Abstract
Tissue-engineered constructs designed to treat large cartilage defects or osteoarthritic lesions may be exposed to significant mechanical loading as well as an inflammatory environment upon implantation in an injured or diseased joint. We hypothesized that a three-dimensionally (3D) woven poly(ε-caprolactone) (PCL) scaffold seeded with bone marrow-derived mesenchymal stem cells (MSCs) would provide biomimetic mechanical properties in early stages of in vitro culture as the MSCs assembled a functional, cartilaginous extracellular matrix (ECM). We also hypothesized that these properties would be maintained even in the presence of the pro-inflammatory cytokine interleukin-1 (IL-1), which is found at high levels in injured or diseased joints. MSC-seeded 3D woven scaffolds cultured in chondrogenic conditions synthesized a functional ECM rich in collagen and proteoglycan content, reaching an aggregate modulus of ~0.75 MPa within 14 days of culture. However, the presence of pathophysiologically relevant levels of IL-1 limited matrix accumulation and inhibited any increase in mechanical properties over baseline values. On the other hand, the mechanical properties of constructs cultured in chondrogenic conditions for 4 weeks prior to IL-1 exposure were protected from deleterious effects of the cytokine. These findings demonstrate that IL-1 significantly inhibits the chondrogenic development and maturation of MSC-seeded constructs; however, the overall mechanical functionality of the engineered tissue can be preserved through the use of a 3D woven scaffold designed to recreate the mechanical properties of native articular cartilage.
Keywords: cytokine, articular cartilage, tissue engineering, textile, stem cell, mesenchymal stem cell, differentiation, fiber, inflammation, fabric
Introduction
Articular cartilage shows little capacity for repair in response to traumatic injury or degenerative conditions such as osteoarthritis. In this regard, there has been increasing interest in the development of tissue engineering strategies for cartilage repair or regeneration using autologous chondrocytes or stem/progenitor cells such as adult bone marrow-derived mesenchymal stem cells (MSCs) or adipose derived stem cells (ASCs). However, there is little evidence of the long-term clinical success of such procedures as compared to less complex surgical methods such as microfracture [1]. While the reasons for these failures are not fully understood, cell-seeded scaffolds generally do not possess appropriate biomechanical functionality at the time of implantation, and they may require significant maturation time in vitro before achieving mechanical properties that can withstand joint loading in vivo (e.g., [2, 3]). In many cases, the repair site shows incomplete cell differentiation and a lack of formation of hyaline cartilage in vivo [4].
One potential cause of graft failure that is not often considered is that injured and osteoarthritic joints exhibit significantly higher levels of pro-inflammatory cytokines. For example, interleukin-1 (IL-1) and tumor necrosis factor alpha (TNF-α), as well as pro-catabolic enzymes and mediators such as metalloproteinases (MMPs), aggrecanases, prostaglandins, and nitric oxide are overexpressed in these joints and can cause tissue degradation, pain, and inflammation [5-7]. While the effects of IL-1 on chondrocytes have been characterized extensively (e.g., [8]), recent studies have also demonstrated the deleterious effects of IL-1 on the chondrogenesis of ASCs [9] and MSCs [10-15], as well as tissue engineered cartilage comprising chondrocytes seeded in scaffolds of agarose [16, 17] or collagen [18, 19]. Furthermore, IL-1 and TNF-α inhibit the integrative repair of cartilaginous tissues such as the meniscus [20], via upregulation of MMPs [21] and the inhibition of cell proliferation [22]. Thus, the inflammatory environment of the injured joint may have similar effects on a tissue-engineered construct in vivo, particularly in severe degenerative conditions such as osteoarthritis.
The development of biomaterial scaffolds that provide appropriate functional biomechanical properties of cartilage, even prior to new tissue formation, may have significant advantages in rapidly restoring and maintaining tissue function in an inflammatory joint environment [23]. In this regard, we have developed a three-dimensional (3D) woven porous scaffold from poly(ε-caprolactone) (PCL) that mimics the nonlinear, anisotropic, compressive, and inhomogeneous mechanical properties of articular cartilage [24]. This scaffold can be readily seeded with cells and can be formed into 3D anatomical shapes to resurface large defects [25-27]. Since PCL degrades slowly, we hypothesized that the combination of the biomimetic scaffold and chondrogenically induced MSCs would provide both the initial functional properties of articular cartilage, and also provide long-term load-bearing properties, even in the conditions in which chondrogenesis was potentially inhibited by pro-inflammatory factors such as IL-1.
The goals of this study were to examine the effects of IL-1 on the chondrogenic differentiation of MSCs within a 3D woven PCL scaffold that possesses biomimetic cartilage mechanical properties. We hypothesized that the use of this 3D woven scaffold would allow continuous maintenance of the biomechanical properties of the construct during in vitro culture, even in the presence of the pro-catabolic and anti-anabolic effects of IL-1. We further examined the hypothesis that chondrogenic differentiation of MSCs would serve a protective role in cartilaginous tissue development in response to a pro-inflammatory environment.
Materials and Methods
3D Woven Scaffold Production
A custom-built weaving machine was used to weave 156 μm diameter multifilament PCL yarns (EMS-Griltech, Domat, Switzerland) into a porous, 3D textile structure. The multilayer architecture consisted of 11 layers of axially oriented yarns, stacked in alternating x- and y-directions, and bound together by an interwoven set of vertically oriented yarns (z-direction) that repeatedly passed through the thickness of the fabric. Yarn spacing within each layer was controlled such that the resulting structure contained interconnected pores measuring approximately 330 μm x 260 μm x 100 μm with a total void fraction of approximately 60% and an overall thickness of approximately 1.4 After weaving, the PCL fabric was soaked in a 4M NaOH bath for 16 hours to clean the fibers and increase their surface hydrophilicity [28]. A 6 mm diameter biopsy punch was used to cut disk-shaped samples from the fabric. Scaffolds were sterilized with ethylene oxide and given a minimum of 1 week to outgas prior to use.
Human Mesenchymal Stem Cells
Human MSCs (hMSCs) were derived from bone marrow aspirates obtained from three healthy adults (two male and one female, average age 39 years) at the Hematopoietic Stem Cell Core Facility at Case Western Reserve University. Informed consent was obtained, and an Institutional Review Board-approved aspiration procedure was used. Briefly, the bone marrow sample was washed with Dulbecco’s modified Eagle’s medium (DMEM-LG, Gibco) supplemented with 10% fetal bovine serum (FBS) from a selected lot [29]. The sample was centrifuged at 460g on a pre-formed Percoll density gradient (1.073 g/mL) to isolate the mononucleated cells. These cells were resuspended in serum-supplemented medium and seeded at a density of 1.8 × 105 cells/cm2 in 10 cm diameter plates. Non-adherent cells were removed after four days by changing the medium. For the remainder of the cell expansion phase, the medium was additionally supplemented with 1 ng/mL of recombinant human fibroblast growth factor-basic (rhFGF-2, Peprotech, Rocky Hill, NJ), and was replaced twice per week. The primary culture was trypsinized after approximately two weeks, and then cryopreserved using Gibco freezing medium.
Tissue Engineered Constructs
hMSCs were thawed and plated at 5500 cells/cm2 and cultured in DMEM-LG supplemented with 10% FBS, 1 ng/mL of rhFGF-2 (Roche Diagnostics, Indianapolis, IN), and 1% penicillin–streptomycin–fungizone (Gibco). Medium was completely replaced every 3 days until cells reached 80% confluence, after which they were passaged and replated. For this study, cells were used after the fourth passage (P4). The PCL scaffolds were placed in 24 well ultra-low attachment plates (Corning, NY) and seeded with 3×105 P4 hMSCs at a density of 10×106 cells/ml. The scaffolds were then placed in a humidified incubator at 37 °C and 5% CO2 for 60 min to allow cell attachment to the scaffold. Subsequently, 1 ml of medium was added per well. Three different culture media were used in this study: (i) “basic culture medium” consisting of DMEM-HG supplemented with 1% ITS+ premix (Collaborative Biomedical, Becton-Dickinson, Bedford, MA), 100 nM dexamethasone (Sigma), 100 U/mL penicillin, 100 U/mL streptomycin and 37.5 μg/ml L-ascorbic acid 2-phosphate, (ii) “chondrogenic culture medium” consisting of basic culture medium plus 1 ng/ml of recombinant human transforming growth factor beta (TGF-β3) (R&D Systems, Minneapolis, MN), and (iii) “pro-inflammatory culture medium” consisting of chondrogenic medium supplemented with 0.1 ng/ml of recombinant human interleukin-1α (IL-1) (R&D Systems). This concentration was based on concentrations of IL-1 measured in the synovial fluid of osteoarthritis patients [30]. These three media formulations were used to make four different test groups: 1) control, 2) chondrogenic, 3) pro-inflammatory, and 4) chondrogenic, pro-inflammatory (chondrogenic culture for days 0 to 28, followed by pro-inflammatory culture for days 28 to 56) (Fig. 1). Medium was completely replaced for all groups every 2-3 days and constructs were harvested at 14, 28, 42, and 56-day time points. Cell-loaded scaffolds were also harvested immediately upon seeding (day 0) to assess baseline properties. Each group included 7 samples per time point; 5 were used for mechanical testing first, followed by biochemical analysis, and 2 were used for histology and immunohistochemistry.
Figure 1.
Experimental timeline depicting culture conditions of each test group over time. Mechanical, biochemical, and compositional properties of all groups were assessed at each time point.
Mechanical Testing
Confined compression tests were performed on 3 mm diameter cylindrical test specimens, cored from the centers of the constructs with a biopsy punch, using an ELF 3200 series materials testing system (Bose, Minnetonka, MN). Specimens (n = 5 per group) were placed in a 3 mm diameter confining chamber in a bath filled with phosphate buffered saline (PBS) and a compressive load was applied using a solid piston against a rigid porous platen (porosity of 50%, pore size of 50–100 μm). Following equilibration of a 10 gf tare load, a step compressive load of 30 gf was applied to the sample and allowed to equilibrate for 2000s. Aggregate modulus (HA) and apparent hydraulic permeability (k) were determined numerically by matching the solution for axial strain to the experimental data for all creep tests using a three-parameter, nonlinear least-squares regression procedure [31] assuming intrinsic incompressibility of the tissue [32]. Unconfined compression tests were performed by applying sequential strains of 4, 8, 12, and 16% to the specimens (n = 5 per group) in a PBS bath after equilibration of a 2 gf tare load. Strain steps were held constant for 900s, which allowed for the specimens to reach equilibrium. Young’s modulus (E) was determined by linear regression on the resulting equilibrium stress–strain plot.
Histology and Immunohistochemistry
Disk-shaped specimens were fixed in 4% paraformaldehyde with 100 mM sodium cacodylate buffer (pH 7.4) for 24 h at 4 °C. From this point on, the constructs were further treated in a fully enclosed tissue processor (Leica ASP300S, Leica Microsystems, Bannockburn, IL). Briefly, constructs were dehydrated in graded ethanol steps, cleared in xylene, and embedded in paraffin wax under vacuum. Embedded paraffin blocks were cut into 8 μm thick sections using a Reichert-Jung microtome and mounted on SuperFrost microscope slides (Microm International AG, Volketswil, Switzerland). Samples were stained for sulfated glycosaminoglycans (s-GAGs) and collagenous matrix using a 0.1% aqueous safranin-O solution and 0.02% fast green solution, respectively, while also using hematoxylin as a nucleus counter-stain. Human osteochondral tissue was used as a positive control. For immunohistochemical analysis (IHC), sections were processed with a fully automated staining system (Bond-Max, Leica Microsystems, Bannockburn, IL). Monoclonal antibodies were used to identify type I collagen (ab6308; Abcam, Cambridge, MA), type II collagen (II-II6B3; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), and chondroitin 4-sulfate (2B6; kind gift from Dr. Virginia Kraus, Duke University Medical Center). Human osteochondral samples were used as positive controls, and negative controls were prepared to rule out nonspecific labeling by omitting the primary antibody incubation step.
Biochemical Analyses
Standard assays for DNA, ortho-hydroxyproline (OHP, an index of total collagen content), and s-GAG (an index of proteoglycan content) were performed (n = 3–4 bisected constructs per time point per group). Values obtained for all day 0 constructs (n=6-8) produced from hMSC-seeded PCL scaffolds were pooled, averaged, and used as a basis for comparison for subsequent (14, 28, 42, and 56-day) constructs. After measuring wet weight, constructs were diced and digested in papain for 12 h at 60°C. DNA was measured using the Quant-iTTM PicoGreen® dsDNA assay (Molecular Probes, Eugene, OR). s-GAG was measured using the Dimethyl-methylene blue assay (DMB) using chondroitin 4-sulfate as a standard and reading the optical density on a plate reader at 595 nm. To measure total collagen, papain digests were hydrolyzed in HCl at 110 °C overnight, dried in a desiccating chamber, reconstituted in acetate–citrate buffer, filtered through activated charcoal, and OHP was quantified by Ehrlich’s reaction. Briefly, hydrolysates were oxidized with chloramine-T, treated with dimethylaminobenzaldehyde, read at 540 nm against a trans-4-hydroxy-L-proline standard curve, and total collagen was calculated by using a ratio of 10 mg of collagen per 1 mg of 4-hydroxyproline. The conversion factor of 10 was selected since immunohistochemical staining showed that type II collagen represented virtually all of the collagen present in the constructs [33].
Statistical Analysis
Two-factor analysis of variance (ANOVA) with Tukey’s HSD post-hoc test was performed to compare the results of mechanical and biochemical tests for each construct between time points (α = 0.05).
Results
Mechanical Testing
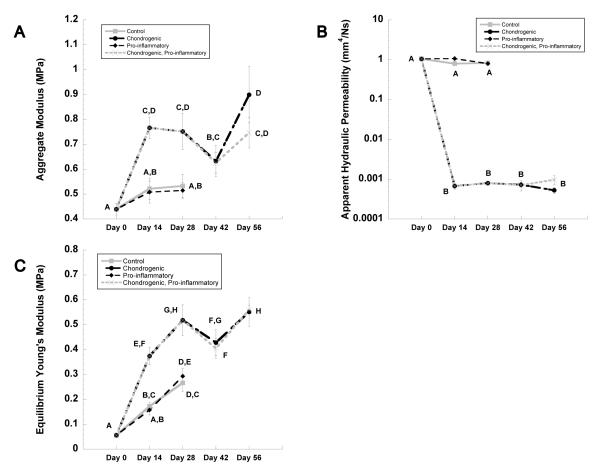
At time zero, constructs exhibited an aggregate modulus of ~0.4 MPa (Fig. 2A). Constructs cultured in chondrogenic medium demonstrated a 31% average increase in aggregate modulus over control values (p < 0.05) at 14 and 28-day time points (Fig. 2A). Chondrogenic culture conditions also resulted in a reduction in apparent hydraulic permeability of 3 orders of magnitude to approximately 0.0008 mm4/N•s (p < 0.05) (Fig. 2B).
Figure 2.
(A-C) Mechanical properties of constructs at day 0, 14, 28, 42 and 56. Groups not sharing the same letter are statistically different from each other (ANOVA, p<0.05). Data represented as mean + SEM.
The presence of IL-1 during the initial 4-week period of chondrogenesis abrogated the effects of TGF-β3, effectively reducing aggregate modulus to that of control constructs with values of approximately 0.5 MPa (Fig. 2A). IL-1 also prevented the TGF-β3-induced decrease in permeability as evidenced of k approaching the value of control at ~ 1 mm4/N•s (Fig. 2B).
Under chondrogenic culture conditions, the aggregate modulus increased significantly during the culture period (Fig. 2C), with a significant 51% average increase at days 14 and 28 to values of approximately 0.75 MPa. However, this increase in modulus was not observed in constructs that were cultured in the presence of the pro-inflammatory medium containing IL-1. For constructs cultured in chondrogenic medium for 28 days prior to IL-1 exposure for a subsequent 14 days (i.e., the chondrogenic, pro-inflammatory condition), there were no differences observed in either aggregate modulus or permeability when compared to constructs that were continually cultured in chondrogenic conditions for up to 56 days (Fig. 2A&C).
Histology and Immunohistochemistry
After 28 days of culture in basic control medium, histological analysis of tissue-engineered constructs revealed a sparsely distributed extracellular matrix (ECM) that stained weakly for collagen (via Fast Green) (Fig. 3). Chondrogenic culture conditions containing TGF-β3 resulted in a dense ECM rich in both s-GAGs (as stained by Safranin-O or chondroitin-4-sulfate) and collagens (as stained by Fast Green) that accumulated and completely filled the interstitial voids within the scaffold.
Figure 3.
Histology and Immunohistochemistry of constructs at day 28 for control, chondrogenic, and pro-inflammatory groups (cross-sectional views). Porcine osteochondral tissue (bottom row) was used as positive control for all staining protocols. Scale bar = 0.5 mm.
Constructs cultured in pro-inflammatory conditions with IL-1 stained positively for the presence of collagens, but showed little or no staining for s-GAGs (Fig. 3). Similar results were observed using immunohistochemical labeling for type II collagen and chondroitin 4-sulfate at day 28 (Fig. 3).
Constructs that had been cultured in chondrogenic conditions 28 days before the addition of IL-1 to the cultures (i.e., chondrogenic, pro-inflammatory group) (Fig. 4B) stained positively for type II collagen with similar intensity to constructs cultured in chondrogenic conditions without IL-1 for the entire 56 days (Fig. 4A). In contrast, constructs cultured in chondrogenic conditions for the duration of the 56-day (Fig. 4C) period revealed markedly darker labeling for chondroitin 4-sulfate than those constructs exposed to IL-1 for days 28 to 56 (Fig. 4D).
Figure 4.
Immunohistochemical staining of type II collagen and chondroitin 4-sulfate at day 56 for: (A,C) continuous chondrogenic culture and (B,D) chondrogenic culture for days 0 to 28, followed by pro-inflammatory culture for days 28 to 56. Scaffold fibers are seen as white spaces. Scale bar = 0.1 mm.
Biochemical Composition
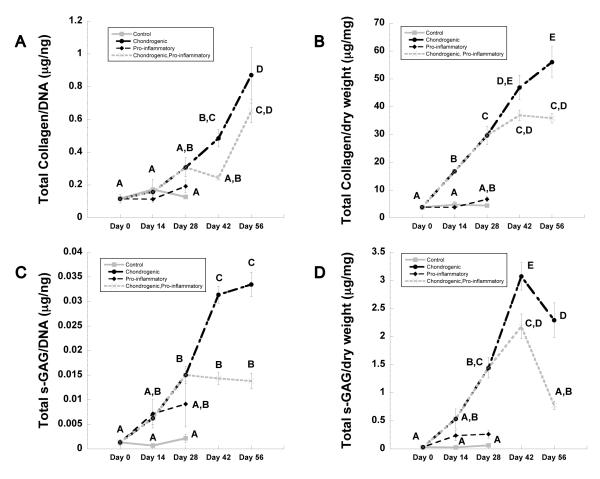
Constructs cultured in chondrogenic conditions showed an increasing trend in collagen content (normalized to DNA) over the first 28 days, but no statistically significant changes were detected between groups up to this time point (Fig. 5A). However, significant increases in total collagen/DNA were observed in both the chondrogenic and chondrogenic, pro-inflammatory conditions from day 42 to 56 (Fig. 5A, p<0.05) to values of approximately 0.8 μg/ng and 0.6 μg/ng respectively. This equates to a 25.3% increase in collagen content in chondrogenic only cultures relative to cultures that were chondrogenically induced but received the IL-1 insult for the final 28 days of culture (i.e., chondrogenic, pro-inflammatory condition). Total s-GAG/DNA was 4-fold higher for constructs cultured in IL-1 conditions at day 28 in comparison to those cultured in control conditions. At the same time point, the s-GAG/DNA content of constructs cultured in chondrogenic conditions was 7-fold higher when compared to control conditions (Fig. 5C, p<0.05) and continued increasing up to day 56 to a final value of 0.0334±0.002 μg/ng. By day 56, constructs that were maintained in chondrogenic conditions for the entire study contained 58.7% more s-GAG/DNA than did chondrogenically-induced constructs that were exposed to IL-1 conditions from day 28 onward.
Figure 5.
Biochemical analysis of constructs over time. (A) Total collagen and (C) total s-GAG normalized to dsDNA, and (B) total collagen and (D) total s-GAG normalized to dry weight. Time points not sharing the same letter are statistically different from each other (ANOVA, p<0.05). Data represented as mean + SEM.
Total collagen content (normalized to the dry weight of the construct) accumulated steadily over time in chondrogenic conditions reaching a maximum of 56.03±5.45 μg/mg at day 56 (Fig. 5B). However, in the presence of IL-1 in the culture at day 28 (chondrogenic, pro-inflammatory group), collagen content peaked at day 42 and showed no significant change at day 56 (Fig. 5B). Total s-GAG/dry weight for constructs cultured in chondrogenic conditions peaked at day 42 (3.07±0.24 μg/mg) and decreased by 25% at day 56. For constructs initially cultured in chondrogenic conditions and changed thereafter to pro-inflammatory conditions at day 28, total s-GAG/dry weight reached a maximum of 2.18±0.22 μg/mg at day 42 and decreased by 64% at day 56 (Fig. 5D, p<0.05).
Discussion
Results of this study show that levels of IL-1 encountered in vivo under pathophysiologic conditions have a significant, deleterious effect on chondrogenesis and the development of mechanical properties in MSC-based cartilage constructs. However, the extent of this effect is highly dependent on stage of chondrogenesis at which the MSCs are exposed to IL-1. Under chondrogenic conditions, the MSC-seeded 3D woven PCL scaffolds show a significant increase in mechanical properties after 4 or 8 weeks in culture. The presence of IL-1 during the initial 28-day culture period strongly inhibited accumulation of ECM components, preventing any increase in compressive mechanical properties over baseline levels. However, constructs cultured for 4 weeks in chondrogenic medium prior to IL-1 exposure maintained their composition and biomechanical properties after IL-1 was added to the medium, although these specimens exhibited lower collagen and proteoglycan content than those that were not exposed to IL-1.
Our findings are consistent with previous studies showing that IL-1 can inhibit chondrogenesis of adult stem cells such as ASCs [9] or MSCs [10, 12-15] in hydrogel or pellet culture. In these cells, IL-1 appears to act through the activation of nuclear factor kappa B (NF-κB), as inhibition of this pathway using a dominant-negative suppressor of I-κB [15], curcumin [13], or resveratrol [34] abrogates the effects of IL-1 on MSCs. Furthermore, relatively high concentrations (100 ng/ml) of bone morphogenetic proteins (BMPs) such as BMP-2 or BMP-9 can partially overcome the effects of IL-1 [10]. Interestingly, other physical and physicochemical factors such as hypoxia [14] or dynamic mechanical loading [16, 35] can also inhibit the deleterious effects of IL-1 on chondrogenesis or tissue repair in bioartificial culture systems.
An important advance of the current study is the measurement of functional mechanical properties of the construct engineered using MSCs, and the effects of IL-1 on the maturation of these properties. We have previously reported the development and characterization of 3D woven textile scaffolds engineered with predefined mechanical properties that mimic the anisotropic, viscoelastic, and nonlinear tension-compression behavior of native articular cartilage [24]. Additionally, we have shown that the use of PCL fibers to weave this structure confers long-term maintenance of initial properties while also supporting cell attachment and tissue synthesis [26, 27, 36]. Biomechanical testing of the MSC-seeded 3D PCL constructs formed in the present study shows an increasing trend in compressive stiffness with culture time, independent of culture conditions (Fig. 2). Consistent with earlier studies, this occurs as accumulating ECM binds the constituent fiber bundles of the scaffold and limits their ability to move and reorient under applied compressive loading, effectively becoming a hybrid composite material, which stiffens the entire structure [36].
Previous studies have also focused on the functional mechanical properties of tissue-engineered cartilaginous constructs using various biomaterials with MSCs as a cell source. For example, MSCs or ASCs cultured in agarose, gelatin, hyaluronic acid, poly(l-lactic acid), Puramatrix, and silk derived scaffolds demonstrated inferior mechanical properties to articular cartilage and also inferior properties relative to primary chondrocytes used in the same hydrogel [3, 37-42]. Conversely, recent studies by Mauck and colleagues have demonstrated greatly improved mechanical properties in MSC-based constructs, effectively acquiring compressive properties that approach that of articular cartilage [43-45]. In these studies, several strategies have been employed to increase the functional properties of the engineered tissue, including transient exposure to growth factors, compressive mechanical stimulation, and the use of in vivo bioreactors [43-45]. Particularly, MSCs seeded in agarose gels and transiently exposed to TGF-β3 resulted in an equilibrium Young’s modulus of ~200 kPa in vitro [43], and implanting MSC-laden hyaluronic acid hydrogels with TGF-β3-loaded microspheres in subcutaneous pockets in nude mice results in a value of ~400 kPa for the equilibrium Young’s modulus after 8 weeks in vivo [45]. Both of these studies represent marked improvements in cartilage tissue engineering using stem cells as a cell source as the target for compressive moduli lies in the range of 400 – 800 kPa [46, 47]. These data are also consistent with the values obtained for equilibrium Young’s modulus in our current study, in which a rapid accumulation of mechanical properties is noted as tissue consolidates the matrix, thereby significantly increasing the Young’s modulus to ~400 kPa after only 2 weeks of culture.
Our data further show that ECM assembly on the 3D PCL scaffold was enhanced by a relatively low, continuous dose of 1 ng/ml TGF-β3, and that this dose significantly improved the mechanical properties of the tissue-engineered constructs over an 8-week culture period. Under these conditions, MSCs produce a robust ECM high in proteoglycan and type II collagen content that penetrate and fills the entire scaffold space. However, the presence of a physiologically relevant dose of IL-1 inhibited the anabolic effect of TGF-β3 by inhibiting the accumulation of collagen and s-GAG, consequently reducing the compressive mechanical properties. Nonetheless, the 3D woven scaffold still provides functional properties similar to those of native cartilage under these catabolic conditions.
Surprisingly, constructs cultured for 4 weeks in chondrogenic conditions prior to the administration of the catabolic agent IL-1 were protected from the IL-1-mediated decrease in mechanical properties. In these chondrogenically “pre-cultured” constructs, s-GAG/DNA content is lower in comparison to scaffolds cultured continuously in chondrogenic medium after week 4. The mechanisms responsible for the apparently lower sensitivity of chondrogenically differentiated MSCs to IL-1 (as compared to that of undifferentiated MSCs) is unknown and may involve several factors. For example, the accumulation of new ECM within the scaffold is likely to significantly reduce the diffusion coefficient and partition coefficient of solutes within the construct [48], thus effectively reducing the concentration of IL-1 to which MSCs are exposed. Alternatively, the sensitivity of MSCs to IL-1 may change during the differentiation process, either through altered expression of cell surface receptors or antagonists of IL-1 [11, 49-52]. For example, previous studies have shown that IL-1 itself can induce IL-Ra production in various cell types [53, 54]. Importantly, growing evidence indicates that MSCs may in fact play an anti-inflammatory role when reintroduced in vivo [50, 55] and enhance repair by producing IL-1 receptor antagonist (IL-1Ra) [51].
Conclusions
In summary, our findings show that IL-1 has significant deleterious effects on chondrogenesis, matrix accumulation, and the maturation of mechanical properties in MSC-seeded 3D woven constructs. However, the impact of the inhibitory effects on the development of functional properties is significantly reduced by the presence of a biomimetic scaffold that exhibits mechanical properties similar to those of native cartilage. Further studies are necessary to determine the mechanisms by which IL-1 acts on MSCs at different stages of differentiation.
Acknowledgments
This study was supported in part by the Department of Veterans Affairs Rehabilitation Research Service, the Arthritis Foundation, the AO Foundation, the Alpha Omicron Pi Foundation, the Fulbright Netherlands America Foundation, and NIH grants AG15768, AR50245, AR48182, AR48852, and AR53622.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Knutsen G, Drogset JO, Engebretsen L, Grontvedt T, Isaksen V, Ludvigsen TC, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007 Oct;89(10):2105–2112. doi: 10.2106/JBJS.G.00003. [DOI] [PubMed] [Google Scholar]
- 2.Freed LE, Langer R, Martin I, Pellis NR, Vunjak-Novakovic G. Tissue engineering of cartilage in space. Proc Natl Acad Sci U S A. 1997 Dec 9;94(25):13885–13890. doi: 10.1073/pnas.94.25.13885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mauck RL, Yuan X, Tuan RS. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage. 2006 Feb;14(2):179–189. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Brun P, Dickinson SC, Zavan B, Cortivo R, Hollander AP, Abatangelo G. Characteristics of repair tissue in second-look and third-look biopsies from patients treated with engineered cartilage: relationship to symptomatology and time after implantation. Arthritis Res Ther. 2008;10(6):R132. doi: 10.1186/ar2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011 Jan;7(1):33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 6.Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011 Sep;23(5):471–478. doi: 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fermor B, Weinberg JB, Pisetsky DS, Misukonis MA, Fink C, Guilak F. Induction of cyclooxygenase-2 by mechanical stress through a nitric oxide-regulated pathway. Osteoarthritis Cartilage. 2002 Oct;10(10):792–798. doi: 10.1053/joca.2002.0832. [DOI] [PubMed] [Google Scholar]
- 8.Sandell LJ, Xing X, Franz C, Davies S, Chang LW, Patra D. Exuberant expression of chemokine genes by adult human articular chondrocytes in response to IL-1beta. Osteoarthritis Cartilage. 2008 Dec;16(12):1560–1571. doi: 10.1016/j.joca.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Estes BT, Fermor B, Guilak F. The influence of interleukin-1 and mechanical stimulation on huan adipose derived adult stem cells undergoing chondrogenesis. Trans Orthop Res Soc. 2004;29:765. [Google Scholar]
- 10.Majumdar MK, Wang E, Morris EA. BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. J Cell Physiol. 2001 Dec;189(3):275–284. doi: 10.1002/jcp.10025. [DOI] [PubMed] [Google Scholar]
- 11.van Buul GM, Villafuertes E, Bos PK, Waarsing JH, Kops N, Narcisi R, et al. Mesenchymal stem cells secrete factors that inhibit inflammatory processes in short-term osteoarthritic synovium and cartilage explant culture. Osteoarthritis Cartilage. 2012 Jul 5; doi: 10.1016/j.joca.2012.06.003. doi:10.1016/j.joca.2012.06.003:[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Heldens GT, Blaney Davidson EN, Vitters EL, Schreurs BW, Piek E, van den Berg WB, et al. Catabolic factors and osteoarthritis-conditioned medium inhibit chondrogenesis of human mesenchymal stem cells. Tissue Eng Part A. 2012 Jan;18(1-2):45–54. doi: 10.1089/ten.TEA.2011.0083. [DOI] [PubMed] [Google Scholar]
- 13.Buhrmann C, Mobasheri A, Matis U, Shakibaei M. Curcumin mediated suppression of nuclear factor-kappaB promotes chondrogenic differentiation of mesenchymal stem cells in a high-density co-culture microenvironment. Arthritis Res Ther. 2010;12(4):R127. doi: 10.1186/ar3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felka T, Schafer R, Schewe B, Benz K, Aicher WK. Hypoxia reduces the inhibitory effect of IL-1beta on chondrogenic differentiation of FCS-free expanded MSC. Osteoarthritis Cartilage. 2009 Oct;17(10):1368–1376. doi: 10.1016/j.joca.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 15.Wehling N, Palmer GD, Pilapil C, Liu F, Wells JW, Muller PE, et al. Interleukin-1beta and tumor necrosis factor alpha inhibit chondrogenesis by human mesenchymal stem cells through NF-kappaB-dependent pathways. Arthritis Rheum. 2009 Mar;60(3):801–812. doi: 10.1002/art.24352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chowdhury TT, Arghandawi S, Brand J, Akanji OO, Bader DL, Salter DM, et al. Dynamic compression counteracts IL-1beta induced inducible nitric oxide synthase and cyclo-oxygenase-2 expression in chondrocyte/agarose constructs. Arthritis Res Ther. 2008;10(2):R35. doi: 10.1186/ar2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lima EG, Tan AR, Tai T, Marra KG, DeFail A, Ateshian GA, et al. Genipin enhances the mechanical properties of tissue-engineered cartilage and protects against inflammatory degradation when used as a medium supplement. J Biomed Mater Res A. 2009 Dec;91(3):692–700. doi: 10.1002/jbm.a.32305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortial D, Gouttenoire J, Rousseau CF, Ronziere MC, Piccardi N, Msika P, et al. Activation by IL-1 of bovine articular chondrocytes in culture within a 3D collagen-based scaffold. An in vitro model to address the effect of compounds with therapeutic potential in osteoarthritis. Osteoarthritis Cartilage. 2006 Jul;14(7):631–640. doi: 10.1016/j.joca.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Scotti C, Osmokrovic A, Wolf F, Miot S, Peretti GM, Barbero A, et al. Response of human engineered cartilage based on articular or nasal chondrocytes to interleukin-1beta and low oxygen. Tissue Eng Part A. 2012 Feb;18(3-4):362–372. doi: 10.1089/ten.tea.2011.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNulty AL, Moutos FT, Weinberg JB, Guilak F. Enhanced integrative repair of the porcine meniscus in vitro by inhibition of interleukin-1 or tumor necrosis factor alpha. Arthritis Rheum. 2007 Sep;56(9):3033–3042. doi: 10.1002/art.22839. [DOI] [PubMed] [Google Scholar]
- 21.McNulty AL, Weinberg JB, Guilak F. Inhibition of matrix metalloproteinases enhances in vitro repair of the meniscus. Clin Orthop Relat Res. 2009 Jun;467(6):1557–1567. doi: 10.1007/s11999-008-0596-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riera KM, Rothfusz NE, Wilusz RE, Weinberg JB, Guilak F, McNulty AL. Interleukin-1, tumor necrosis factor-alpha, and transforming growth factor-beta 1 and integrative meniscal repair: influences on meniscal cell proliferation and migration. Arthritis Res Ther. 2011;13(6):R187. doi: 10.1186/ar3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freed LE, Engelmayr GC, Jr., Borenstein JT, Moutos FT, Guilak F. Advanced material strategies for tissue engineering scaffolds. Advanced materials. 2009 Sep 4;21(32-33):3410–3418. doi: 10.1002/adma.200900303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moutos FT, Freed LE, Guilak F. A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nature materials. 2007 Feb;6(2):162–167. doi: 10.1038/nmat1822. [DOI] [PubMed] [Google Scholar]
- 25.Abrahamsson CK, Yang F, Park H, Brunger JM, Valonen PK, Langer R, et al. Chondrogenesis and mineralization during in vitro culture of human mesenchymal stem cells on three-dimensional woven scaffolds. Tissue Eng Part A. 2010 Dec;16(12):3709–3718. doi: 10.1089/ten.tea.2010.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moutos FT, Guilak F. Functional properties of cell-seeded three-dimensionally woven poly(epsilon-caprolactone) scaffolds for cartilage tissue engineering. Tissue engineering Part A. 2010 Apr;16(4):1291–1301. doi: 10.1089/ten.tea.2009.0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valonen PK, Moutos FT, Kusanagi A, Moretti MG, Diekman BO, Welter JF, et al. In vitro generation of mechanically functional cartilage grafts based on adult human stem cells and 3D-woven poly(epsilon-caprolactone) scaffolds. Biomaterials. 2010 Mar;31(8):2193–2200. doi: 10.1016/j.biomaterials.2009.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuji H, Ishida T, Fukuda N. Surface hydrophilicity and enzymatic hydrolyzability of biodegradable polyesters: 1. Effects of alkaline treatment. Polymer International. 2003 May;52(5):843–852. [Google Scholar]
- 29.Lennon DPHS, Bruder SP, Jaiswall N, Caplan AI. Human and animal mesenchymal progenitor cells from bone marrow: identification of serum for optimal selection and proliferation. In Vitro Cell Dev Biol. 1996;32:602–611. [Google Scholar]
- 30.Hopkins SJ, Humphreys M, Jayson MI. Cytokines in synovial fluid. I. The presence of biologically active and immunoreactive IL-1. Clin Exp Immunol. 1988 Jun;72(3):422–427. [PMC free article] [PubMed] [Google Scholar]
- 31.Mow VC, Kuei SC, Lai WM, Armstrong CG. Biphasic creep and stress relaxation of articular cartilage in compression? Theory and experiments. J Biomech Eng. 1980 Feb;102(1):73–84. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- 32.Bachrach NM, Mow VC, Guilak F. Incompressibility of the solid matrix of articular cartilage under high hydrostatic pressures. J Biomech. 1998 May;31(5):445–451. doi: 10.1016/s0021-9290(98)00035-9. [DOI] [PubMed] [Google Scholar]
- 33.Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961 May;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 34.Lei M, Liu SQ, Liu YL. Resveratrol protects bone marrow mesenchymal stem cell derived chondrocytes cultured on chitosan-gelatin scaffolds from the inhibitory effect of interleukin-1beta. Acta Pharmacol Sin. 2008 Nov;29(11):1350–1356. doi: 10.1111/j.1745-7254.2008.00880.x. [DOI] [PubMed] [Google Scholar]
- 35.McNulty AL, Estes BT, Wilusz RE, Weinberg JB, Guilak F. Dynamic loading enhances integrative meniscal repair in the presence of interleukin-1. Osteoarthritis Cartilage. 2010 Jun;18(6):830–838. doi: 10.1016/j.joca.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moutos FT, Estes BT, Guilak F. Multifunctional hybrid three-dimensionally woven scaffolds for cartilage tissue engineering. Macromolecular bioscience. 2010 Nov 10;10(11):1355–1364. doi: 10.1002/mabi.201000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Awad HA, Wickham MQ, Leddy HA, Gimble JM, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004 Jul;25(16):3211–3222. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 38.Erickson IE, Huang AH, Chung C, Li RT, Burdick JA, Mauck RL. Differential maturation and structure-function relationships in mesenchymal stem cell- and chondrocyte-seeded hydrogels. Tissue engineering Part A. 2009 May;15(5):1041–1052. doi: 10.1089/ten.tea.2008.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung C, Beecham M, Mauck RL, Burdick JA. The influence of degradation characteristics of hyaluronic acid hydrogels on in vitro neocartilage formation by mesenchymal stem cells. Biomaterials. 2009 Sep;30(26):4287–4296. doi: 10.1016/j.biomaterials.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janjanin S, Li WJ, Morgan MT, Shanti RM, Tuan RS. Mold-shaped, nanofiber scaffold-based cartilage engineering using human mesenchymal stem cells and bioreactor. J Surg Res. 2008 Sep;149(1):47–56. doi: 10.1016/j.jss.2007.12.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hofmann S, Knecht S, Langer R, Kaplan DL, Vunjak-Novakovic G, Merkle HP, et al. Cartilage-like tissue engineering using silk scaffolds and mesenchymal stem cells. Tissue Eng. 2006 Oct;12(10):2729–2738. doi: 10.1089/ten.2006.12.2729. [DOI] [PubMed] [Google Scholar]
- 42.Bian L, Zhai DY, Zhang EC, Mauck RL, Burdick JA. Dynamic compressive loading enhances cartilage matrix synthesis and distribution and suppresses hypertrophy in hMSC-laden hyaluronic acid hydrogels. Tissue engineering Part A. 2012 Apr;18(7-8):715–724. doi: 10.1089/ten.tea.2011.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang AH, Stein A, Tuan RS, Mauck RL. Transient exposure to transforming growth factor beta 3 improves the mechanical properties of mesenchymal stem cell-laden cartilage constructs in a density-dependent manner. Tissue engineering Part A. 2009 Nov;15(11):3461–3472. doi: 10.1089/ten.tea.2009.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang AH, Farrell MJ, Kim M, Mauck RL. Long-term dynamic loading improves the mechanical properties of chondrogenic mesenchymal stem cell-laden hydrogel. European cells & materials. 2010;19:72–85. doi: 10.22203/ecm.v019a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bian L, Zhai DY, Tous E, Rai R, Mauck RL, Burdick JA. Enhanced MSC chondrogenesis following delivery of TGF-beta3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials. 2011 Sep;32(27):6425–6434. doi: 10.1016/j.biomaterials.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Athanasiou KA, Agarwal A, Dzida FJ. Comparative study of the intrinsic mechanical properties of the human acetabular and femoral head cartilage. J Orthop Res. 1994 May;12(3):340–349. doi: 10.1002/jor.1100120306. [DOI] [PubMed] [Google Scholar]
- 47.Jurvelin JS, Buschmann MD, Hunziker EB. Optical and mechanical determination of Poisson’s ratio of adult bovine humeral articular cartilage. J Biomech. 1997 Mar;30(3):235–241. doi: 10.1016/s0021-9290(96)00133-9. [DOI] [PubMed] [Google Scholar]
- 48.Leddy HA, Awad HA, Guilak F. Molecular diffusion in tissue-engineered cartilage constructs: effects of scaffold material, time, and culture conditions. J Biomed Mater Res B Appl Biomater. 2004 Aug 15;70(2):397–406. doi: 10.1002/jbm.b.30053. [DOI] [PubMed] [Google Scholar]
- 49.Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell Physiol. 1996 Mar;166(3):585–592. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 50.Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012 Jan;20(1):14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A. 2007 Jun 26;104(26):11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Charoenpanich A, Wall ME, Tucker CJ, Andrews DM, Lalush DS, Loboa EG. Microarray analysis of human adipose-derived stem cells in three-dimensional collagen culture: osteogenesis inhibits bone morphogenic protein and Wnt signaling pathways, and cyclic tensile strain causes upregulation of proinflammatory cytokine regulators and angiogenic factors. Tissue Eng Part A. 2011 Nov;17(21-22):2615–2627. doi: 10.1089/ten.tea.2011.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Venteclef N, Delerive P. Interleukin-1 receptor antagonist induction as an additional mechanism for liver receptor homolog-1 to negatively regulate the hepatic acute phase response. J Biol Chem. 2007 Feb 16;282(7):4393–4399. doi: 10.1074/jbc.M608993200. [DOI] [PubMed] [Google Scholar]
- 54.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011 Apr 7;117(14):3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006 Aug 1;98(5):1076–1084. [Google Scholar]