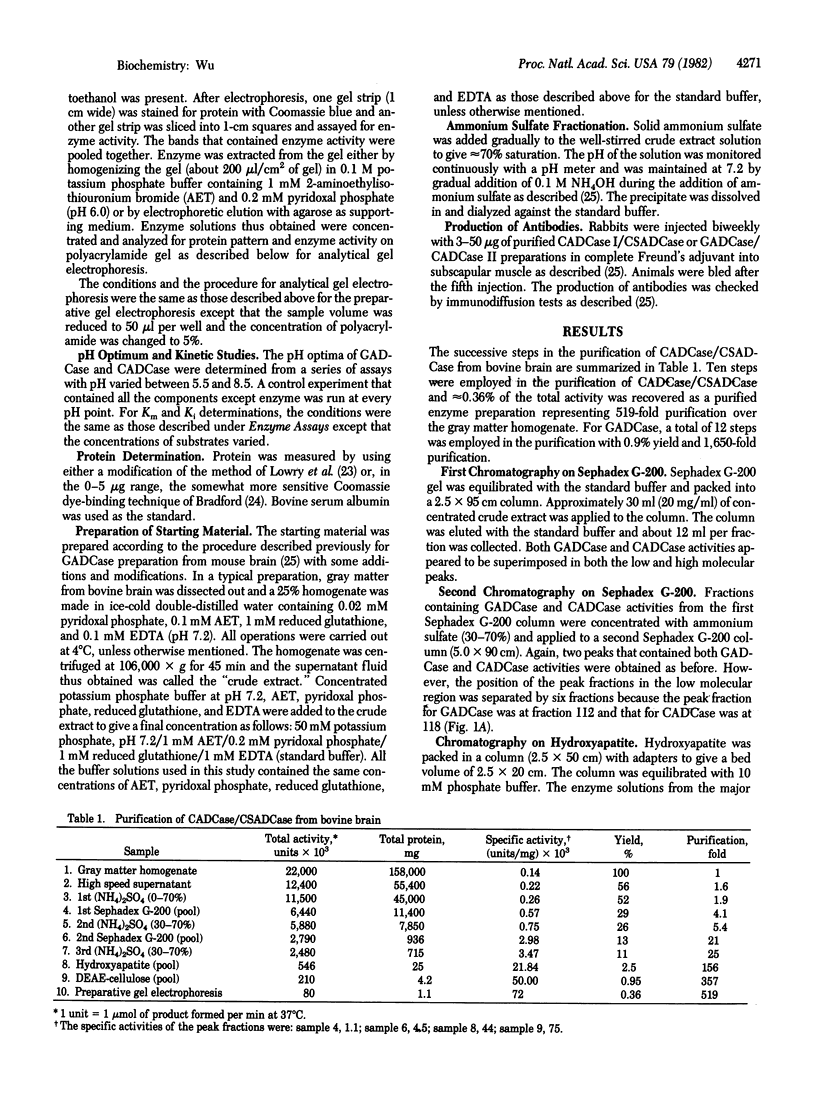
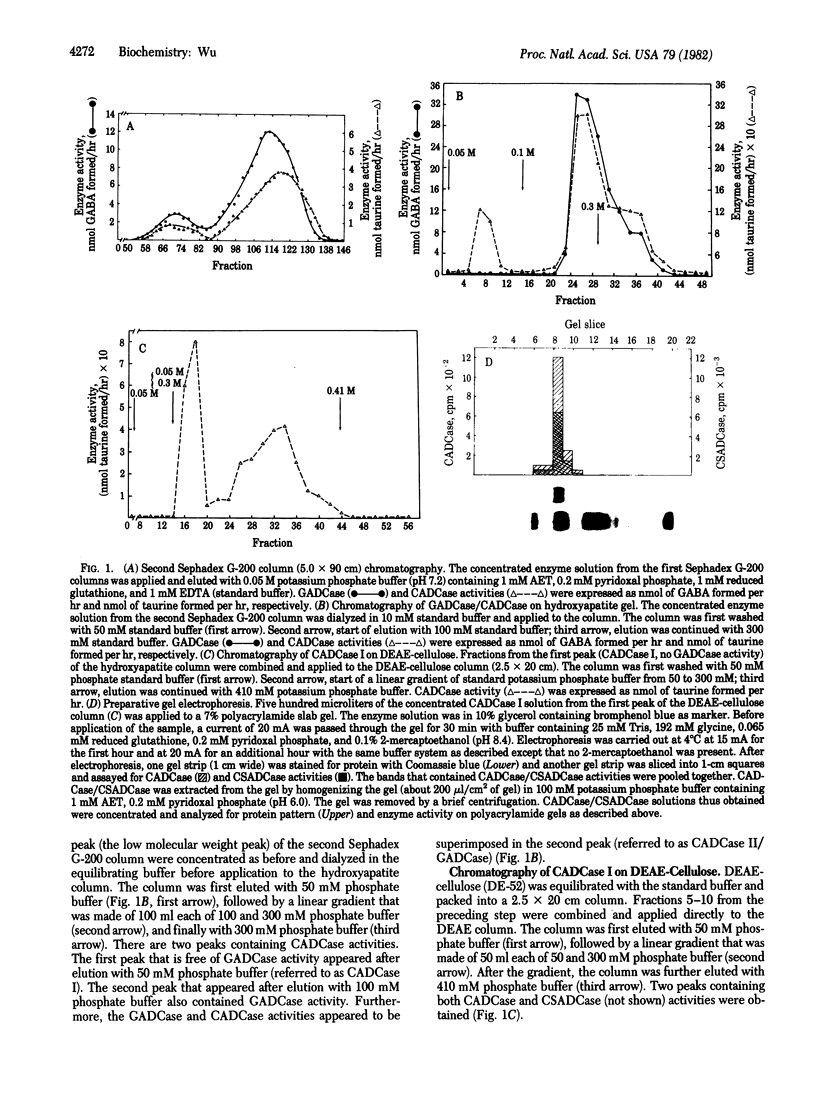
Abstract
L-Cysteic and cysteine sulfinic acids decarboxylase (CADCase/CSADCase) and L-glutamic acid decarboxylase (GADCase), the synthetic enzymes for taurine and gamma-aminobutyric acid, respectively, have been purified to homogeneity from bovine brain. Although CADCase/CSADCase and GADCase copurified through various column procedures, these two enzymes can be clearly separated by a hydroxyapatite column. The purification procedures involve ammonium sulfate fractionation, column chromatographies on Sephadex G-200, hydroxyapatite, DEAE-cellulose, and preparative polyacrylamide gel electrophoresis. The Km values for CADCase/CSADCase are 0.22 and 0.18 mM with L-cysteic and cysteine sulfinic acids as substrates, respectively. CADCase/CSADCase cannot use L-glutamate as substrate. GADCase can use L-glutamate, L-cysteic, and cysteine sulfinic acid as substrates with Km values of 1.6, 5.4, and 5.2 mM, respectively. Antibodies against CADCase/CSADCase do not crossreact with GADCase preparations and vice versa. It is concluded that CADCase/CSADCase and GADCase are two distinct enzyme entities and they are responsible for the biosynthesis of taurine and gamma-aminobutyric acid, respectively.
Full text
PDF
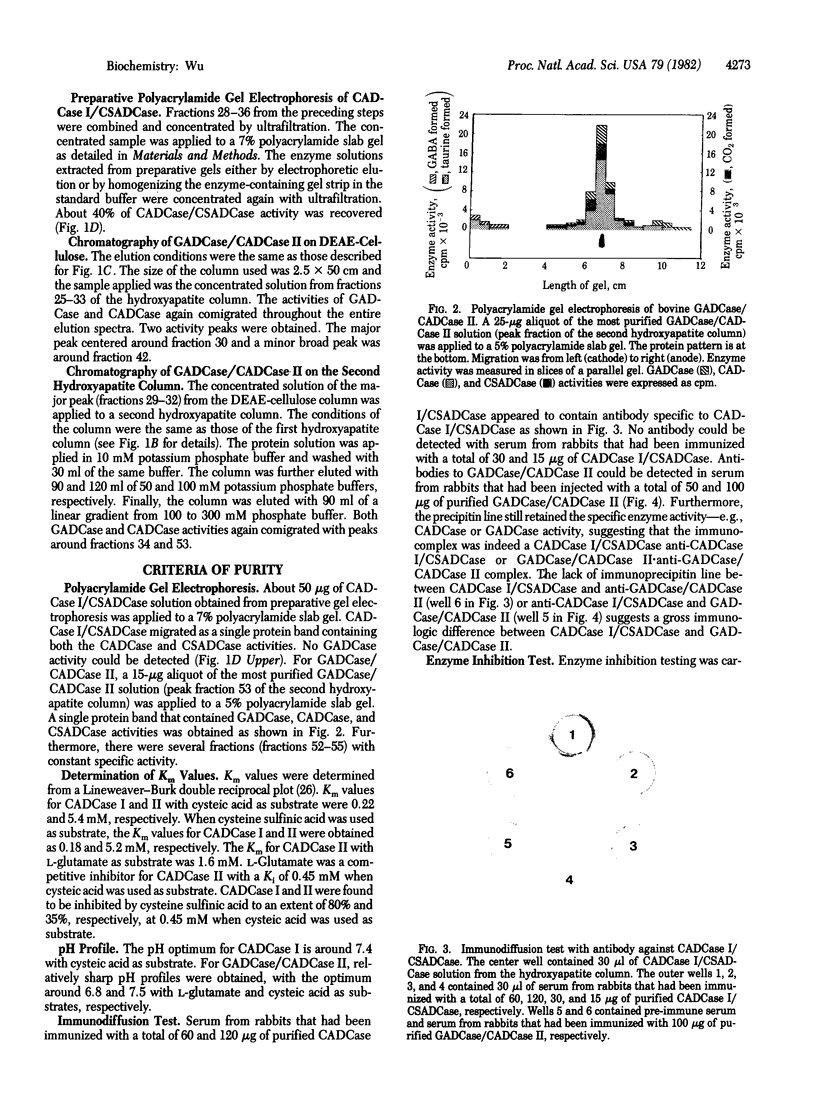

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos W. B. An apparatus for microelectrophoresis in polyacrylamide slab-gels. Anal Biochem. 1976 Feb;70(2):612–615. doi: 10.1016/0003-2697(76)90487-5. [DOI] [PubMed] [Google Scholar]
- Bachelard H. S. Biochemistry of central active amino acids. Adv Biochem Psychopharmacol. 1981;29:475–497. [PubMed] [Google Scholar]
- Blindermann J. M., Maitre M., Ossola L., Mandel P. Purification and some properties of L-glutamate decarboxylase from human brain. Eur J Biochem. 1978 May;86(1):143–152. doi: 10.1111/j.1432-1033.1978.tb12293.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V., Lin C. T., Palay S., Yamamoto M., Wu J. Y. Taurine in the mammalian cerebellum: demonstration by autoradiography with [3H]taurine and immunocytochemistry with antibodies against the taurine-synthesizing enzyme, cysteine-sulfinic acid decarboxylase. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2695–2699. doi: 10.1073/pnas.79.8.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guion-Rain M-C, Portemer C., Chatagner F. Rat liver cysteine sulfinate decarboxylase: purification, new appraisal of the molecular weight and determination of catalytic properties. Biochim Biophys Acta. 1975 Mar 28;384(1):265–276. doi: 10.1016/0005-2744(75)90115-1. [DOI] [PubMed] [Google Scholar]
- HOPE D. B. Pyridoxal phosphate as the coenzyme of the mammalian decarboxylase for L-cysteine sulphinic and L-cysteic acids. Biochem J. 1955 Mar;59(3):497–500. doi: 10.1042/bj0590497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable R. J. Does taurine have a function? Introduction. Fed Proc. 1980 Jul;39(9):2678–2679. [PubMed] [Google Scholar]
- Jacobsen J. G., Smith L. H. Biochemistry and physiology of taurine and taurine derivatives. Physiol Rev. 1968 Apr;48(2):424–511. doi: 10.1152/physrev.1968.48.2.424. [DOI] [PubMed] [Google Scholar]
- Kuriyama K. Taurine as a neuromodulator. Fed Proc. 1980 Jul;39(9):2680–2684. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin Y. C., DeMeio R. H., Metrione R. M. Purification and properties of rat liver cysteine sulfinate decarboxylase. Biochim Biophys Acta. 1971 Dec 15;250(3):558–567. doi: 10.1016/0005-2744(71)90256-7. [DOI] [PubMed] [Google Scholar]
- SORBO B., HEYMAN T. On the purification of cysteinesulfinic acid decarboxylase and its substrate specificity. Biochim Biophys Acta. 1957 Mar;23(3):624–627. doi: 10.1016/0006-3002(57)90385-2. [DOI] [PubMed] [Google Scholar]
- Saito K., Wu J. Y., Matsuda T., Roberts E. Immunochemical comparisons of vertebrate glutamic acid decarboxylase. Brain Res. 1974 Jan 11;65(2):277–285. doi: 10.1016/0006-8993(74)90039-0. [DOI] [PubMed] [Google Scholar]
- Su Y. Y., Wu J. Y., Lam D. M. Purification of L-glutamic acid decarboxylase from catfish brain. J Neurochem. 1979 Jul;33(1):169–179. doi: 10.1111/j.1471-4159.1979.tb11719.x. [DOI] [PubMed] [Google Scholar]
- Wu J. Y., Moss L. G., Chen M. S. Tissue and regional distribution of cysteic acid decarboxylase. A new assay method. Neurochem Res. 1979 Apr;4(2):201–212. doi: 10.1007/BF00964144. [DOI] [PubMed] [Google Scholar]