Abstract
A method was developed to identify specific protein-lipid interactions of complex lipid mixtures and to assess their effect upon the arrangement of such complexes in monolayers at an air-water interface. Its application to striated muscle alpha-actinin revealed that just two lipids selectively interact with alpha-actinin. One molecule of glyceride and one molecule of fatty acid were found to be associated in a constant stoichiometry with one molecule of the alpha-actinin dimer. In the presence of both glycerides and fatty acids unexpectedly rigid monolayer areas formed. This lipid specificity could be confirmed by brief protease digestion of alpha-actinin liposome mixtures followed by peptide analysis; the peptide patterns of alpha-actinin depended on the presence or absence of only these two lipids. Possible implications of these findings are discussed in the context of Z-line formation.
Full text
PDF
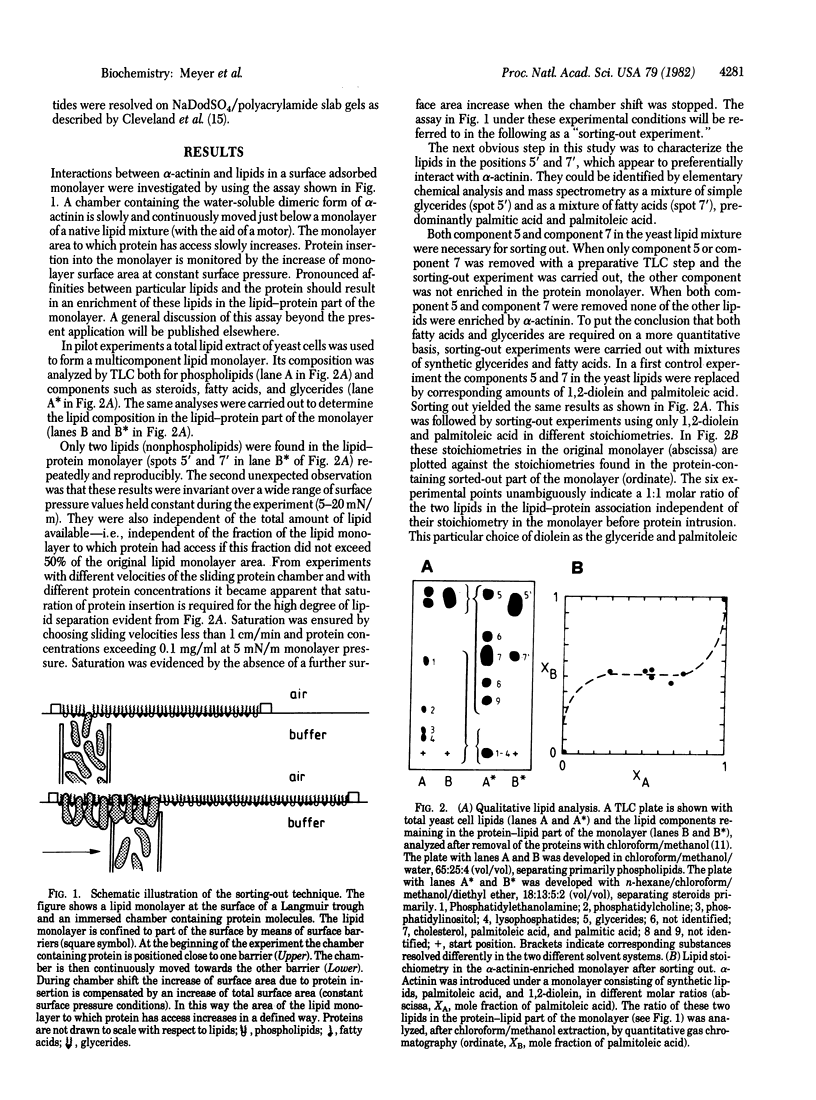


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bretscher A., Vandekerckhove J., Weber K. alpha-Actinins from chicken skeletal muscle and smooth muscle show considerable chemical and immunological differences. Eur J Biochem. 1979 Oct;100(1):237–243. doi: 10.1111/j.1432-1033.1979.tb02054.x. [DOI] [PubMed] [Google Scholar]
- Burridge K., McCullough L. The association of alpha-actinin with the plasma membrane. J Supramol Struct. 1980;13(1):53–65. doi: 10.1002/jss.400130106. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Geiger B., Singer S. J. The participation of alpha-actinin in the capping of cell membrane components. Cell. 1979 Jan;16(1):213–222. doi: 10.1016/0092-8674(79)90202-2. [DOI] [PubMed] [Google Scholar]
- Geiger B., Tokuyasu K. T., Dutton A. H., Singer S. J. Vinculin, an intracellular protein localized at specialized sites where microfilament bundles terminate at cell membranes. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4127–4131. doi: 10.1073/pnas.77.7.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockusch B. M., Burger M. M., DaPrada M., Richards J. G., Chaponnier C., Gabbiani G. alpha-Actinin attached to membranes of secretory vesicles. Nature. 1977 Dec 15;270(5638):628–629. doi: 10.1038/270628a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lane B. P., Elias J., Drummond E. Immunoelectronmicroscopic localization of alpha-actinin in skeletal muscle cells. J Histochem Cytochem. 1977 Jan;25(1):69–72. doi: 10.1177/25.1.833423. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Granger B. L. Fluorescent localization of membrane sites in glycerinated chicken skeletal muscle fibers and the relationship of these sites to the protein composition of the Z disc. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3683–3687. doi: 10.1073/pnas.75.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loor F. Cell surface design. Nature. 1976 Nov 18;264(5583):272–273. doi: 10.1038/264272a0. [DOI] [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S. Covalent binding of fatty acid to the transferrin receptor in cultured human cells. J Biol Chem. 1981 May 25;256(10):4715–4718. [PubMed] [Google Scholar]
- Osborn M., Weber K. Microfilament-associated proteins in tissue culture cells viewed by stereo immunofluorescence microscopy. Eur J Cell Biol. 1979 Oct;20(1):28–36. [PubMed] [Google Scholar]
- Papahadjopoulos D., Poste G., Schaeffer B. E. Fusion of mammalian cells by unilamellar lipid vesicles: inflluence of lipid surface charge, fluidity and cholesterol. Biochim Biophys Acta. 1973 Sep 27;323(1):23–42. doi: 10.1016/0005-2736(73)90429-x. [DOI] [PubMed] [Google Scholar]
- Podlubnaya Z. A., Tskhovrebova L. A., Zaalishtsbvili M. M., Stefanenko G. A. Electron microscopic study of alpha-actinin. J Mol Biol. 1975 Feb 25;92(2):357–359. doi: 10.1016/0022-2836(75)90234-x. [DOI] [PubMed] [Google Scholar]
- Robson R. M., Goll D. E., Arakawa N., Stromer M. H. Purification and properties of alpha-actinin from rabbit skeletal muscle. Biochim Biophys Acta. 1970 Feb 17;200(2):296–318. doi: 10.1016/0005-2795(70)90173-x. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J., Magee A. I., Schmidt M. F. Fatty acid acylation of proteins in cultured cells. J Biol Chem. 1980 Nov 10;255(21):10021–10024. [PubMed] [Google Scholar]
- Schmidt M. F., Schlesinger M. J. Fatty acid binding to vesicular stomatitis virus glycoprotein: a new type of post-translational modification of the viral glycoprotein. Cell. 1979 Aug;17(4):813–819. doi: 10.1016/0092-8674(79)90321-0. [DOI] [PubMed] [Google Scholar]
- Stromer M. H., Goll D. E. Studies on purified -actinin. II. Electron microscopic studies on the competitive binding of -actinin and tropomyosin to Z-line extracted myofibrils. J Mol Biol. 1972 Jun 28;67(3):489–494. doi: 10.1016/0022-2836(72)90465-2. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Goll D. E., Singh I., Allen R. E., Robson R. M., Stromer M. H. Some properties of purified skeletal muscle alpha-actinin. J Biol Chem. 1976 Nov 10;251(21):6860–6870. [PubMed] [Google Scholar]
- Wehland J., Osborn M., Weber K. Cell-to-substratum contacts in living cells: a direct correlation between interference-reflexion and indirect-immunofluorescence microscopy using antibodies against actin and alpha-actinin. J Cell Sci. 1979 Jun;37:257–273. doi: 10.1242/jcs.37.1.257. [DOI] [PubMed] [Google Scholar]



