, chronically inflamed Ldlr−/− mice fed HFHS diet with added cholesterol only. (A) Aortic intimal and (B) sinus lesions. (C) Quantification of oil red-O staining and (D) Mac2 staining of aortic root lesions. (E) Representative photomicrographs of aortic root Oil Red-O and (F) Mac 2 staining. Open bars represent diets without ezetimibe; hatched bars represent diets admixed with ezetimibe (EZE). n = 10–15 per group, aP < 0.05 versus chow; bP < 0.001 versus chow; cP < 0.001 versus HFHS; dP < 0.001 versus HFHS+C by two-way ANOVA and post-hoc Bonferroni test.
DISCUSSION
The major finding in this study was that inhibition of intestinal cholesterol absorption using ezetimibe led to a marked reduction in the extent of atherosclerosis in Ldlr−/− mice fed a diabetogenic diet rich in saturated fat and sucrose with a moderate amount of added cholesterol. Such diabetogenic diets, when fed to Ldlr−/− mice, result in the development of many features of the metabolic syndrome, which are exacerbated by the addition of dietary cholesterol (6). The reduction in atherosclerosis seen in the present study occurred without major changes in adipose tissue inflammation or insulin sensitivity. The profound effect on atherosclerosis was confined to mice that received a moderate amount of cholesterol added to their diets and was not observed in mice fed the same diet without added cholesterol.
Obesity, both diet-induced and genetic, leads to the accumulation of macrophages in adipose tissue (2, 17), particularly visceral fat (6). Adipose tissue macrophage accumulation is in turn associated with insulin resistance (18–21), which has been thought to be a major contributor to the development of atherosclerosis (22, 23). Various cytokines produced by adipose tissue macrophages have been postulated to inhibit insulin action and lead to insulin resistance (19, 24). We previously have shown that the addition of 0.15% cholesterol to the diet results in worsening of many of the features of the metabolic syndrome in this mouse model (6). We therefore hypothesized that inhibition of dietary cholesterol absorption would lead to a reduction in adipose tissue macrophage accumulation, insulin resistance, and subsequently, atherosclerosis. We chose to test this hypothesis by using the widely available intestinal cholesterol absorption inhibitor ezetimibe in Ldlr−/− mice fed high-fat, high-sucrose diets with or without added cholesterol. We had anticipated that inhibition of dietary cholesterol absorption would be accompanied by a reduction in macrophage accumulation in adipose tissue, as we previously had shown that the addition of cholesterol to the diet exacerbated macrophage accumulation in adipose depots (6). However, adipose inflammatory gene expression and glucose tolerance did not improve in the mice that received ezetimibe. Consistent with our original hypothesis, the administration of ezetimibe did indeed lead to improvement in circulating inflammatory protein markers SAA and SAP, especially against a background of added cholesterol, and a dramatic reduction in atherosclerosis in this group only, but this result was unrelated to changes in adipose tissue inflammation or insulin resistance. The lack of effect of adipose tissue inflammation that we observed was similar to our findings in TLR4-deficient mice on a LDLR-deficient background, in which we observed changes in atherosclerosis related to lipids and lipoproteins, without changes in adipose tissue inflammation (25). The absence of significant changes in adipose tissue suggests that the effect of ezetimibe in improving lipoprotein profiles is a critical factor in decreasing atherosclerosis rather than decreasing adipose tissue inflammation or insulin resistance in this mouse model. Thus, based on our results, it is unclear whether inhibition of intestinal cholesterol absorption, both exogenous and endogenous, plays a role in obesity-associated adipose inflammation and insulin resistance.
However, we observed a blunting of weight gain with ezetimibe only in the mice on the high-fat, high-sucrose diet with added cholesterol. Similar findings have been observed by others. Reduced absorption of saturated fatty acids and resistance to diet-induced obesity and diabetes was seen in both ezetimibe-treated and NPC1L1-deficient mice (26). Others also have observed an improvement in insulin sensitivity with ezetimibe in mice (27), which is different from our observations, especially considering the fact the mice on the diet that contained added cholesterol had reduced weight gain. Blunting of weight gain with ezetimibe has been reported in mice on a high-fat diet but to a lesser extent than blunting of weight gain with a very high dose of atorvastatin (28). Although the cause for this observation is unknown, fat malabsorption may be a contributing factor (26), as suggested by the increase in fecal lipid content. We speculate that the efficiency of fatty acid absorption by the intestine is determined by its solubilization in mixed micelles. Thus, in the setting of cholesterol added to the diet, ezetimibe-induced cholesterol malabsorption may have created cholesterol-saturated micelles, which were less effective in solubilizing the more hydrophobic saturated fatty acids. This in turn could have impaired their absorption and attenuated weight gain. An additional beneficial effect of ezetimibe may be secondary to increased energy expenditure, as mice that received dietary cholesterol and the drug gained significantly less weight despite equivalent or slightly increased caloric intake per gram body weight. Reasons for this effect of ezetimibe are unclear, and further studies are required to investigate the effect of ezetimibe on body weight and energy expenditure.
Addition of cholesterol to the high-fat, high-sucrose diet resulted in hypercholesterolemia of roughly equal magnitude to that seen in mice fed the diet without added cholesterol. Plasma triglyceride levels were higher in the mice with cholesterol added to their diets. This difference was the result of accumulation of lipoproteins in the VLDL/IDL density range. Ezetimibe led to modest reduction of LDL in mice fed the high-fat, high-sucrose diet without added cholesterol. This group had an increase in atherosclerosis, and ezetimibe did not significantly decrease atherosclerotic lesion area. Although this may be related to statistical power, a likely explanation is that lesion burden in these animals was not as profound to cause a significant change as in the mice that received added cholesterol in the diet. In the presence of added dietary cholesterol, a reduction in both LDL and particularly VLDL/IDL was observed with ezetimibe treatment. Atherosclerosis was reduced by ezetimibe only in this group of animals, which strongly suggests that the reduction in these triglyceride-rich lipoproteins played an important role in determining the extent of atherosclerotic lesions, especially as lesion size showed a strongly positive correlation with plasma triglyceride levels. Ezetimibe has previously been shown to reduce atherosclerosis in apoE-deficient mice, in which it also led to a marked reduction of VLDL/IDL-like particles (11, 29), which are the main lipoproteins that accumulate in that mouse model. HDL also increased in the apoE-deficient mice with ezetimibe treatment (11, 29), an effect that was not observed in the Ldlr−/− mice in our study.
Inflammatory markers, such as C-reactive protein and SAA, are increased in human subjects with the metabolic syndrome (30, 31). We previously have observed increases in SAA in obese mice fed high-fat or diabetogenic diets (6, 12). In the present study, ezetimibe improved levels of circulating SAA in both groups of obese mice. Fatty liver and nonalcoholic steatohepatitis (NASH) also are common features of the metabolic syndrome in humans (32). Changes resembling fatty liver and NASH also have been observed in several mouse models (33–35), including Ldlr−/− mice fed the diabetogenic diets used in this study (14). Moreover, the addition of cholesterol led to a worsening of features of NASH in Ldlr−/− mice fed a high-fat, high-sucrose diet (14). In the current study, liver weight was reduced with ezetimibe administration, and liver triglyceride content was decreased to levels seen in chow-fed animals. Interestingly, hepatic cholesterol concentration also was reduced as a result of ezetimibe administration. Several other studies also have shown a reduction of liver fat in several mouse models of fatty liver, including diet-induced obesity (36) and db/db mice (37). This effect appears to be exacerbated when ezetimibe is combined with acarbose (38). Further evidence for cholesterol absorption playing a role in fatty liver disease is provided by the observation that liver fat is markedly reduced in mice deficient in NPC1L1 (39), which plays a critical role in the absorption of cholesterol from the gut.
How these findings in mice relate to the use of intestinal cholesterol absorption inhibitors, such as ezetimibe, or possibly other mechanisms for blocking intestinal cholesterol absorption in humans is speculative. Although the dramatic effect of ezetimibe on triglyceride levels due to a reduction in levels of VLDL/IDL has not been reported in human subjects receiving ezetimibe treatment, a few small clinical studies have evaluated effects of ezetimibe on plasma triglycerides and VLDL/IDL levels. In subjects with primary hypercholesterolemia, ezetimibe monotherapy decreased LDL-C concentrations and resulted in increased catabolism of VLDL, IDL, and LDL apoB particles (40, 41). Short-term ezetimibe treatment decreased plasma triglycerides, postprandial triglyceride excursion, and endothelial function in healthy individuals (42). Similar results have been observed in type 2 diabetes (43). Another possible mechanism for the reduction in triglycerides is the potential effect of ezetimibe on VLDL secretion rate, which is suggested by the reduction in hepatic triglyceride levels that we observed in the added cholesterol group. However, existing human studies do not show a consistent effect of ezetimibe on VLDL secretion (40, 41). Coupled with weight loss, ezetimibe did not further decrease VLDL secretion rate (44). Our observation that atherosclerosis was only reduced in mice with elevations of VLDL/IDL strongly suggests that the effect of ezetimibe on these lipoprotein fractions was a major factor in the reduction of atherosclerosis seen in that group of mice. Long-term studies on the effect of ezetimibe on the cardiovascular disease events in humans are awaited with interest; a large ongoing clinical trial will hopefully provide definitive information (45). Studies in humans have not reported a consistent effect of ezetimibe on body weight or insulin resistance. However, a recent study demonstrated that ezetimibe therapy significantly improved nonalcoholic fatty liver disease (NAFLD)-related metabolic parameters, including visceral fat area, fasting insulin, homeostasis model assessment of insulin resistance, triglycerides, total cholesterol, and LDL cholesterol in newly diagnosed patients with biopsy-proven NAFLD (46). Ezetimibe plus weight loss resulted in a greater reduction in liver triglycerides and inflammatory markers than weight loss alone (44). Thus, consistent with findings in our mouse model, strategies to inhibit cholesterol absorption, such as the use of ezetimibe, might be a novel way to lower hepatic triglycerides, especially if combined with weight loss.
Acknowledgments
The authors would like to acknowledge Kevin Weitz for technical assistance.
Footnotes
Abbreviations:
- ALT
- alanine aminotransferase
- HFHS
- high-fat, high-sucrose
- HFHS+C
- high-fat, high-sucrose with cholesterol
- IL-6
- interleukin-6
- LDLR
- LDL receptor
- NPC1L1
- Niemann-Pick C1 Like 1
- SAA
- serum amyloid A
- SAP
- serum amyloid P
- TNFα
- tumor necrosis factor-α
This work was supported by National Institutes of Health Grants HL-094352, HL-092969, and DK-035816, and by an investigator-initiated grant from Merck. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Després J-P., Moorjani S., Lupien P. J., Tremblay A., Nadeau A., Bouchard C. 1990. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis. 10: 497–511 [DOI] [PubMed] [Google Scholar]
- 2.Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., Ferrante A. W., Jr 2003. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112: 1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leinonen E., Hurt-Camejo E., Wiklund O., Hulten L. M., Hiukka A., Taskinen M. R. 2003. Insulin resistance and adiposity correlate with acute-phase reaction and soluble cell adhesion molecules in type 2 diabetes. Atherosclerosis. 166: 387–394 [DOI] [PubMed] [Google Scholar]
- 4.Yoon J., Subramanian S., Ding Y., Wang S., Goodspeed L., Sullivan B., Kim J., O'Brien K. D., Chait A. 2011. Chronic insulin therapy reduces adipose tissue macrophage content in LDL-receptor-deficient mice. Diabetologia. 54: 1252–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schenk S., Saberi M., Olefsky J. M. 2008. Insulin sensitivity: modulation by nutrients and inflammation. J. Clin. Invest. 118: 2992–3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subramanian S., Han C. Y., Chiba T., McMillen T. S., Wang S. A., Haw A., 3rd, Kirk E. A., O'Brien K. D., Chait A. 2008. Dietary cholesterol worsens adipose tissue macrophage accumulation and atherosclerosis in obese LDL receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 28: 685–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shekelle R. B., Shryock A. M., Paul O., Lepper M., Stamler J., Liu S., Raynor W. J., Jr 1981. Diet, serum cholesterol, and death from coronary heart disease. The Western Electric study. N. Engl. J. Med. 304: 65–70 [DOI] [PubMed] [Google Scholar]
- 8.Shekelle R. B., Stamler J. 1989. Dietary cholesterol and ischaemic heart disease. Lancet. 1: 1177–1179 [DOI] [PubMed] [Google Scholar]
- 9.Davidson M. H. 2003. Ezetimibe: a novel option for lowering cholesterol. Expert Rev. Cardiovasc. Ther. 1: 11–21 [DOI] [PubMed] [Google Scholar]
- 10.Davis H. R., Veltri E. P. 2007. Zetia: inhibition of Niemann-Pick C1 Like 1 (NPC1L1) to reduce intestinal cholesterol absorption and treat hyperlipidemia. J. Atheroscler. Thromb. 14: 99–108 [DOI] [PubMed] [Google Scholar]
- 11.Davis H. R., Jr, Compton D. S., Hoos L., Tetzloff G. 2001. Ezetimibe, a potent cholesterol absorption inhibitor, inhibits the development of atherosclerosis in ApoE knockout mice. Arterioscler. Thromb. Vasc. Biol. 21: 2032–2038 [DOI] [PubMed] [Google Scholar]
- 12.Lewis K. E., Kirk E. A., McDonald T. O., Wang S., Wight T. N., O'Brien K. D., Chait A. 2004. Increase in serum amyloid a evoked by dietary cholesterol is associated with increased atherosclerosis in mice. Circulation. 110: 540–545 [DOI] [PubMed] [Google Scholar]
- 13.Folch J., Lees M., Sloane-Stanley G. H. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226: 497–509 [PubMed] [Google Scholar]
- 14.Subramanian S., Goodspeed L., Wang S., Kim J., Zeng L., Ioannou G. N., Haigh W. G., Yeh M. M., Kowdley K. V., O'Brien K. D., et al. 2011. Dietary cholesterol exacerbates hepatic steatosis and inflammation in obese LDL receptor-deficient mice. J. Lipid Res. 52: 1626–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uhlar C. M., Whitehead A. S. 1999. Serum amyloid A, the major vertebrate acute-phase reactant. Eur. J. Biochem. 265: 501–523 [DOI] [PubMed] [Google Scholar]
- 16.Han C. Y., Subramanian S., Chan C. K., Omer M., Chiba T., Wight T. N., Chait A. 2007. Adipocyte-derived serum amyloid A3 and hyaluronan play a role in monocyte recruitment and adhesion. Diabetes. 56: 2260–2273 [DOI] [PubMed] [Google Scholar]
- 17.Xu H., Barnes G. T., Yang Q., Tan G., Yang D., Chou C. J., Sole J., Nichols A., Ross J. S., Tartaglia L. A., et al. 2003. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112: 1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hotamisligil G. S., Arner P., Caro J. F., Atkinson R. L., Spiegelman B. M. 1995. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Invest. 95: 2409–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hotamisligil G. S., Shargill N. S., Spiegelman B. M. 1993. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 259: 87–91 [DOI] [PubMed] [Google Scholar]
- 20.Berg A. H., Scherer P. E. 2005. Adipose tissue, inflammation, and cardiovascular disease. Circ. Res. 96: 939–949 [DOI] [PubMed] [Google Scholar]
- 21.Lionetti L., Mollica M. P., Lombardi A., Cavaliere G., Gifuni G., Barletta A. 2009. From chronic overnutrition to insulin resistance: the role of fat-storing capacity and inflammation. Nutr. Metab. Cardiovasc. Dis. 19: 146–152 [DOI] [PubMed] [Google Scholar]
- 22.Cersosimo E., DeFronzo R. A. 2006. Insulin resistance and endothelial dysfunction: the road map to cardiovascular diseases. Diabetes Metab. Res. Rev. 22: 423–436 [DOI] [PubMed] [Google Scholar]
- 23.Sowers J. R., Frohlich E. D. 2004. Insulin and insulin resistance: impact on blood pressure and cardiovascular disease. Med. Clin. North Am. 88: 63–82 [DOI] [PubMed] [Google Scholar]
- 24.Hotamisligil G. S., Murray D. L., Choy L. N., Spiegelman B. M. 1994. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc. Natl. Acad. Sci. USA. 91: 4854–4858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding Y., Subramanian S., Montes V. N., Goodspeed L., Wang S., Han C., Teresa A. S., 3rd, Kim J., O'Brien K. D., Chait A. 2012. Toll-like receptor 4 deficiency decreases atherosclerosis but does not protect against inflammation in obese low-density lipoprotein receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 32: 1596–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Labonté E. D., Camarota L. M., Rojas J. C., Jandacek R. J., Gilham D. E., Davies J. P., Ioannou Y. A., Tso P., Hui D. Y., Howles P. N. 2008. Reduced absorption of saturated fatty acids and resistance to diet-induced obesity and diabetes by ezetimibe-treated and Npc1l1-/- mice. Am. J. Physiol. Gastrointest. Liver Physiol. 295: G776–G783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muraoka T., Aoki K., Iwasaki T., Shinoda K., Nakamura A., Aburatani H., Mori S., Tokuyama K., Kubota N., Kadowaki T., et al. 2011. Ezetimibe decreases SREBP-1c expression in liver and reverses hepatic insulin resistance in mice fed a high-fat diet. Metabolism. 60: 617–628 [DOI] [PubMed] [Google Scholar]
- 28.Paraskevas K. I., Pantopoulou A., Vlachos I. S., Agrogiannis G., Illiopoulos D. G., Karatzas G., Tzivras D., Mikhailidis D. P., Perrea D. N. 2011. Comparison of fibrate, ezetimibe, low- and high-dose statin therapy for the dyslipidemia of the metabolic syndrome in a mouse model. Angiology. 62: 144–154 [DOI] [PubMed] [Google Scholar]
- 29.Nakagami H., Osako M. K., Takami Y., Hanayama R., Koriyama H., Mori M., Hayashi H., Shimizu H., Morishita R. 2009. Vascular protective effects of ezetimibe in ApoE-deficient mice. Atherosclerosis. 203: 51–58 [DOI] [PubMed] [Google Scholar]
- 30.Ridker P. M., Buring J. E., Cook N. R., Rifai N. 2003. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 107: 391–397 [DOI] [PubMed] [Google Scholar]
- 31.Kotani K., Asahara-Satoh N., Kato Y., Araki R., Himeno A., Yamakage H., Koyama K., Tanabe M., Oishi M., Okajima T., et al. 2011. Remnant-like particle cholesterol and serum amyloid A-low-density lipoprotein levels in obese subjects with metabolic syndrome. J. Clin. Lipidol. 5: 395–400 [DOI] [PubMed] [Google Scholar]
- 32.Kotronen A., Yki-Jarvinen H. 2008. Fatty liver: a novel component of the metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 28: 27–38 [DOI] [PubMed] [Google Scholar]
- 33.Browning J. D., Horton J. D. 2004. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Invest. 114: 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiri-Sverdlov R., Wouters K., van Gorp P. J., Gijbels M. J., Noel B., Buffat L., Staels B., Maeda N., van Bilsen M., Hofker M. H. 2006. Early diet-induced non-alcoholic steatohepatitis in APOE2 knock-in mice and its prevention by fibrates. J. Hepatol. 44: 732–741 [DOI] [PubMed] [Google Scholar]
- 35.Schattenberg J. M., Galle P. R. 2010. Animal models of non-alcoholic steatohepatitis: of mice and man. Dig. Dis. 28: 247–254 [DOI] [PubMed] [Google Scholar]
- 36.Zheng S., Hoos L., Cook J., Tetzloff G., Davis H., Jr, van Heek M., Hwa J. J. 2008. Ezetimibe improves high fat and cholesterol diet-induced non-alcoholic fatty liver disease in mice. Eur. J. Pharmacol. 584: 118–124 [DOI] [PubMed] [Google Scholar]
- 37.Fukuda M., Nakamura T., Kataoka K., Nako H., Tokutomi Y., Dong Y. F., Yasuda O., Ogawa H., Kim-Mitsuyama S. 2010. Ezetimibe ameliorates cardiovascular complications and hepatic steatosis in obese and type 2 diabetic db/db mice. J. Pharmacol. Exp. Ther. 335: 70–75 [DOI] [PubMed] [Google Scholar]
- 38.Nozaki Y., Fujita K., Yoneda M., Wada K., Shinohara Y., Takahashi H., Kirikoshi H., Inamori M., Kubota K., Saito S., et al. 2009. Long-term combination therapy of ezetimibe and acarbose for non-alcoholic fatty liver disease. J. Hepatol. 51: 548–556 [DOI] [PubMed] [Google Scholar]
- 39.Jia L., Ma Y., Rong S., Betters J. L., Xie P., Chung S., Wang N., Tang W., Yu L. 2010. Niemann-Pick C1-Like 1 deletion in mice prevents high-fat diet-induced fatty liver by reducing lipogenesis. J. Lipid Res. 51: 3135–3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tremblay A. J., Lamarche B., Hogue J. C., Couture P. 2009. Effects of ezetimibe and simvastatin on apolipoprotein B metabolism in males with mixed hyperlipidemia. J. Lipid Res. 50: 1463–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tremblay A. J., Lamarche B., Cohn J. S., Hogue J. C., Couture P. 2006. Effect of ezetimibe on the in vivo kinetics of apoB-48 and apoB-100 in men with primary hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 26: 1101–1106 [DOI] [PubMed] [Google Scholar]
- 42.Yunoki K., Nakamura K., Miyoshi T., Enko K., Kohno K., Morita H., Kusano K. F., Ito H. 2011. Ezetimibe improves postprandial hyperlipemia and its induced endothelial dysfunction. Atherosclerosis. 217: 486–491 [DOI] [PubMed] [Google Scholar]
- 43.Bozzetto L., Annuzzi G., Corte G. D., Patti L., Cipriano P., Mangione A., Riccardi G., Rivellese A. A. 2011. Ezetimibe beneficially influences fasting and postprandial triglyceride-rich lipoproteins in type 2 diabetes. Atherosclerosis. 217: 142–148 [DOI] [PubMed] [Google Scholar]
- 44.Chan D. C., Watts G. F., Gan S. K., Ooi E. M., Barrett P. H. 2010. Effect of ezetimibe on hepatic fat, inflammatory markers, and apolipoprotein B-100 kinetics in insulin-resistant obese subjects on a weight loss diet. Diabetes Care. 33: 1134–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cannon C. P., Giugliano R. P., Blazing M. A., Harrington R. A., Peterson J. L., Sisk C. M., Strony J., Musliner T. A., McCabe C. H., Veltri E., et al. 2008. Rationale and design of IMPROVE-IT (IMProved Reduction of Outcomes: Vytorin Efficacy International Trial): comparison of ezetimbe/simvastatin versus simvastatin monotherapy on cardiovascular outcomes in patients with acute coronary syndromes. Am. Heart J. 156: 826–832 [DOI] [PubMed] [Google Scholar]
- 46.Park H., Shima T., Yamaguchi K., Mitsuyoshi H., Minami M., Yasui K., Itoh Y., Yoshikawa T., Fukui M., Hasegawa G., et al. 2011. Efficacy of long-term ezetimibe therapy in patients with nonalcoholic fatty liver disease. J. Gastroenterol. 46: 101–107 [DOI] [PubMed] [Google Scholar]