Abstract
Optimized facile syntheses and highly desirable spectroscopic properties of two isomorphic fluorescent pyrimidines, comprising a 1,2,4-triazine motif conjugated to a thiophene (1a) or a furan (1b), are described. Although structurally related to their 5-modified uridine counterparts, these modified 6-aza-uridines reveal dramatically improved fluorescence properties and a remarkable sensitivity to polarity and pH changes. The thiophene derivative 1a has an absorption maximum around 335 nm, which upon excitation yields visible emission with a polarity sensitive maximum and fluorescence quantum yield ranging from 415 nm (Φ = 0.8) to 455 nm (Φ= 0.2) in dioxane and water, respectively. Nucleoside 1a also displays susceptibility to acidity. Correlating emission intensity and solution pH yields a pKa value of 6.7–6.9, reasonably close to physiological pH values. The results illustrate that highly sought-after fluorescence features (brightness and responsiveness) are not necessarily the trait of large fluorophores alone, but can be observed with probes that meet stringent isomorphic design criteria.
Keywords: Fluorescent Probes, Nucleosides, Photophysics Donor-acceptor Systems, Acidity
1. Introduction
The sensitivity and simplicity of fluorescence spectroscopy has made it one of the most useful tools for the study of biomolecules. Despite numerous technological and instrumental advances, the quality of fluorescence-based analyses ultimately relies on the fluorescent probes themselves. Countless fluorescent tags, labels and probes have been designed over the years, but only a limited fraction meets the stringent design criteria of isomorphicity.[1] Useful isomorphic probes are characterized by a high structural resemblance to the molecular building blocks they mimic, while maintaining distinct spectroscopic properties. This imposes structural and application-specific constraints, where probes capable of innocently replacing membranes, proteins, or nucleic acid components have to be fabricated.[1] The design of such probes is further challenged by the fact that the relationship between structure and photophysical properties is hard to deduce, and can typically only be elucidated experimentally.
To explore nucleic acids, the non-emissive canonical nucleosides need to be structurally modified to endow them with useful fluorescent properties. Such alterations cannot impede native W-C base-pairing and subsequent helix formation.[2,3] Successful nucleoside probes reconcile these structural and photophysical demands with an exclusively excitable wavelength, red-shifted from the ~260 nm absorption band of the native nucleobases. Responsive emissive nucleoside probes, unlike fluorescent tags or labels, are characterized by spectroscopic properties, which are sensitive to changes in the local environment. Several groups, including ours, have reported a rich variety of isomorphic fluorescent nucleoside analogs.[1,4,5] The basic design principle of our first generation emissive nucleobase analogs has relied on conjugating five membered aromatic heterocycles to the pyrimidine core at the 5-position.[2] The modification at this position appears to be structurally benign, with no impact on the anti orientation or sugar pucker of the nucleosides.[6] The resulting nucleosides emit in the visible range (390–443 nm), have rather large Stokes shifts (8400–9700 cm−1), but suffer from relatively low emission quantum efficiency (F=0.01–0.035).[6] These nucleosides behave like molecular rotors, with the 5-membered heterocycles and the pyrimidine core acting as the “donor and acceptor”, respectively.[7] Rotation around the biaryl linkage provides an effective channel for non-radiative torsional relaxation, thus leading to low emission quantum yield in non-viscous media.[7,8] We hypothesized that altering the electronics by replacing the pyrimidine with the corresponding 1,2,4-triazine core (i.e., 6-aza-pyrimidine)[9] would enhance the charge transfer character, ideally yielding a bathochromic shift as well as a hyperchromic effect and higher brightness. Herein we report a simplified synthetic pathway to thiophene and furan-modified 6-azauridines, discuss their most relevant photophysical properties, and reveal their responsiveness to environmental polarity and pH.
2. Chromophore Design
As articulated above, replacing the uridine core with 6-azauracil, a more electron deficient heterocycle, while keeping the electron rich thiophene and furan moieties in the 5-position, was expected to maintain an extended π-system while augmenting the donor-acceptor nature of this motif (Scheme 1). This enhanced “push-pull” interaction is expected to affect a red-shift of the absorption maxima and likely a concomitant red-shifted emission maxima. Such a donor–acceptor interplay is also frequently associated with enhanced sensitivity toward environmental polarity.[10–13] The single bond linkage between the 5-membered heterocycle and the 6-membered nucleobase introduces a molecular rotor element likely to render 1a and 1b sensitive toward viscosity (molecular crowding) as demonstrated for other 5-aryl modified pyrimidines.[7,8] Chromophores possessing such a feature typically reveal much higher emission intensity in viscous compared to non-viscous media. Viscosity (molecular crowding) hampers formation of a twisted excited state thereby limiting the rotational relaxation, a non-emissive decay pathway, to the ground state.[14,15]
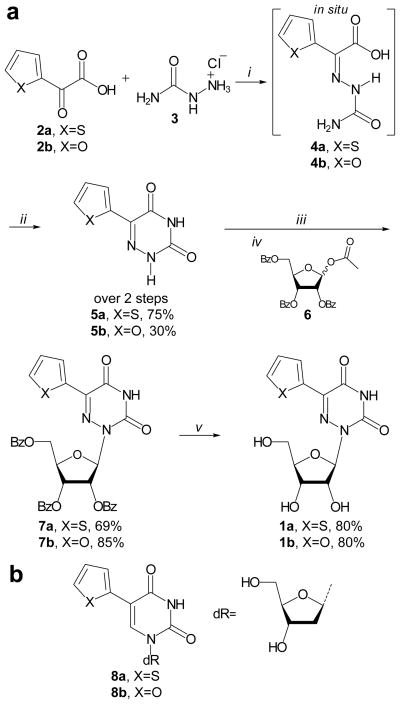
Scheme 1.
a) Syntheses of 1a and 1b, reagents and conditions: i) semicarbazide hydrochloride (3) (1.1 equiv.), H2O, 60°C, 17h, ii) add. 1 M NaOH aq. (3 equiv.), 60°C, 4h, iii) BSA (3 equiv.), CH3CN, rt, 30′, iv) β-D-ribofuranose 1-acetate 2,4,5-tribenzoate (6) (1.1 equiv.), TMSOTf (1.1 equiv.), CH3CN, 85°C, 30′, v) NH3/MeOH, 60°C, 24h; and b) structures of reported 5-(thiophene-2-yl)-2′-deoxyuridine (8a) and 5-(furan-2-yl)-2′-deoxyuridine (8b).
Only a few fluorescent nucleosides have been shown to be pH sensitive.[8,16,17] 8-Aza-guanosine, an isomorphic fluorescent purine analog,[18] shows its pH-dependent emission characteristics as a nucleoside[19] and after enzymatic incorporation into RNA.[20] This feature was exploited in the investigation of the catalytic function of RNA,[21] An application reveals interesting opportunities for fluorescent nucleosides that display spectral sensitivity toward (de)protonation. No emissive pyrimidines with pKa values near physiological pHs have, however, been documented. Importantly, the presence of the nitrogen atom at position 6 has a profound influence on the acidity of the NH, lowering its pKa to 6.8 vs. a pKa value of 9.3 for the parent uridine.[22] Like uridine, 6-aza-uridine has no appreciable fluorescence properties hampering visualization of (de)protonation events with fluorescence spectroscopy. Assuming the emissive features discovered for the parent 5-modified uridines translate to (or are enhanced in) the 6-aza motif, deprotonation and protonation events are likely to be revealed by changes in the emission profile. This new motif would therefore constitute the first example of a pH responsive emissive pyrimidine with a pKa value near physiological pHs.
3. Results
3.1. Synthesis
Condensation of commercially available thiophene glyoxylic acid (2a) with semicarbazide (3) gave imine 4a which, in situ, cyclized upon heating under basic conditions to furnish 5a in good yields. The same approach, starting from 2b, provided 5b. Glycosylation of 5a and 5b was performed in a two-step one-pot procedure. First, the nucleobase was activated by reaction with bis(trimethylsilyl)acetamide (BSA) in acetonitrile for ~30 minutes at rt, turning the initial suspension into a clear solution. Secondly, the reaction mixture was brought to 85°C after which β-D-ribofuranose 1-acetate 2,4,5-tribenzoate (6) was added, immediately followed by addition of trimethylsilyltriflate (TMSOTf). The reaction was allowed to run for 30 minutes after which TLC analysis indicated full conversion of the nucleobase to the desired glycosylated product. An aqueous work-up followed by purification with column chromatography yielded 7a and 7b in good yields. Deprotection with methanolic ammonia at 60°C, followed by column chromatographic purification gave desired 1a and 1b.
3.2. Photophysics
3.2.1. Basic photophysics in water
In unbuffered deionized water, 1a is characterized by a red-shifted absorption maximum at 332 nm, which upon excitation gives a visible fluorescence signal, peaking at 455 nm, with a respectable quantum yield (Φ = 0.20) and a fluorescence lifetime of 4.9 ns (Table 1). In contrast, a solution in dioxane yields an absorption maximum at 335 nm, an emission maximum at 415 nm (Φ = 0.80) and a slightly longer fluorescence lifetime of 5.4 ns. The small change in absorption maximum, but 40 nm difference between the emission maxima in the two solvents prompted us to study the sensitivity to polarity in greater detail (see below).
Table 1.
Selected spectroscopic properties of 1a and 1b.[a]
| Solvent | Absorption | Fluorescence | Brightness | Stokes shift | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| λmax nm | ε ×104 M−1cm−1 | λmax nm | Φ | τ ns | Φ×ε ×103 | νabs-νem cm−1 | ||
|
| ||||||||
| 1a | Dioxane | 335 | 1.3 | 415 | 0.80 | 5.4 | 10.4 | 6025 |
| Water | 332 | 1.1 | 455 | 0.20 | 4.9 | 2.2 | 8492 | |
|
| ||||||||
| pH 2.55 | 336 | 1.2 | 462 | 0.13 | 3.0b | 1.6 | 8485 | |
| pH 10.13 | 325 | 1.2 | 426 | 0.39 | 6.6b | 4.7 | 7588 | |
|
| ||||||||
| MeOH | 334 | 1.1 | 433 | 0.50 | 6.6 | 5.5 | 7332 | |
| Glycerol | 339 | 1.0 | 444 | 0.66 | 7.9 | 6.6 | 7372 | |
|
| ||||||||
| 1b | Dioxane | 327 | 1.2 | 414 | 0.60 | 5.6 | 7.2 | 6824 |
| Water | 320 | 1.0 | 443 | 0.05 | 3.1 | 0.5 | 9107 | |
|
| ||||||||
| pH 2.55 | 329 | 1.1 | 485 | <0.01 | <1b | <0.1 | 9861 | |
| pH 10.13 | 319 | 1.1 | 446 | 0.11 | 2.8b | 1.2 | 9273 | |
|
| ||||||||
| MeOH | 326 | 1.1 | 442 | 0.04 | 1.6 | 0.4 | 8585 | |
| Glycerol | 331 | 1.1 | 434 | 0.46 | 2.7 | 5.1 | 7574 | |
The Stokes shifts are calculated with corrected emission maxima obtained using: Intensity [ν] = λ2 × Intensity [λ].[10]
Values for pH 2.55 and pH 10.13 (aq. 10 mM phosphate, 0.1 M NaCl buffers) represent the lower- and upper plateau values of the sigmoidal fit (Figure 2). All values represent averages, see SI for error analysis.
3.2.2. Sensitivity to polarity
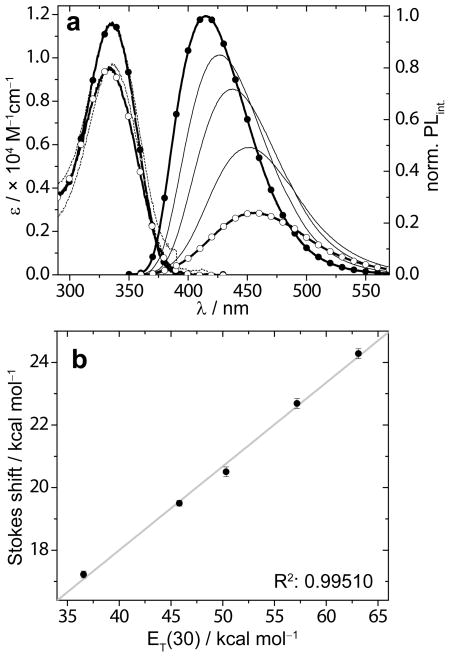
Influence of polarity on spectroscopic parameters of fluorescent probes was studied using samples in dioxane (ET(30) = 36.0 kcal/mol), water (ET(30) = 63.1 kcal/mol) and mixtures thereof.[23] Water–dioxane samples of 1a and 1b were prepared from a concentrated DMSO stock solution and subjected to an absorption and steady state emission spectroscopy study (Figure 1 and Table 1). The small amount of DMSO has been established to have negligible impact on polarity.[24]
Figure 1.
a) Absorption (dashed line) and emission (solid line) spectra of 1a in dioxane (bold black line, solid circles), water (bold black line, open circles), and binary mixtures of 10, 30, and 70 v% water in dioxane (black lines). Samples’ concentration is 7.9 × 10−6 M, emission is recorded after excitation at 335 nm. b) A plot of the Stokes shift (in kcal/mol) vs. ET(30) values (in kcal/mol) obtained from dioxane–water mixtures (data points with error bars) and linearization (grey line). Absorption and emission spectra of each sample are recorded in duplicate; only one set is shown.
The Stokes shifts (νabs–νem) were calculated for the samples in dioxane, water, and mixtures thereof and plotted, after conversion from cm−1 to kcal/mol, as a function of the experimentally-determined samples’ ET(30) values (Figure 3). The data points clearly line-up linearly as was established by a good linear fit with a slope value of 0.268, quantifying the sensitivity to polarity (Table 2). In addition to a poorer linear fit, the sensitivity to polarity for 1b was lower (Table 2 and SI).
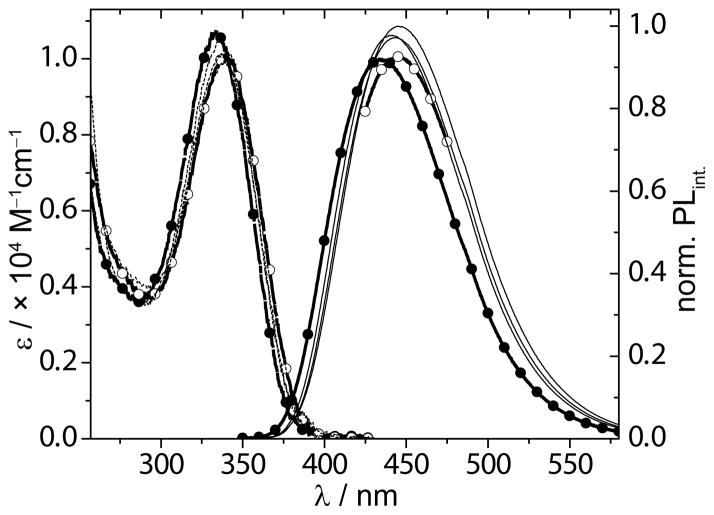
Figure 3.
Absorption (dashed lines) and emission (solid lines) curves for 1a, corrected for small differences in the O.D. at the λex(340 nm), in methanol (solid circles), glycerol (open circles) and mixtures thereof (black lines).
Table 2.
Sensitivity to polarity[a]
| Slope | Y-int. | Slope | Y-int. | R2 | |
|---|---|---|---|---|---|
|
| |||||
| cm−1/kcal mol−1 | cm−1 | - | kcal mol−1 | ||
| 1a | 93.7 | 2548 | 0.268 | 7.29 | 0.99510 |
| 8a | 63.8 | 5188 | 0.182 | 14.83 | 0.86159 |
|
| |||||
| 1b | 75.4 | 4290 | 0.216 | 12.27 | 0.84211 |
| 8b | 79.5 | 4437 | 0.227 | 12.69 | 0.96922 |
Polarity sensitivity equals the slope of the linearized Stokes shift vs. ET(30) plot. Values for 5-(thiophen-2-yl)-2dU 8a and 5-(fur-2-yl)-2dU 8b in cm−1/kcal mol−1 have been reported.[7, 25] A unitless value for polarity sensitivity is obtained by conversion of the Stokes shift value from cm−1 to kcal mol and replotting it as a function of the sample ET(30) value (in kcal mol−1). The adjusted R2 represents the goodness of the linear fit.
3.2.3. Sensitivity to pH
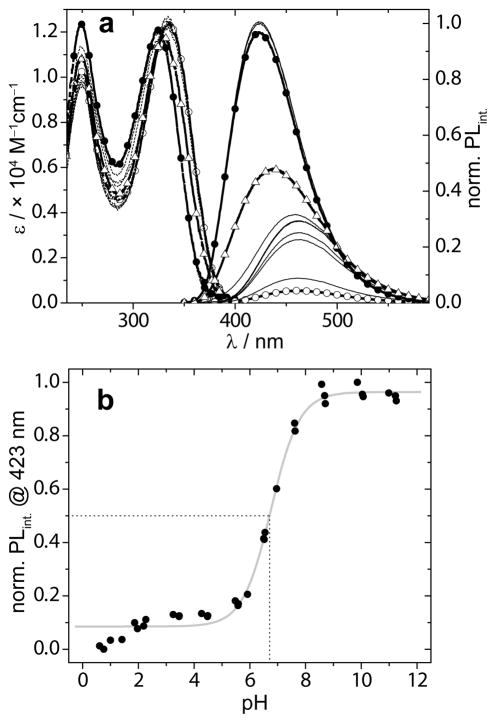
The sensitivity of the emissive 6-aza-U chromophores to pH was studied in aqueous phosphate buffers with pH values ranging from 0.5 to 12. Absorption spectra only showed small differences in maxima (λmax = 326 nm), while the corresponding emission spectra revealed a strong influence of pH on the position as well as intensity of the emission maximum (Figure 2a, Table 1). The emission maximum shifted from ~426 nm (Φ = 0.39) to ~462 nm (Φ = 0.13), going from pH 10.13 to 2.55, respectively. Curve-fitting the normalized fluorescence intensity as a function of pH gave a sigmoidal curve from which a pKa, value of 6.7 was calculated (Figure 2b). Using the same approach a pKa value of 6.6 for probe 1b was determined.[25] The difference between the quantum yield under acidic and basic conditions was also reflected by the difference in fluorescence lifetimes, 3.0 and 6.6 ns, respectively (Table 1).
Figure 2.
a) Absorption (dashed lines) and emission plots (solid lines), corrected for small differences in the O.D. at the λex(335 nm) of 1a at pH 0.61 (open circles), pH 10.99 (solid circles), pH 6.55 (open triangles) and intermediate pH values (black lines). b) Plot of the normalized emission intensity as function of sample pH (solid circles) fitted to a sigmoidal curve (grey line). The dashed lines illustrate a graphical determination of the pKa value.
3.2.4. Sensitivity to viscosity
Absorption and emission spectra of 1a in samples of methanol, glycerol and mixtures thereof reveal virtually no responsiveness toward viscosity for 1a (Figure 3), but indicate a significant sensitivity to viscosity for 1b.[25] A double log plot of the fluorescence intensity as a function of sample viscosity for 1b, however, reveals poor linearity.[25] The high fluorescence quantum yields for 1a in methanol (0.50) and glycerol (0.66), are accompanied by the longest lifetimes observed in this study, 6.6 and 7.9 ns, respectively.
4. Discussion
4.1. Synthesis
The earliest scheme described for the synthesis of 5b, is a two-pot two-step procedure starting from furanglyoxylic acid (2b), which was reacted with semicarbazide hydrochloride (3) to give the corresponding formic acid semicarbazone (4b). This, in turn, yielded 5b upon refluxing in a mixture of propylene glycol–ethanol in the presence of sodium ethoxide.[26] Later, Walker et. al. reported a procedure for the synthesis of 2a, but used thio-semicarbazide instead of semicarbazide (3), necessitating a third step to convert the thiocarbonyl into a carbonyl moiety.[27] To shorten the synthesis of 5a and 5b we started from semicarbazide and convert the original two-pot two-step synthesis[26] to a simplified one-pot two-step procedure.[28] Importantly, this streamlined approach works well for the synthesis of 5a and, albeit with lower yield, also for 5b, while the three-step three-pot synthesis by Walker et. al. works for the synthesis of 5a it but proved very challenging for the synthesis of 5b.[27,29] Glycosylation of 5-substituted 6-aza uracils has been reported.[27,30,31] We used a slightly modified procedure involving activation of the nucleobase with BSA in CH3CN instead of TMSCl and Et3N in benzene. We performed the glycosylation directly in situ after BSA treatment, activating the ribose (6) with TMSOTf, instead of changing the solvent from benzene to dichloroethane and using stannic chloride to activate the ribose (6). Our procedure proved facile, quick, and high yielding for the synthesis of 7a, and 7b. Deprotection of the benzoyl groups to give 1a and 1b was performed using methanolic ammonia for 24 hours at 60°C in a pressure vessel. Deprotection also succeeded with aqueous ammonia in methanol in 17 hours at 40°C, albeit in lower yield. Both 1a and 1b were synthesized in 3 steps from commercially available starting materials in 41% and 20% overall yield, respectively.
4.2. Fluorescence Characteristics and their Sensitivity to Polarity
Diverse events can impact the structure of nucleic acids (e.g., (un)folding, binding of low MW ligand, lesions), which often result in changes in the local polarity. Nucleic acid probes able to detect such changes in environmental polarity have proven to be useful tools.[1,24,32–39] We therefore thoroughly evaluate the influence of polarity on a probe’s photophysical properties. We regularly use water–dioxane mixtures, as they not only vary in polarity, but also present changes in hydrogen bonding donor and acceptor composition prone to exert specific solvent–solute interactions.[23] Moreover, the polarity window obtainable with such water–dioxane mixtures likely reflects most biological relevant environmental polarities. Using a microenvironmental polarity parameter such as the ET(30) scale with values ranging from 36.0 to 63.1 kcal/mol for pure dioxane to water, respectively,[11] typically provides good correlation with spectral features like Stokes shifts.[23]
While the quantum yield for 1a in water is modest (Φ = 0.20) and low for 1b (Φ = 0.05), the quantum yield displayed by 1a in dioxane (Φ = 0.80) is among the highest values reported for an isomorphic nucleoside (Table 1).[1] Both the quantum yield in water and dioxane represent a huge improvement compared to the values reported for the corresponding 2′-deoxyuridine modified with either a thiophene (8a) (Φwater = 0.01) or furan (8b) (Φwater = 0.03) in the five position (Scheme 1).[2,6] Interestingly, uridine analogs 8a and 8b display the opposite behavior with even lower quantum yield in apolar organic media.[2,6,24] Incidentally, this remarkable difference in fluorescence quantum yield between these isoelectronic nucleoside analogs shows that photophysical properties are difficult to predict from the molecular structure alone.
It is worth noting that we previously reported the sensitivity to polarity for 8a and 8b in cm−1/kcal mol−1 (Table 2). To obtain unitless values, the values for the Stokes shift in cm−1 were converted to kcal/mol, and replotted as a function of the samples’ ET(30) value. The slope for the linear relationship, the polarity sensitivity, for modified uridines 8a and 8b was determined to be 0.182 and 0.227,[25] respectively, both significantly less sensitive than 1a (Table 2). These substantial differences illustrate how the enhanced push-pull motif present in 1a and 1b, as compared to 8a and 8b indeed results in the expected enhanced susceptibility to polarity.
4.3. Sensitivity to pH
Sensitivity to pH as a characteristic of fluorescent nucleosides is relatively uncommon, although a couple of examples have been reported.[8,17,19] In these cases the absorption spectra show a two-state transition with an isosbestic point. The influence of pH on the emission properties is reflected by a strong increase or decrease of the fluorescence intensity not necessarily accompanied by significant shifts of the emission maxima.
Nucleoside 1a reveals its pH sensitivity by small changes in absorption, but large changes in emission intensity and maxima (Figure 2a). Changing from basic to acidic conditions the fluorescence curve of 1a shifts from a maximum located at 426 nm to 462 nm with a concurrent drop in the fluorescence quantum yield from 0.39 to 0.13, respectively (Figure 2a, Table 1). Fluorescence decay analysis reveals that the fluorescence lifetime in basic conditions is more than doubled compared to the value in acidic conditions. The drop in normalized fluorescence intensity as a function of pH gives, after a Boltzmann fit of the data points, an S-curve indicative of a two-state (de)protonation process with a pKa of 6.7 and 6.6 for 1a, and 1b respectively (Figure 2b and SI). These values are in agreement with a reported value for 6-aza-uridine (pKa = 6.8).[22] It further shows that the nature of the modification in the five position of the six membered heterocycle has little effect on the pKa of the NH as its electronic influence is limited. The observed pKa values significantly deviate from the reported values for the parent uridine (9.3 – 9.5),[22,40,41] demonstrating that the additional nitrogen renders the NH ~1000 fold more acidic. This finding is corroborated by others who have reported that substitution of H-6 in native uracil with an electron withdrawing chloride has a spectacular influence on the pKa value causing a drop from 9.5 to 5.8.[42] Closer inspection of Figure 2a shows a downward trend of the fluorescent intensity at very low pHs. Although speculative at this stage, this might suggest that the 6-aza nitrogen gets protonated at pHs far below 2. A normalized plot excluding these low pH data points (pH < 2.5) gives a slightly higher pKa value of 6.85 for the heterocycle’s NH (Figure S4.1).[25] Regardless of this putative second transition, 1a shows fluorescence intensity as well as emission maximum sensitivity with a pKa close to physiological pH. This makes 1a an attractive probe for studies involving (de)protonation events on RNA, as there are no known isomorphic pyrimidine analogs with pKa values at this range.[21]
4.4. Sensitivity to Viscosity
The nucleobase moiety in 1a and 1b can be described as a molecular rotor, being comprised of a single bond linking two π systems.[7] Such molecules can lose their excitation energy via a non-emissive rotational decay from a twisted excited state. Molecular crowding effects such as the one induced by viscous solvents can hinder formation of a twisted excited state, thereby limiting the non-emissive rotational decay process resulting in an increased emission intensity.[43] The contribution of this non-emissive decay pathway can be studied by plotting the emission intensity as a function of sample viscosity.[44] A practical approach to controlling sample viscosity is the use of binary mixtures of solvents with very low and very high viscosities that differ minimally in polarity.[7,8,45] Suitable non-viscous and highly viscous solvents are methanol (η20°C = 0.583 cp), and glycerol (η20°C = 1317 cp),[7, 46] respectively. They can be mixed in all ratios to control sample viscosity which can be calculated based on the weight fraction and viscosity values of its pure components.[47]
Clearly, 1a shows no fluorescence intensification upon increasing viscosity, suggesting that 1a does not adopt a twisted excited state upon excitation (Figure 3a). This contrasts the significant sensitivity for viscosity observed for 1b.[25] The difference can perhaps be explained by their respective quantum yields in solvents of low viscosity (Table 1). Samples in non-viscous, protic solvents such as water and methanol display quantum yields of 0.20 and 0.50 for 1a, but 0.05 and 0.04 for 1b, respectively. The already much higher quantum yields for 1a suggest that rotational relaxation might not be a major non-radiative pathway for this chromophore’s electronic decay. The very low initial quantum yields of 1b, however, benefit greatly from curtailment of this non-emissive decay pathway.
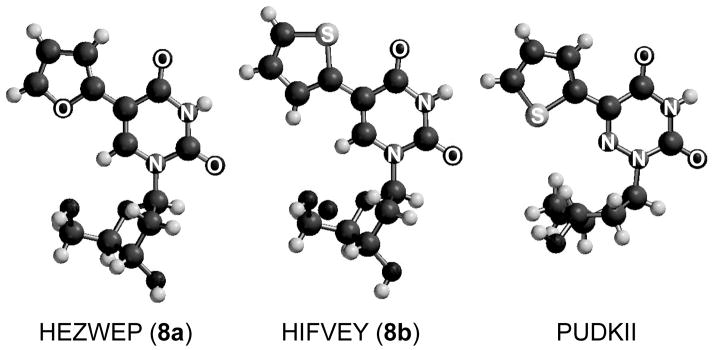
Explaining the distinct differences between the two related chromophores is challenging and must be sought in searching for differences in the relative ground state orientation of the thiophene (or furan) with respect to the nitrogenous nucleobase. In the reported crystal structure of the deoxy-ribose form of 1a, the sulfur atom of the thiophene moiety, which is coplanar with the nucleobase, points away (“anti”) from the 4-carbonyl thereby facing (“syn”) the 6-aza nitrogen (Figure 4).[48] This orientation is opposite to the one observed in our reported crystal structure of 8a, in which the thiophene moiety, also coplanar with the nucleobase, is oriented with its sulfur atom pointing toward the 4-carbonyl.[2,6] The crystal structure of 8b shows again the opposite configuration, with the furan oxygen facing away from the 4-carbonyl (Figure 4).[2] In lieu of a crystal structure of 1b, we can only speculate on the orientation of its furan oxygen. While differences between solid and solution states could exist, the distinct orientational preferences of the thiophene and furan units in the crystal structures might also reflect their ground state orientation in solution. The electronic nature of the atom in the 6-position, CH (8a and 8b) or N (1a and 1b) seems, therefore, to influence the orientation of the 5-membered heterocycle. This, in turn, could have a profound influence on the excited state geometry and hence sensitivity toward viscosity.
Figure 4.
Crystal structures of 8a,[6] 8b, [6] and the 2′-deoxy-ribose form of 1a[48]. Note: For clarity, the hetero atoms of the nucleobases are labled.
5. Summary
The 6-aza-uridine motif, decorated at the 5-position with either a thiophene (1a) or furan (1b), is a synthetically accessible pyrimidine analog with highly desirable photophysical properties. The Stokes shift of 1a is very sensitive to polarity. The pH sensitive fluorescence intensity starkly increases upon deprotonation with a pKa value of 6.7–6.9, close to physiological pHs. Most importantly, the fluorescence quantum yield remains sufficient (ΦWater(pH 2.55) = 0.13) to excellent (Φdioxane = 0.80) under all conditions and is thereby amongst the highest reported for isomorphic nucleoside probes.[1] The robust fluorescence quantum yield in neutral protic solvents (Φwater = 0.20, ΦMeOH = 0.50, and Φglycerol = 0.66) suggest minimal population of non-radiative decay pathways, which is arguably the cause for the lack of sensitivity to viscosity. The structurally related nucleoside 1b, displays relatively low quantum yields in protic solvents and sensitivity to viscosity, marked by a steep increasing fluorescence quantum yield with increasing medium viscosity. Interestingly, 1b shows almost identical pH sensitivity to 1a, but a weaker sensitivity to polarity. Despite their differences, both 1a and 1b exhibit more desirable photophysical properties than their reported 2′-deoxyuridine analogs (8a and 8b), making the 6-aza motif one of the most attractive and promising emissive pyrimidines.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health for their generous support (grant number GM 069773), and the National Science Foundation (instrumentation grant CHE-0741968).
Footnotes
Supporting information for this article is available on the WWW under http://www.chemphyschem.org or from the author
References
- 1.Sinkeldam RW, Greco NJ, Tor Y. Chem Rev. 2010;110:2579–2619. doi: 10.1021/cr900301e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greco NJ, Tor Y. J Am Chem Soc. 2005;127:10784–10785. doi: 10.1021/ja052000a. [DOI] [PubMed] [Google Scholar]
- 3.Shin D, Sinkeldam RW, Tor Y. J Am Chem Soc. 2011;133:14912–14915. doi: 10.1021/ja206095a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selected reviews. Hawkins ME. Meth Enzym. 2008;450:201–231. doi: 10.1016/S0076-6879(08)03410-1.Dodd DW, Hudson RHE. Mini-Rev Org Chem. 2009;6:378–391.Tor Y. Pure Appl Chem. 2009;81:263–272.Wilhelmsson LM. Q Rev Biophys. 2010;43:159–183. doi: 10.1017/S0033583510000090.Kimoto M, Cox RSI, Hirao I. Expert Rev Mol Diagn. 2011;11:321–331. doi: 10.1586/erm.11.5.
- 5.Selected individual contributions. Ward DC, Reich E, Stryer L. J Biol Chem. 1969;244:1228–1237.Liu CH, Martin CT. J Mol Biol. 2001;308:465–475. doi: 10.1006/jmbi.2001.4601.Tor Y, Del Valle S, Jaramillo D, Srivatsan SG, Rios A, Weizman H. Tetrahedron. 2007;63:3608–3614.Srivatsan SG, Greco NJ, Tor Y. Angew Chem Int Ed Engl. 2008;47:6661–6665. doi: 10.1002/anie.200802199.Angew Chem. 2008;120:6763–6767.Xie Y, Dix AV, Tor Y. J Am Chem Soc. 2009;131:17605–17614. doi: 10.1021/ja905767g.Xie Y, Maxson T, Tor Y. J Am Chem Soc. 2010;132:11896–11897. doi: 10.1021/ja105244t.g) Ref [2] h) Ref. [3].
- 6.Greco NJ, Tor Y. Tetrahedron. 2007;63:3515–3527. doi: 10.1016/j.tet.2007.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinkeldam RW, Wheat AJ, Boyaci H, Tor Y. ChemPhysChem. 2011;12:567–570. doi: 10.1002/cphc.201001002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinkeldam RW, Marcus P, Uchenik D, Tor Y. ChemPhysChem. 2011;12:2260–2265. doi: 10.1002/cphc.201100315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Although a trivial name, we refer to the nucleosides as 6-aza uridines since their features are compared to their corresponding modified pyrimidines.
- 10.Lakowicz JR. Principles of fluorescence spectroscopy. 3. Springer; New York: 2006. [Google Scholar]
- 11.Reichardt C. Chem Rev. 1994;94:2319–2358. [Google Scholar]
- 12.Berezin MY, Achilefu S. Chem Rev. 2010;110:2641–2684. doi: 10.1021/cr900343z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lobo BC, Abelt CJ. J Phys Chem A. 2003;107:10938–10943. [Google Scholar]
- 14.Oster G, Nishijima Y. J Am Chem Soc. 1956;78:1581–1584. [Google Scholar]
- 15.Loutfy RO, Arnold BA. J Phys Chem. 1982;86:4205–4211. [Google Scholar]
- 16.Wilhelmsson LM, Sandin P, Holmen A, Albinsson B, Lincoln P, Norden B. J Phys Chem B. 2003;107:9094–9101. [Google Scholar]
- 17.Sun KM, McLaughlin CK, Lantero DR, Manderville RA. J Am Chem Soc. 2007;129:1894–1895. doi: 10.1021/ja068416l. [DOI] [PubMed] [Google Scholar]
- 18.Roblin RO, Lampen JO, English JP, Cole QP, Vaughan JR. J Am Chem Soc. 1945;67:290–294. [Google Scholar]
- 19.Wierzchowski J, Wielgus-Kutrowska B, Shugar D. Biochim Biophys Acta, Gen Subj. 1996;1290:9–17. doi: 10.1016/0304-4165(95)00181-6. [DOI] [PubMed] [Google Scholar]
- 20.Da Costa CP, Fedor MJ, Scott LG. J Am Chem Soc. 2007;129:3426–3432. doi: 10.1021/ja067699e. [DOI] [PubMed] [Google Scholar]
- 21.Liu L, Cottrell JW, Scott LG, Fedor MJ. Nat Chem Biol. 2009;5:351–357. doi: 10.1038/nchembio.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seela F, Chittepu P. J Org Chem. 2007;72:4358–4366. doi: 10.1021/jo0702903. [DOI] [PubMed] [Google Scholar]
- 23.Sinkeldam RW, Tor Y. Org Biomol Chem. 2007;5:2523–2528. doi: 10.1039/b707719j. [DOI] [PubMed] [Google Scholar]
- 24.Sinkeldam RW, Greco NJ, Tor Y. Chem Bio Chem. 2008;9:706–709. doi: 10.1002/cbic.200700714. [DOI] [PubMed] [Google Scholar]
- 25.SI, See SI for details.
- 26.Hayes K. J Med Chem. 1964;7:819–820. doi: 10.1021/jm00336a037. [DOI] [PubMed] [Google Scholar]
- 27.Basnak I, Coe PL, Walker RT. Nucleosides and Nucleotides. 1994;13:163–175. [Google Scholar]
- 28.A 5-(fur-3-yl)-6-azauridine motif has also been published.[31].
- 29.Burch HA. J Med Chem. 1970;13:288–291. doi: 10.1021/jm00296a029. [DOI] [PubMed] [Google Scholar]
- 30.Cristescu C. Rev Roum Chim. 1975;20:1287–1293. [Google Scholar]
- 31.Mitchell WL, Ravenscroft P, Hill ML, Knutsen LJS, Judkins BD, Newton RF, Scopes DIC. J Med Chem. 1986;29:809–816. doi: 10.1021/jm00155a035. [DOI] [PubMed] [Google Scholar]
- 32.Jin R, Breslauer KJ. Proc Nat Acad Sci USA. 1988;85:8939–8942. doi: 10.1073/pnas.85.23.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barawkar DA, Ganesh KN. Biochem Biophys Res Commun. 1994;203:53–58. doi: 10.1006/bbrc.1994.2147. [DOI] [PubMed] [Google Scholar]
- 34.Barawkar DA, Ganesh KN. Nucleic Acids Res. 1995;23:159–164. doi: 10.1093/nar/23.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimura T, Kawai K, Majima T. Org Lett. 2005;7:5829–5832. doi: 10.1021/ol052473m. [DOI] [PubMed] [Google Scholar]
- 36.Kimura T, Kawai K, Majima T. Chem Commun. 2006:1542–1544. doi: 10.1039/b600026f. [DOI] [PubMed] [Google Scholar]
- 37.Jadhav VR, Barawkar DA, Ganesh KN. J Phys Chem B. 1999;103:7383–7385. [Google Scholar]
- 38.Okamoto A, Tainaka K, Saito I. Bioconjugate Chem. 2005;16:1105–1111. doi: 10.1021/bc050077i. [DOI] [PubMed] [Google Scholar]
- 39.Tainaka K, Tanaka K, Ikeda S, Nishiza K, Unzai T, Fujiwara Y, Saito I, Okamoto A. J Am Chem Soc. 2007;129:4776–4784. doi: 10.1021/ja069156a. [DOI] [PubMed] [Google Scholar]
- 40.Shugar D, Fox JJ. Biochim Biophys Acta. 1952;9:199–218. doi: 10.1016/0006-3002(52)90147-9. [DOI] [PubMed] [Google Scholar]
- 41.Fox JJ, Shugar D. Biochim Biophys Acta. 1952;9:369–384. doi: 10.1016/0006-3002(52)90181-9. [DOI] [PubMed] [Google Scholar]
- 42.Pfleider W, Deiss H. Isr J Chem. 1968;6:603–614. [Google Scholar]
- 43.Abdelmottaleb MSA, Loutfy RO, Lapouyade R. J Photochem Photobiol A-Chem. 1989;48:87–93. [Google Scholar]
- 44.Förster T, Hoffmann G. Z Phys Chem. 1971;75:63–76. [Google Scholar]
- 45.Haidekker MA, Theodorakis EA. Org Biomol Chem. 2007;5:1669–1678. doi: 10.1039/b618415d. [DOI] [PubMed] [Google Scholar]
- 46.DIPPR Project 801. Design Institute for Physical Properties, AIChE; 2009. [Google Scholar]
- 47.Haidekker MA, Brady TP, Lichlyter D, Theodorakis EA. Bioorg Chem. 2005;33:415–425. doi: 10.1016/j.bioorg.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Bansak I, Sun M, Hamor TA, Focher F, Verri A, Spadari S, Wroblowski B, Herdewijn P, Walker RT. Nucleosides and Nucleotides. 1998;17:187–206. doi: 10.1080/07328319808005169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.