Abstract
Tuberculosis (TB) is the worldwide leading cause of death among HIV-infected individuals, accounting for more than half of AIDS-related deaths. A high risk of tuberculosis (TB) has been shown in early stages of the HIV disease, even in the presence of normal CD4+ cell counts. Moreover, the factors that determine protective immunity vs. susceptibility to M. tuberculosis cannot be fully explained by simple changes in IFNγ levels or a shift from Th1 to Th2 cytokines. This work investigated the relationship between cytokine expression profiles in peripheral blood mononuclear cells (PBMC) and susceptibility to M. tuberculosis in ten HIV+ women who went on to develop TB. RNA transcripts for IL-4, IL-4δ2, IL-10, IL-12(p35), IL-13, IL-17A, IFNγ and TNFα were measured by real-time quantitative PCR in unstimulated or TB peptide antigen-stimulated PBMCs from ten HIV+ women with positive tuberculin skin tests (TST) and compared with HIV-seropositive and seronegative women without previous TB and negative TST. Stimulated PBMC cultures showed significantly lower expression of IL-12p35 (p=0.004) and IL-10 (p=0.026) in the HIV+TB+ group six to twelve months before onset of TB compared to HIV+TB− women. Unstimulated PBMC from HIV+TB+ women also had lower expression of Th2 cytokines [IL-4 (p=0.056) and IL-13 (p=0.050)] compared to HIV+TB− women. These results suggest that lower IL-12 production by PBMC in response to TB antigens and lower levels of both Th1 and Th2 cytokines by PBMC correlate with future development of TB in HIV-infected women and may be responsible for their increased susceptibility.
Keywords: Interferon-γ(IFNγ), Interleukin-4 (IL-4), Interleukin-12 (IL-12), Human Immunodeficiency Virus (HIV), Tuberculosis (TB)
1. INTRODUCTION
Tuberculosis (TB) is the worldwide leading cause of death among HIV-infected individuals [1,2], in fact, it is estimated that TB is the cause of as many half of AIDS-related deaths [3]. In the absence of anti-retroviral therapy, HIV-infected individuals with latent tuberculosis infection have 5–10% annual risk of TB in contrast to 10% during the life-time in HIV negative individuals [4,5]. A high risk of TB has been shown in early stages of the HIV disease, even in the presence of normal CD4+ cell counts [6,7]. The factors contributing to this increased risk are not clear, but may include a compromised response by CD4+ T cells, defective innate immunity and/or other elements of the host response against M. tuberculosis [8,9]. Because of the increased susceptibility to TB in infected individuals, HIV infection is a unique model to investigate factors and mechanisms facilitating the progression of TB disease.
Many studies have examined the roles of Th1 (IFNγ and IL-12) and Th2 (IL-4 and IL-10)-related cytokines in the immunity against M. tuberculosis infection [9–11] and protective immunity has been generally associated with strong Th1 responses [8,9]. Indeed, their key role in protective immunity against M. tuberculosis is supported by observations in genetically-modified mice or in humans affected by genetic defects. For example, mutations in the genes that encode IFNγ, IL-12 (subunits p40 or p35) or the receptors for these two cytokines greatly increase the susceptibility to infections by M. tuberculosis and other mycobacteria [8,12]. Consistently, HIV-infected individuals have been reported to have impaired production of IFNγ by mycobacteria-specific CD4+ T cells despite long-term highly-active antiretroviral therapy [4].
The role of Th2 responses in immunity against M. tuberculosis is more controversial [9]. Because of their ability to antagonize many of the effects of IFNγ, Th2 cytokines such as IL-4 and IL-10 have been generally regarded as contributing factors [10,11]. Many studies have found a relationship between increased production of these cytokines and disease progression or susceptibility to TB [10,13,14]. For example, increased IL-4 levels have been associated to a poor outcome to anti-TB treatment [15] or to development of overt TB in a group of healthcare workers [16]. The role of IL-4 may involve inhibition of bactericidal functions of macrophages, increased toxicity by TNFα and pulmonary fibrosis [10]. Some of the confusion about the role of IL-4 in TB has been due to the existence of an alternatively-spliced variant, IL-4δ2, which acts as a natural IL-4-antagonist [17]. There is evidence that IL-4δ2 expression is increased in patients with TB [18] and that elevated IL-4δ2 levels, in addition to Th1 cytokines, is associated with the ability of infected individuals to control latent M. tuberculosis infection [19].
Given the central importance of cytokines in the immunity against TB, it is very likely that HIV-associated defects or alterations in cytokine responses may contribute to increased susceptibility or non-protective immunity against M. tuberculosis. There are, however, multiple factors involved in the immunity to M. tuberculosis and thus it is not surprising that protection vs. susceptibility hasn’t been fully explained by simple changes in IFNγ levels or a shift from Th1 to Th2 cytokine patterns [8,9]. Thus, further study of cytokine responses/profiles preceding onset of the disease in HIV-infected patients may enhance our understanding of the factors that determine protective immunity or susceptibility to TB. The aim of this study was to examine the cytokine expression profiles in PBMC from HIV-infected patients both preceding and following TB, and to compare such profiles with those of HIV-infected patients without TB and a control group without HIV or TB.
2. MATERIAL AND METHODS
2.1 Study Design
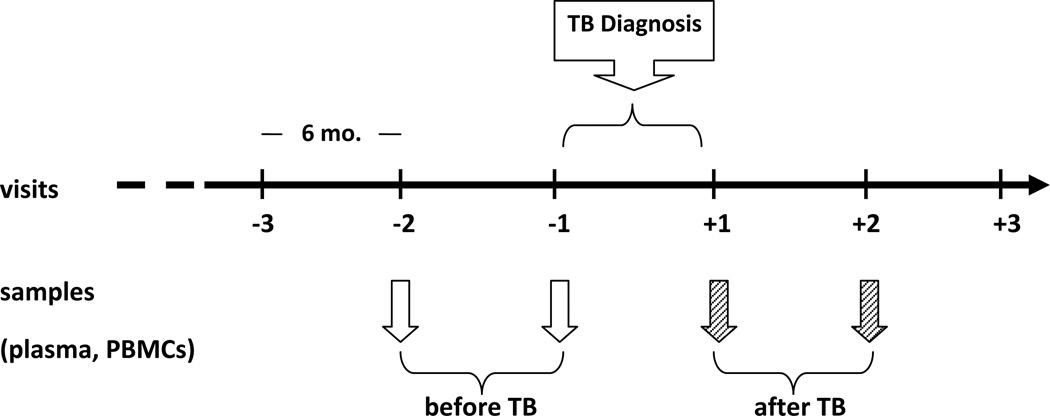
The work described in this article was approved by the University of Louisville Institutional Review Board (08.0355). Informed consent was obtained from all participants and human experimentation guidelines followed those of the U.S. Department of Health and Human Services. This was a retrospective longitudinal study to investigate cytokine profiles in plasma and peripheral blood mononuclear cells (PBMCs) in the context of tuberculosis in HIV-seropositive (HIV+) women enrolled in the Women Interagency HIV Study (WIHS). WIHS is a multicenter prospective cohort study established in 1994 to investigate the natural history of HIV infection and effects of therapy in women. Details of study methodology have been previously described [20,21]. Participant women visit WIHS sites every 6 months for extraction of information related to HIV infection and collection of blood. HIV-infected women receive anti-retroviral therapy according to guideline indications [21]. Ten HIV-infected women with TB were identified upon searching the WIHS database for cases with proven TB. These women received standard anti-tuberculous drug therapy for six months following diagnosis. Dates of diagnosis and anti-TB treatment were confirmed from at least one of the following: medical record abstractions, registry matches and death certificates. There were no records indicating that these women had been diagnosed with LTBI and/or had received treatment for LTBI. Plasma and PBMC specimens collected from these women during their two visits previous (≤ 1 year) to the diagnosis of TB, corresponding to the stage of latent M. tuberculosis infection (LTBI), and their two visits after (≤ 1 year) diagnosis and treatment of TB were obtained from the WIHS repository. Because reactivation of LTBI in HIV+ patients is common and the selected samples studied in our HIV+TB+ population were taken less than 6 and 12 months previous to their development of active TB, our studies assume that these patients already had LTBI. This group was designated as [HIV+TB+]. Figure 1 depicts the sample collection strategy for the study.
Figure 1.
Design for sample collection for the HIV+TB+ group. Plasma and PBMC samples collected during the two visits before and after development of active TB were obtained from the WIHS repository. For the control groups, samples from two consecutive visits were obtained from both HIV+TB− and HIV−TB− WIHS-participant women that closely matched the HIV+TB+ samples in terms of age, race, date of collection, CD4+ T cell numbers and HIV viral load.
2.2 Study Controls
Control group 1 included 10 WIHS-participant HIV-seropositive women without previous TB and negative skin tuberculin test (TST). This group was designated as [HIV+TB-]. Control group 2 included 10 WIHS-participant HIV-seronegative women without previous TB and negative TST. This group was designated as [HIV−TB−]. Specimens taken on two consecutive visits were obtained from the WIHS repository for each control participant in order to match the HIV+TB+ cases as close as possible. Case and Controls were matched for age, race, CD4+ cell counts, plasma HIV-viral load, and approximate date of collection. In the case of race and ethnicity, individual cases were matched as Caucasian, African American or Hispanic. Table 1 shows a summary of the characteristics of the patient groups.
TABLE 1.
Characteristics of Study Groups
| GROUP | Age (years) |
CD4+ T cell counts (cells/µl) |
HIV viral load (copies/µl) |
|---|---|---|---|
| HIV+ before TB | 38.2 (23.9–49.3) | 255 (184 –353) | 3,654 (1,100 – 12,136) |
| HIV+ after TB | 38.7 (24.4–50.3) | 244 (180 – 329) | 4,376 (1,145 – 16,720) |
| HIV+ TB− | 38.2 (30.4–46.7) | 281 (213 – 371) | 3,272 (924 – 11,590) |
| HIV− TB− | 36.5 (21.9–46.7) | 596 (433 – 820) | 0 |
Values denote the arithmetic mean and range (in parentheses) for age and geometric mean and 95% Confidence Interval (in parentheses) for CD4+ T cell counts and HIV viral loads. The HIV+ before and after TB groups include the same 10 subjects. Each subject included at least two samples obtained at consecutive visits to a WIHS center.
2.3 RNA isolation and Reverse Transcription
After thawing, PBMCs were either immediately lysed or cultured in the presence of a mixture of M. tuberculosis peptide antigens (ESAT-6 and CFP-10 peptides; QuantiFERON-TB Gold, Cellestis, Australia) before lysis. Total RNA was isolated using a RNAqueous kit (Ambion, Austin, TX) and purity and quantity assessed spectrophotometrically using a Nanodrop 2000 apparatus (Thermo Scientific, Wilmington, DE). The reverse-transcription step was carried out using a High capacity RNA-to-cDNA kit according to manufacturer’s protocol (Applied Biosystems, San Diego, CA).
2.4 Stimulation of PBMC
Aliquots of PBMC (~1–2 × 106 cells) were cultured in 24-well plates using complete medium (RPMI 1640 supplemented with 10% FCS, 100 U/ml penicillin, 100 mg/ml streptomycin, 2mM L-glutamine, 1 mM sodium pyruvate and 0.1 mM non-essential amino acids) in the presence of a mixture of M. tuberculosis ESAT-6 and CFP-10 peptides (10 mg/ml; Cellestis, Australia), for 48 hours at 37° C/5% CO2. The cells were then harvested and immediately lysed using RNAqueous lysis reagent.
2.5 Real-time PCR analysis of cytokine-specific transcripts
Expression of transcripts specific for IL-4, IL-4δ2, IL-10, IL-12(p35), IL-13, IL-17A, IFNγ and TNFα in unstimulated and stimulated PBMCs was measured by real-time quantitative polymerase chain reaction (PCR). Following the reverse-transcription step, cDNA aliquots were amplified in an ABI prism 7300 sequence detection system (Applied Biosystems, Foster City, CA) using TaqMan Gene expression assays and Master mix (Applied Biosystems). The expression of the different cytokine transcripts was normalized to that of the housekeeping gene control, β-actin, in all the experiments (ΔCt). The levels of the different cytokine transcripts were also expressed as Arbitrary Units, calculated by multiplying the ratio of the expression of each cytokine transcript to that of β-actin by 106.
2.6 Measurement of cytokine levels in plasma samples
Levels of eight different cytokines (IL-4, IL-6, IL-10, IL-12 (p70), IL-17, IFNγ and TNFα) in plasma were measured using a Bioplex-Pro multianalyte assay (Bio-Rad, Hercules, CA) according to the manufacturer’s protocol. Readings were done in a Luminex 100 system (Luminex, Austin, TX).
2.7 Statistical Methods
Due to the longitudinal nature of the data, the repeated measures ANOVA model was utilized to evaluate the differences in 8 cytokine profile measures (IL-4, IL-4δ2, IL-10, IL-17, IL-12, IL-13, IFNγ and TNFα) and 4 ratios (IL-4δ2/IL-4, IFNγ/IL-4, IFNγ/IL-10 and IL-12/IL-10). This type of modeling takes into account both within repeated measures and between study groups subject variability. Initially, we calculated summary statistics for each of the cytokines by HIV-TB group and TB status using the ΔCt data. Then, we examined the differences in cytokine profiles (pre-TB and post-TB) in the group that developed TB. Next, we ran the repeated measures one-way ANOVA with HIV-TB group to examine the differences in group means between HIV+TB− vs. HIV+TB+ (pre-TB) and HIV−TB− vs. HIV+TB+ (pre-TB) groups. Finally, an ANCOVA model was fit with possible predictors of cytokine outcomes, CD4+ cell count and/or HIV-RNA load as covariates. Analysis of cytokine expression in stimulated PBMC cultures was done in similar fashion. All statistical tests were based on 5% significance level.
3. RESULTS
3.1 Cytokine Profiles in Unstimulated PBMC
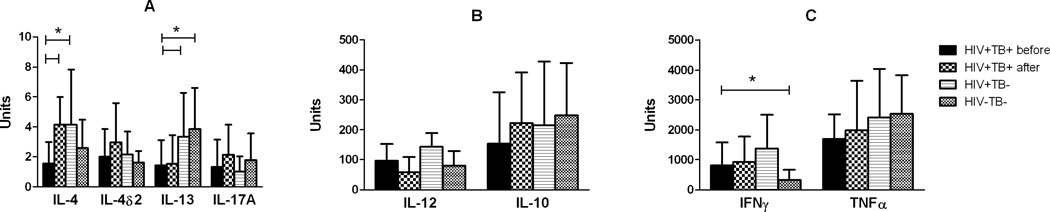
The cytokine expression profiles for unstimulated PBMC are shown in Figure 2 (raw ΔCt data is summarized in the Supplemental Data, Table SD-1). Because of their wide range of expression, Figure 2 divides cytokines in three different groups based on expression levels (2A: <10; 2B: 10–500; and 2C: 500–5000 units). The expression levels were compared in HIV+TB+ patients, both before and after development of TB, and then with the HIV+TB− and HIV−TB− control groups. Analysis of the levels of cytokine expression in HIV+TB+ patients showed moderate increases in the expression of both Th1 and Th2 cytokines in samples taken after development and treatment of TB. The expression of IL-4 transcripts increased approximately 2.57-fold in comparison to levels before TB (p<0.05). The expression of transcripts for IL-4δ2 (1.41-fold), IFNγ(1.72-fold) and IL-10 (1.37-fold) also showed a tendency to increase after TB, but the differences did not reach statistically significance. There were no significant changes observed in the expression of IL-12(p35), IL-13, IL-17A or TNFα. When compared with the control groups, the expression of IFNγ in both HIV+ groups was considerably higher than in HIV− controls (p<0.05), suggesting that HIV infection leads to an enhanced production of this cytokine. The levels in HIV+TB+ patients before developing TB were lower compared with HIV+TB− patients (2.5- vs. 4.2-fold over HIV−TB− controls), although this difference did not reach statistical significance. Interestingly, differences in the expression levels of the Th2 cytokines, IL-4 and IL-13, between the HIV+TB+ (before TB) group and the HIV+TB− group were statistically significant (p<0.05). In both cases, transcripts for these two cytokines were lower in HIV+ patients that went on to develop TB compared to HIV+ patients without TB.
Figure 2.
Cytokine transcript expression in unstimulated PBMCs. Frozen PBMC samples were obtained from the WIHS repository from a group of HIV+ women before and after diagnosis and treatment of TB (HIV+TB+ before and HIV+TB+ after, respectively); a group of matched HIV+TB− women and another control group consisting of matched HIV−TB− women. Total RNA was isolated from PBMC samples following thawing as indicated in Materials and Methods. Cytokine expression was estimated by quantitative real-time PCR. Relative expression was calculated based on ΔCt values and expressed as Arbitrary Units, calculated by multiplying the ratio of the expression of each cytokine transcript to that of β-actin by 106. The three graphs (A-C) group different cytokines according to expression levels (A: <10; B: 10–500; C: >500 Arbitrary Units). Bars represent the geometric mean of expression each group (in Arbitrary Units) and error bars represent 95% C.I. (* p<0.05).
3.2 Cytokine Profiles in Stimulated PBMC
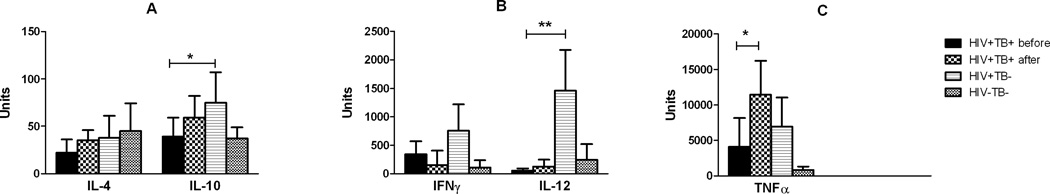
The cytokine expression profiles for stimulated PBMC are shown in Figure 3 (raw ΔCt data is summarized in the Supplemental Data, Table SD-2). This figure is also divides in three different groups based on expression levels (3A: <100; 3B: 100–1500; and 3C: >1500 units). Transcripts for IL-4δ2, IL-13 and IL-17A were not consistently detected in these samples and were not included in the analyses. Similarly to the unstimulated PBMCs, the relative levels of expression for most cytokines were higher in the HIV+TB+ samples taken after development of TB, particularly in the cases of IL-12(p35) and TNFα, which showed increases of 3.71- and 2.83-fold compared to levels before TB. In the case of TNFα, this difference was statistically significant (p<0.05). Comparison of HIV+TB+ (before TB) with the HIV+TB− group showed that cytokine expression levels were consistently lower in the HIV+ group that went on to develop TB. As shown in Figure 3B, the most dramatic difference observed was that for the expression of IL-12(p35), which was much lower in HIV+TB+ patients (p<0.01). Expression of IL-10 was also significantly lower in the HIV+TB+ compared with the HIV+TB− group (p<0.05). Although expression of IFNγ followed the same pattern (lower in the HIV+ group before TB), differences with the HIV+TB− group were not statistically significant. Likewise, expression of IL-4 and TNFα, tended to be lower in the HIV+TB+ group, but the differences with the HIV+TB− group were not significant.
Figure 3.
Cytokine transcript expression in PBMCs stimulated with ESAT-6 and CFP-10 peptides (10 µg/ml) for 48 hours, as indicated in Materials and Methods. Groups are the same as those described under Figure 1. Total RNA was isolated from PBMC following culture and cytokine expression was estimated by quantitative real-time PCR. Relative expression was calculated based on ΔCt values and expressed as Arbitrary Units, calculated by multiplying the ratio of the expression of each cytokine transcript to that of β-actin by 106. The three graphs (A-C) group different cytokines according to expression levels (A: < 100; B: 100–1,500; C: >1,500 Arbitrary Units). Bars represent the geometric mean of each group (in Arbitrary Units) and error bars represent 95% C.I. (* p<0.05; ** p<0.01).
3.3 Comparison of cytokine expression ratios
In order to investigate whether differences in expression ratios rather than absolute expression existed in HIV+ patients before or after TB or between HIV+ patients that went on to develop TB vs. those without TB, we also calculated and compared the relative expression ratios of IL-4δ2/IL-4, IFNγIL-4, IFNγ/IL-10 and IL-12/IL-10 (Table 2). We did not detect any significant changes in IL-4δ2/IL-4 expression ratios among the different groups in unstimulated PBMCs. However, because of the lower expression levels of IFNγ in HIV−TB− controls, the IFNγ/IL-4 ratio in this group was significantly different from that of the HIV+TB+ patients before development of TB. Moreover, the IFNγ/IL-10 ratio in the HIV−TB− control groups was significantly smaller compared with the other groups. No significant differences were observed in IL-12/IL-10 ratios in unstimulated PBMCs. However, in the case of TB peptide-stimulated PBMCs, the IL-12/IL-10 ratios were significantly lower (p<0.05) compared to those of HIV+TB− and HIV−TB− controls, most likely the result of the lower levels of IL-12p35 expression.
TABLE 2.
Expression of cytokine transcripts in unstimulated PBMC
| GROUP | IL-4 | IL-4δ2 | IFNγ | IL-10 | IL-12(p35) | IL-13 | IL-17A | TNFα |
|---|---|---|---|---|---|---|---|---|
| HIV+ before TB | 19.29 ± 1.61 | 18.92 ± 1.69 | 10.26 ± 1.78 | 12.67 ± 2.03 | 13.33 ± 1.22 | 19.43 ± 1.87 | 19.51 ± 1.83 | 8.81 ± 1.88 |
| HIV+ after TB | 17.93 ± 1.02 | 18.33 ± 2.24 | 9.68 ± 1.89 | 12.25 ± 1.63 | 13.66 ± 1.08 | 19.42 ± 1.80 | 19.16 ± 1.85 | 9.10 ± 1.78 |
| HIV+ TB- | 17.88 ± 1.77 | 18.83 ± 1.77 | 9.51 ± 1.96 | 12.18 ± 2.25 | 12.75 ± 0.86 | 18.20 ± 1.95 | 19.94 ± 1.80 | 8.70 ± 1.68 |
| HIV− TB− | 18.56 ± 1.56 | 19.23 ± 1.19 | 11.58 ± 2.32 | 11.98 ± 1.75 | 13.62 ± 1.55 | 17.98 ± 1.60 | 19.11 ± 2.11 | 8.62 ± 1.33 |
Results expressed as the Mean ± SD of ΔCt values calculated by real-time PCR using the expression of β-actin as housekeeping gene control. Total RNA was isolated from unstimulated PBMC samples.
3.4 Cytokine levels in Plasma
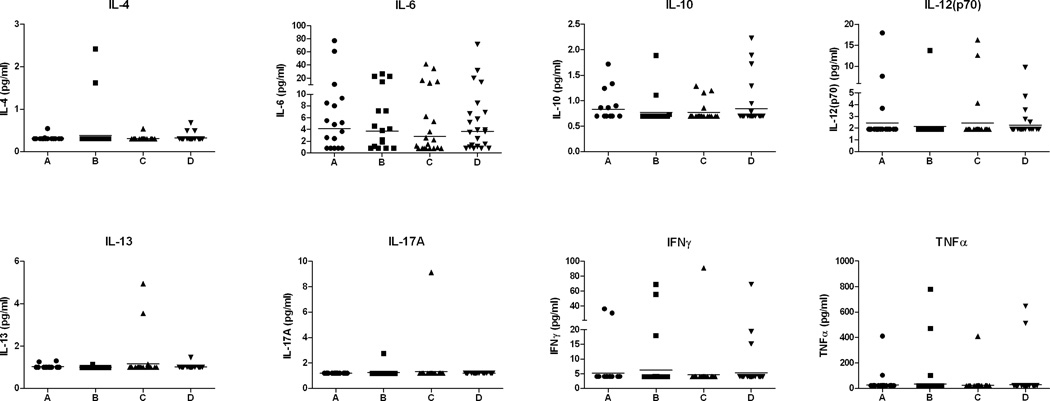
In order to investigate potential differences in circulating cytokine levels, the concentrations of IL-4, IL-6, IL-10, IL-12 (p70), IL-13, IL-17A, IFNγ and TNFα in plasma samples of the same subjects were also investigated. However, for many of these cytokines, most of the samples had levels at or below the detectable range, as can be appreciated in Figure 4, which shows the distribution of plasma concentrations of the different cytokines. Even for those cytokines that were more consistently detected (e.g., IL-6, IL-10, IL-12, IFNγ and TNFα) no statistically significant differences were observed when comparing the different groups.
Figure 4.
Distribution of cytokine levels in plasma samples of groups of HIV+ women before (A) and after (B) reactivation of TB; HIV+ women without TB (C) and a control group of HIV−TB− women (D). Cytokine levels were assayed using a Bioplex bead array and expressed as pg/ml. Lower limits of detection for each of the assayed cytokines were: IL-4 (0.3 pg/ml); IL-6 (0.8 pg/ml); IL-10 (0.7 pg/ml); IL-12p70 (1.9 pg/ml); IL-13 (1.0 pg/ml); IL-17A (1.2 pg/ml); IFNγ (4.0 pg/ml) and TNFα (20 pg/ml). Horizontal bars represent the geometric mean of each group.
4. DISCUSSION
These studies, although preliminary, were able to identify differences in cytokine expression patterns in HIV+ patients that may potentially play a role in their development of TB. Main among these findings, was the significantly lower expression of IL-12(p35) transcripts by PBMCs stimulated with M. tuberculosis peptides in HIV+ patients before development of TB, when compared to those of HIV+ women without TB. The significance of this observation was maintained even after controlling for CD4+ T cell counts and HIV-RNA load. The weak IL-12 response seen in our study correlates with reports of defective IL-12 being a major factor contributing to the development of TB in the setting of HIV infection [7,8]. In this regard, a major role for IL-12 function in the immune response to M. tuberculosis is supported by reports that IL-12 receptor deficiency was found in otherwise healthy individuals with mycobacterial infections [22]. Moreover, IL-12 receptor-induced IFNγ production has been shown to correlate with a protective Th1 response in TB and other mycobacterial infections [23,24] and IL-12 has been described to be a successful adjuvant in the treatment of TB [25]. Thus, considering that HIV-infected individuals have been reported to have impairments in the IL-12/IFNγ axis [26,27] coupled with the key role of IL-12 in protective immunity against M. tuberculosis, it is not then surprising that their risk to develop TB is greatly enhanced.
The reasons for the compromised ability to produce IL-12 in response to M. tuberculosis peptide antigens in the HIV-infected patients that developed TB (6–12 months before) are not clear. IL-12 is a key component of the innate immune response and produced by macrophages stimulated by bacteria and other pathogens or their components [28]. However, M. tuberculosis and derived products, including ESAT-6, have been shown to be able to reduce secretion of both IL-12 and IL-15 in macrophages [29,30], which could be a strategy to circumvent a strong Th1 immunity.
Although the largest difference occurred in terms of IL-12(p35) expression, it is significant that the expression of IL-10 transcripts by stimulated PBMCs was also reduced in HIV+ patients before development of TB. Because the levels of IL-12 and IL-10 in HIV+TB+ individuals were not different from the HIV−TB− controls, it would appear that HIV infection lead to dysregulated production of both IL-12 and IL-10. In the presence of TB infection, however, the production of both cytokines was reduced. Because macrophages are also important sources of IL-10 [31], this finding suggests that the defect in HIV-infected individuals who later developed TB may not be restricted to reduced IL-12 (a cytokine promoting Th1 responses), but may also include other macrophage-derived cytokines and even macrophage function. Understanding the mechanisms responsible for this defect in IL-12 expression would help explain the greater susceptibility of HIV+ patients for TB.
It appeared from results of Figure 3, that although TNFα production was increased in HIV-infected individuals in comparison with the HIV−TB− group, latent TB infection may have also affected the ability of PBMCs to produce this cytokine, a defect that was mitigated after treatment for TB. We believe that these results point out to a defect of APCs related to the TB infection which may lead to dysregulation of cytokine production, resulting in a weakened IL-12/IFNγ/TNFα axis and perhaps other macrophage-related functions, thus facilitating the development of TB.
Although not statistically-significant, our results showed a tendency for reduced expression of IFNγ among HIV-infected patients who went on to develop TB, which would be consistent with a compromised IL-12/IFNγ axis. However, our results also showed that expression of two Th2 cytokines (IL-4 and IL-13) were also lower in PBMC from HIV+ women before development of TB. Interestingly, production of these three cytokines increased after TB to levels that were similar to those of the HIV+TB− group. These results suggest that potential alterations to the cytokine profiles of the HIV+ patients before development of TB cannot be simply explained by an inversion of the Th1/Th2 ratio. In fact, rather than an alteration of the Th1/Th2 balance, the results are more consistent with a general lower reactivity of PBMC. It is interesting to note that IL-4 and IL-13 have been reported to potentiate the transcription of the genes encoding both p40 and p35 subunits of IL-12, and that in mononuclear cells of HIV-infected patients, priming with these two cytokines potentiated in vitro IL-12 production [32,33].
Although previous studies had associated high levels of IL-4 and low levels of IL-4δ2 at the time of TB [2,14,16], such pattern was not found in our study. However, a study by Dheda et al. [18], noted that expression of IL-4δ2 was restricted to the lungs but not the peripheral blood of HIV-TB co-infected patients. Another potential explanation is that the previously reported changes of IL-4 and IL-4δ2 may occur around the time of TB, and not 6–12 months before, which is more consistent with our studies.
Despite some reports of the importance of IL-17 in M. tuberculosis infection, mostly in the maintenance of the inflammatory response [34], we did not observe any significant changes in the expression of this cytokine. Moreover, although we expected the samples from HIV+TB+ patients to have higher levels of expression of T-cell-derived cytokines (such as IFNγ) in response to TB peptides compared to HIV+TB− patients, this was not the case. The reasons for this finding are not clear, but might have resulted from an overall lack of responsiveness, to lower production of IL-12 by monocyte/macrophages, or the inhibitory effects of ESAT-6 in vitro.
Recent studies by Berry et al. [35 and Maertzdorf et al. [36] have analyzed whole blood gene expression profiles in patients with active and latent TB as well as uninfected controls. The former study was able to identify a 393-transcript signature for active TB which correlated with radiological extent of the disease and reverted to that of healthy controls after treatment. The most over-represented pathway in patients with active TB was IFN-signaling, including genes downstream of both IFNγ and type I IFN. In our study, we found that expression of IFNγ was higher in both HIV+ groups compared to HIV− controls, probably representing increased IFNγ production due to the viral infection. However, the HIV+TB+ group tended to have comparatively lower levels of IFNγ. The second study found unique expression profiles in active TB vs. LTBI, with a cluster of genes involved in apoptosis regulation showing reduced expression in active TB and a cluster of genes involved in host defense responses and mainly active in granulocytes and macrophage function and differentiation expressed at lower levels in LTBI compared to active TB or even non-infected donors. Although none of the reported genes included the cytokines analyzed in our study, the pattern observed in the HIV+TB+ patients, particularly before development of TB (LTBI stage), appears consistent with compromised macrophage function (e.g., reduced IL-12, TNFα, IL-10 production).
Among the limitations to our study are the lack of prospective study design and small patient sample that may have limited an optimal evaluation of the cytokine profile in relation to TB and HIV infection. Given the small numbers of patients, our findings need to be validated with prospective studies with larger numbers of patients and including also HIV-seropositive, TST-positive patients who do not develop TB to make sure that the abnormal cytokine responses are indeed associated with increased susceptibility. Another potential limitation is the working assumption that all of the HIV+TB+ cases were due to reactivation of LTBI. Although we consider that based on epidemiological data [37] and the time at which the PBMC samples were obtained prior to the development of TB, our assumption is solid, we cannot completely rule out that some cases may have represented primary TB. Finally, the relatively long storage period of some of the PBMC samples used in our studies, may have affected cell viability upon thawing. In order to minimize such an effect and in addition to matching samples for storage length, we chose to stimulate our cultures with M. tuberculosis-derived peptide antigens based on reports that long-term storage affects T cell responsiveness to peptides to a lesser extent than to whole proteins (antigens) [38]. Nevertheless, changes in cell viability and composition in the thawed/cultured PBMCs may have been responsible for the lower arbitrary expression units when comparing stimulated (Fig. 3) vs. unstimulated (Fig. 2) samples.
In summary, our study revealed lower levels of IL-12 production by PBMC in response to TB antigens and lower levels of both Th1 and Th2 cytokine expression by T cells at 6 – 12 months in HIV+ women that went on to develop TB compared with a group of matched HIV+ women without TB. Our data suggest that defective production of IL-12 may contribute to the increased risk of developing TB in individuals with HIV infection. Prospective studies are needed to validate our study results.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grant #50520 from the Basic Grant Program, University of Louisville School of Medicine and a grant from the Research on Women Grant Program, University of Louisville. Data in this manuscript were collected by the Women's Interagency HIV Study (WIHS). Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington, DC, Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (AlexandraLevine); Chicago Consortium (Mardge Cohen); Data Coordinating Center (Stephen Gange). The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (UO1-HD-32632). The study is co-funded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is also provided by the National Center for Research Resources (UCSF-CTSI Grant Number UL1 RR024131). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Markowitz N, Hansen NI, Hopewell PC, Glassroth J, Kvale PA, Mangura BT, Wilcosky TC, Wallace JM, Rosen MJ, Reichman LB. Incidence of tuberculosis in the United States among HIV-infected persons. The Pulmonary Complications of HIV Infection Study Group. Ann. Intern. Med. 1997;126:123–132. doi: 10.7326/0003-4819-126-2-199701150-00005. [DOI] [PubMed] [Google Scholar]
- 2.Corbett EL, Watt CJ, Walker N, Maher DBM, Williams BG, Raviglione MC, Dye C. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 3.UNAIDS. Frequently asked questions about tuberculosis and HIV. 2006 http://data.unaids.org/pub/FactSheet/2006/tb_hiv_qa.pdf.
- 4.Rook GA, Dheda K, Zumla A. Immune responses to tuberculosis in developing countries: implications for new vaccines. Nat. Rev. Immunol. 2005;5:661–667. doi: 10.1038/nri1666. [DOI] [PubMed] [Google Scholar]
- 5.Selwyn PA, Hartel D, Lewis VA, Schoenbaum EE, Vermund SH, Klein RS, Walker AT, Friedland GH. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N. Engl. J. Med. 1989;320:545–550. doi: 10.1056/NEJM198903023200901. [DOI] [PubMed] [Google Scholar]
- 6.Sonnenberg P, Glynn JR, Fielding K, Murray J, Godfrey-Faussett P, Shearer S. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in south african gold miners. J. Infect. Dis. 2005;191:150–158. doi: 10.1086/426827. [DOI] [PubMed] [Google Scholar]
- 7.Benson CA, Kaplan JE, Masur H, Pau A, Holmes KK. Treating opportunistic infections among HIV-infected adults and adolescents. M.M.W.R. 2004;53(RR15):1–112. [PubMed] [Google Scholar]
- 8.North RJ, Jung YJ. Immunity to tuberculosis. Annu. Rev. Immunol. 2004;22:599–623. doi: 10.1146/annurev.immunol.22.012703.104635. [DOI] [PubMed] [Google Scholar]
- 9.Van Crevel R, Ottenhoff THM, van der Meer JWM. Innate immunity to Mycobacterium tuberculosis . Clin. Microbiol. Rev. 2002;15:294–309. doi: 10.1128/CMR.15.2.294-309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rook GAW, Hernandez-Pando R, Dheda K, Seah GT. IL-4 in tuberculosis: implications for vaccine design. TRENDS Immunol. 2004;25:483–488. doi: 10.1016/j.it.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Bhatt K, Salgame P. Host innate immune response to Mycobacterium tuberculosis . J. Clin. Immunol. 2007;27:347–362. doi: 10.1007/s10875-007-9084-0. [DOI] [PubMed] [Google Scholar]
- 12.Howie S, Ramage R, Hewson T. Innate immune system damage in human immunodeficiency virus type 1 infection. Implications for acquired immunity and vaccine design. Am. J. Respir. Crit. Care Med. 2000;162:S141–S145. doi: 10.1164/ajrccm.162.supplement_3.15tac1. [DOI] [PubMed] [Google Scholar]
- 13.Seah GT, Rook GAW. High levels of mRNA encoding IL-4 in unstimulated peripheral blood mononuclear cells from tuberculosis patients revealed by quantitative nested reverse transcriptase-polymerase chain reaction; correlations with serum IgE levels. Scand. J. Infect. Dis. 2001;33:106–109. doi: 10.1080/003655401750065472. [DOI] [PubMed] [Google Scholar]
- 14.Dlugovitzky D, Bay ML, Rateni L, Urizar L, Rondelli CF, Largacha C, Farroni MA, Molteni O, Bottasso OA. In vitro synthesis of interferon-gamma, interleukin-4, transforming growth factor-beta and interleukin-1 beta by peripheral blood mononuclear cells from tuberculosis patients: relationship with the severity of pulmonary involvement. Scand. J. Immunol. 1999;49:210–217. doi: 10.1046/j.1365-3083.1999.00492.x. [DOI] [PubMed] [Google Scholar]
- 15.Marchant A, Amedei A, Azzurri A, Vekemans J, Benagiano M, Tamburini C, Lienhardt C, Corrah T, McAdam KP, Romagnani S, D'Elios MM, Del Prete G. Polarization of PPD-specific T-cell response of patients with tuberculosis from Th0 to Th1 profile after successful antimycobacterial therapy or in vitro conditioning with interferon-α or interleukin-12. Am. J. Respir. Cell. Mol. Biol. 2001;24:187–194. doi: 10.1165/ajrcmb.24.2.4274. [DOI] [PubMed] [Google Scholar]
- 16.Ordway D, Costa L, Martins M, Silveira H, Amaral L, Arroz MJ, Ventura FA, Dockrell HM. Increased IL-4 production by CD8 and γδ cells in health care workers is associated with the subsequent development of active tuberculosis. J. Infect. Dis. 2004;190:756–766. doi: 10.1086/422532. [DOI] [PubMed] [Google Scholar]
- 17.Atamas SP, Choi J, Yurovsky VV, White B. An alternative splice variant of IL-4, IL-4 delta 2, inhibits IL-4-stimulated T cell proliferation. J. Immunol. 1996;156:534–541. [PubMed] [Google Scholar]
- 18.Dheda K, Chang JS, Breen RAM, Kim LU, Haddock JA, Huggett JF, Johnson MA, Rook GA, Zumla A. Expression of a novel cytokine, IL-4delta2, in HIV and HIV-tuberculosis co-infection. AIDS. 2005;19:1601–1606. doi: 10.1097/01.aids.0000183520.52760.ef. [DOI] [PubMed] [Google Scholar]
- 19.Demissie A, Abebe M, Aseffa A, Rook G, Fletecher H, Zumla A, Weldingh K, Brock I, Andersen P, Doherty TM. VACSEL Study Group. Healthy individuals that control a latent infection with Mycobacterium tuberculosis express higher levels of Th1 cytokines and the IL-4 antagonist IL-4 δ2. J. Immunol. 2004;172:6938–6943. doi: 10.4049/jimmunol.172.11.6938. [DOI] [PubMed] [Google Scholar]
- 20.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, Young M, Greenblatt R, Sacks H, Feldman J. The Women's Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- 21.Bacon M, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, Gange S, Barranday Y, Holman S, Weber K, Young MA. The Women's Interagency HIV Study: an Observational Cohort Brings Clinical Sciences to the Bench. Clin. Diagn. Lab. Immunol. 2005;12:1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu. Rev. Immunol. 2002;20:581–620. doi: 10.1146/annurev.immunol.20.081501.125851. [DOI] [PubMed] [Google Scholar]
- 23.Zhang M, Gong J, Presky DH, Xue W, Barnes PF. Expression of the IL-12 receptor β1 and β2 subunits in human tuberculosis. J. Immunol. 1999;162:2441–2447. [PubMed] [Google Scholar]
- 24.Altare F, Durandy A, Lammas D, Emile JF, Lamhamedi S, Le Deist F, Drysdale P, Jouanguy E, Doffinger R, Bernaudin F, Jeppsson O, Gollob JA, Meinl E, Segal AW, Fischer A, Kumararatne D, Casanova JL. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280:1432–1435. doi: 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- 25.Greinert U, Ernst M, Schlaak M, Entzian P. Interleukin-12 as successful adjuvant in tuberculosis treatment. Eur. Respir. J. 2001;17:1049–1051. doi: 10.1183/09031936.01.17510490. [DOI] [PubMed] [Google Scholar]
- 26.Chehimi J, Starr SE, Frank I, D'Andrea A, Ma X, MacGregor RR, Sennelier J, Trinchieri G. Impaired interleukin 12 production in human immunodeficiency virus-infected patients. J. Exp. Med. 1994;179:1361–1366. doi: 10.1084/jem.179.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marshall JD, Chehimi J, Gri G, Kostman JR, Montaner LJ, Trinchieri G. The interleukin-12-mediated pathway of immune events is dysfunctional in human immunodeficiency virus-infected individuals. Blood. 1999;94:1003–1011. [PubMed] [Google Scholar]
- 28.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nature Rev. Immunol. 2003;2:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 29.Nau GJ, Richmond JFL, Schlesinger A, Jennings EG, Lander ES, Young RA. Human macrophage activation programs induced by bacterial pathogens. Proc. Natl. Acad. Sci. USA. 2002;99:1503–1508. doi: 10.1073/pnas.022649799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanley SA, Raghavan S, Hwang WW, Cox JS. Acute infection and macrophage subversion by Mycobacterium tuberculosis require a specialized secretion system. Proc. Natl. Acad. Sci. USA. 2003;100:13001–13006. doi: 10.1073/pnas.2235593100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nature Rev. Immunol. 2010;10:170–182. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 32.D'Andrea A, Ma X, Aste-Amegaza M, Paganin C, Trinchieri G. Stimulatory and inhibitory effects of interleukin (IL)-4 and IL-13 on the production of cytokines by peripheral blood mononuclear cells: priming for IL-12 and tumor-necrosis factor-α production. J. Exp. Med. 1995;181:537–546. doi: 10.1084/jem.181.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marshall JD, Robertson SE, Trinchieri G, Chehimi J. Priming with IL-4 and IL-13 during HIV-1 infection restores in vitro IL-12 production by mononuclear cells of HIV-infected patients. J. Immunol. 1997;159:5705–5714. [PubMed] [Google Scholar]
- 34.Khader SA, Cooper AM. IL-23 and IL-17 in tuberculosis. Cytokine. 2008;41:79–83. doi: 10.1016/j.cyto.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berry MPR, Graham CM, McNab FW, Xu Z, Bloch SAA, Oni T, Wilkinson KA, Banchereau R, Skinner J, Wilkinson RJ, Quinn C, Blankenship D, Dhawan R, Cush JJ, Mejias A, Ramilo O, Kon OM, Pascual V, Banchereau J, Chaussabel D, O'Garra A. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–979. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maertzdorf J, Repsilber D, Parida SK, Stanley K, Roberts T, Black G, Walzl G, Kaufmann SHE. Human gene expression profiles of susceptibility and resistance in tuberculosis. Genes Immun. 2011;12:15–22. doi: 10.1038/gene.2010.51. [DOI] [PubMed] [Google Scholar]
- 37.Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis. A general review. Bibl. Tuberc. 1970;26:28–106. [PubMed] [Google Scholar]
- 38.Owen RE, Sinclair E, Emu B, Heitman JW, Hirschkorn DF, Epling CL, Tan QX, Custer B, Harris JM, Jacobson MA, McCune JM, Martin JN, Hecht FM, Deeks SG, Norris PJ. Loss of T cell responses following long-term cryopreservation. J. Immunol. Methods. 2007;326:93–115. doi: 10.1016/j.jim.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.