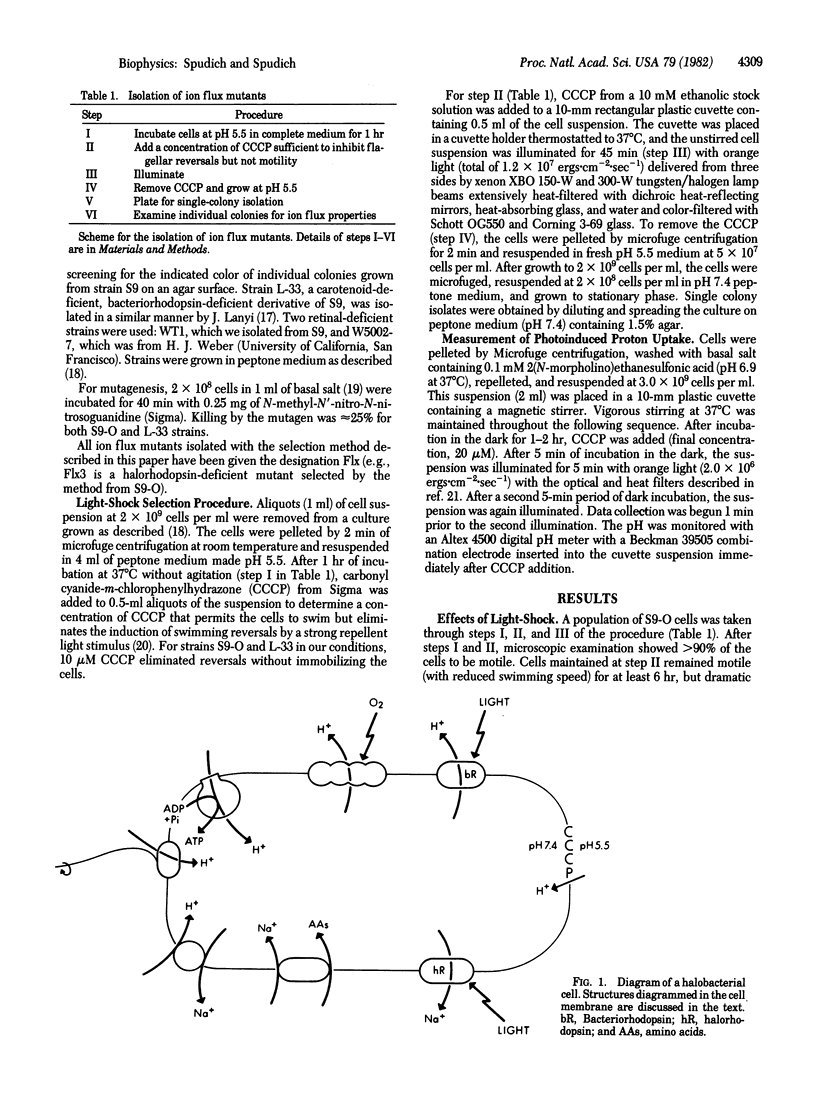
Abstract
We describe a selection method for mutants altered in the generation and regulation of transmembrane ion flux in Halobacterium halobium. The method is based on experimental control of ion fluxes by a combination of light, ionophore, and external pH to generate an imbalance in the cells' proton circulation through their membranes. The steady-state proton circulation is increased by the introduction of a small inward proton leak with a protonophore. The cells are then illuminated to excite halorhodopsin, which hyperpolarizes the membrane and drives protons into the cells. As a result, wild-type cells suffer cytoplasmic acidification, which causes a dramatic loss of motility and suppresses their growth. These properties can be used to select for mutants that escape cytoplasmic acidification because either they lack halorhodopsin function or they have a greater capacity to eject protons during the illumination. In a popular selected by this method, 97% of the individual cells were demonstrably altered in ion flux properties. Cells were selected with alterations in the halobacterial rhodopsin, specifically with deficiencies in membrane potential generation by halorhodopsin and with increased cellular proton ejection by bacteriorhodopsin. We describe properties of one of the halorhodopsin-deficient strains, Flx37.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bogomolni R. A., Baker R. A., Lozier R. H., Stoeckenius W. Action spectrum and quantum efficiency for proton pumping in Halobacterium halobium. Biochemistry. 1980 May 13;19(10):2152–2159. doi: 10.1021/bi00551a024. [DOI] [PubMed] [Google Scholar]
- Danon A., Stoeckenius W. Photophosphorylation in Halobacterium halobium. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1234–1238. doi: 10.1073/pnas.71.4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dencher N. A., Hildebrand E. Sensory transduction in Halobacterium halobium: retinal protein pigment controls UV-induced behavioral response. Z Naturforsch C. 1979 Sep-Oct;34(9-10):841–847. doi: 10.1515/znc-1979-9-1030. [DOI] [PubMed] [Google Scholar]
- Khan S., Macnab R. M. The steady-state counterclockwise/clockwise ratio of bacterial flagellar motors is regulated by protonmotive force. J Mol Biol. 1980 Apr 15;138(3):563–597. doi: 10.1016/s0022-2836(80)80018-0. [DOI] [PubMed] [Google Scholar]
- Kushwaha S. C., Kates M., Weber H. J. Exclusive formation of all-trans-phytoene by a colorless mutant of Halobacterium halobium. Can J Microbiol. 1980 Aug;26(8):1011–1014. doi: 10.1139/m80-171. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. Light energy conversion in Halobacterium halobium. Microbiol Rev. 1978 Dec;42(4):682–706. doi: 10.1128/mr.42.4.682-706.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanyi J. K., MacDonald R. E. Existence of electrogenic hydrogen ion/sodium ion antiport in Halobacterium halobium cell envelope vesicles. Biochemistry. 1976 Oct 19;15(21):4608–4614. doi: 10.1021/bi00666a010. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K., MacDonald R. E. Light-induced transport in Halobacterium halobium. Methods Enzymol. 1979;56:398–407. doi: 10.1016/0076-6879(79)56038-8. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K., Weber H. J. Spectrophotometric identification of the pigment associated with light-driven primary sodium translocation in Halobacterium halobium. J Biol Chem. 1980 Jan 10;255(1):243–250. [PubMed] [Google Scholar]
- Lindley E. V., MacDonald R. E. A second mechanism for sodium extrusion in Halobacterium halobium: a light-driven sodium pump. Biochem Biophys Res Commun. 1979 May 28;88(2):491–499. doi: 10.1016/0006-291x(79)92075-8. [DOI] [PubMed] [Google Scholar]
- MacDonald R. E., Greene R. V., Clark R. D., Lindley E. V. Characterization of the light-driven sodium pump of Halobacterium halobium. Consequences of sodium efflux as the primary light-driven event. J Biol Chem. 1979 Dec 10;254(23):11831–11838. [PubMed] [Google Scholar]
- MacDonald R. E., Greene R. V., Lanyi J. K. Light-activated amino acid transport systems in Halobacterium halobium envelope vesicles: role of chemical and electrical gradients. Biochemistry. 1977 Jul 12;16(14):3227–3235. doi: 10.1021/bi00633a029. [DOI] [PubMed] [Google Scholar]
- Matsuno-Yagi A., Mukohata Y. ATP synthesis linked to light-dependent proton uptake in a rad mutant strain of Halobacterium lacking bacteriorhodopsin. Arch Biochem Biophys. 1980 Jan;199(1):297–303. doi: 10.1016/0003-9861(80)90284-2. [DOI] [PubMed] [Google Scholar]
- Matsuno-Yagi A., Mukohata Y. Two possible roles of bacteriorhodopsin; a comparative study of strains of Halobacterium halobium differing in pigmentation. Biochem Biophys Res Commun. 1977 Sep 9;78(1):237–243. doi: 10.1016/0006-291x(77)91245-1. [DOI] [PubMed] [Google Scholar]
- Mukohata Y., Kaji Y. Light-induced membrane-potential increase, ATP synthesis, and proton uptake in Halobacterium halobium, R1mR catalyzed by halorhodopsin: Effects of N,N'-dicyclohexylcarbodiimide, triphenyltin chloride, and 3,5-di-tert-butyl-4-hydroxybenzylidenemalononitrile (SF6847). Arch Biochem Biophys. 1981 Jan;206(1):72–76. doi: 10.1016/0003-9861(81)90067-9. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Functions of a new photoreceptor membrane. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2853–2857. doi: 10.1073/pnas.70.10.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich E. N., Spudich J. L. Photosensitive phosphoproteins in Halobacteria: regulatory coupling of transmembrane proton flux and protein dephosphorylation. J Cell Biol. 1981 Dec;91(3 Pt 1):895–900. doi: 10.1083/jcb.91.3.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Wagner G., Hartmann R., Oesterhelt D. Potassium uniport and ATP synthesis in Halobacterium halobium. Eur J Biochem. 1978 Aug 15;89(1):169–179. doi: 10.1111/j.1432-1033.1978.tb20909.x. [DOI] [PubMed] [Google Scholar]


