Abstract
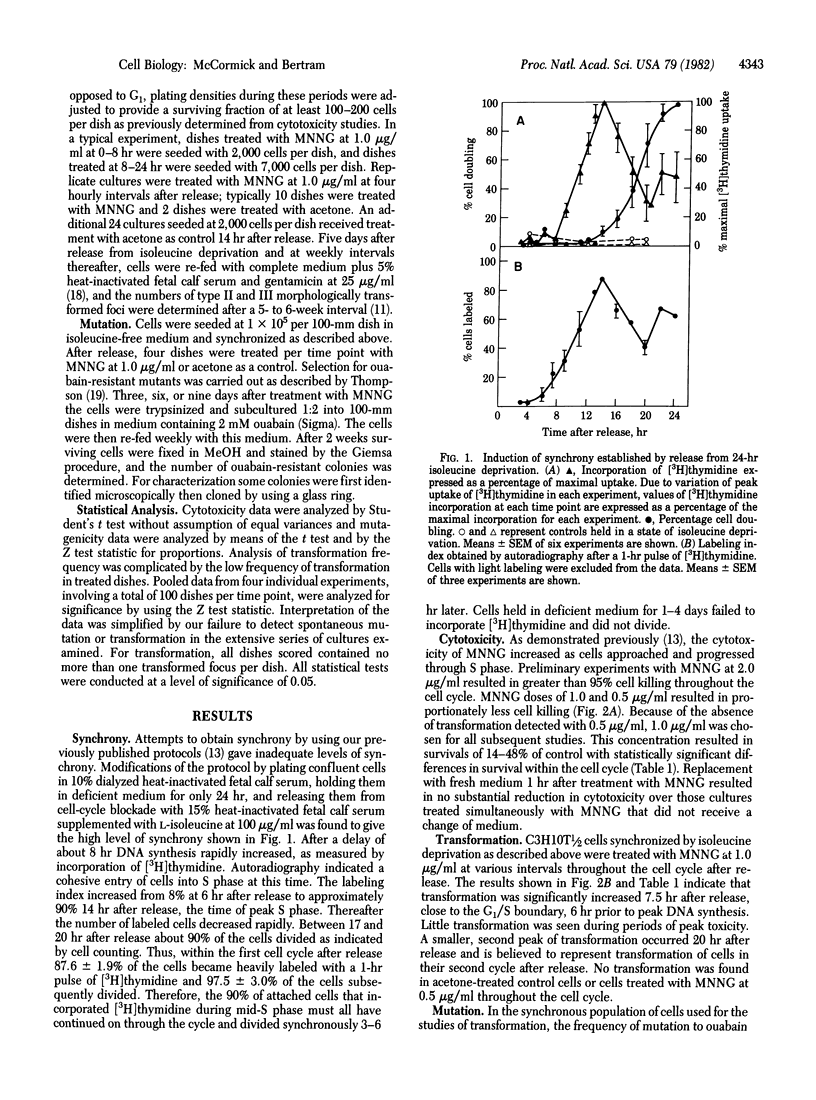
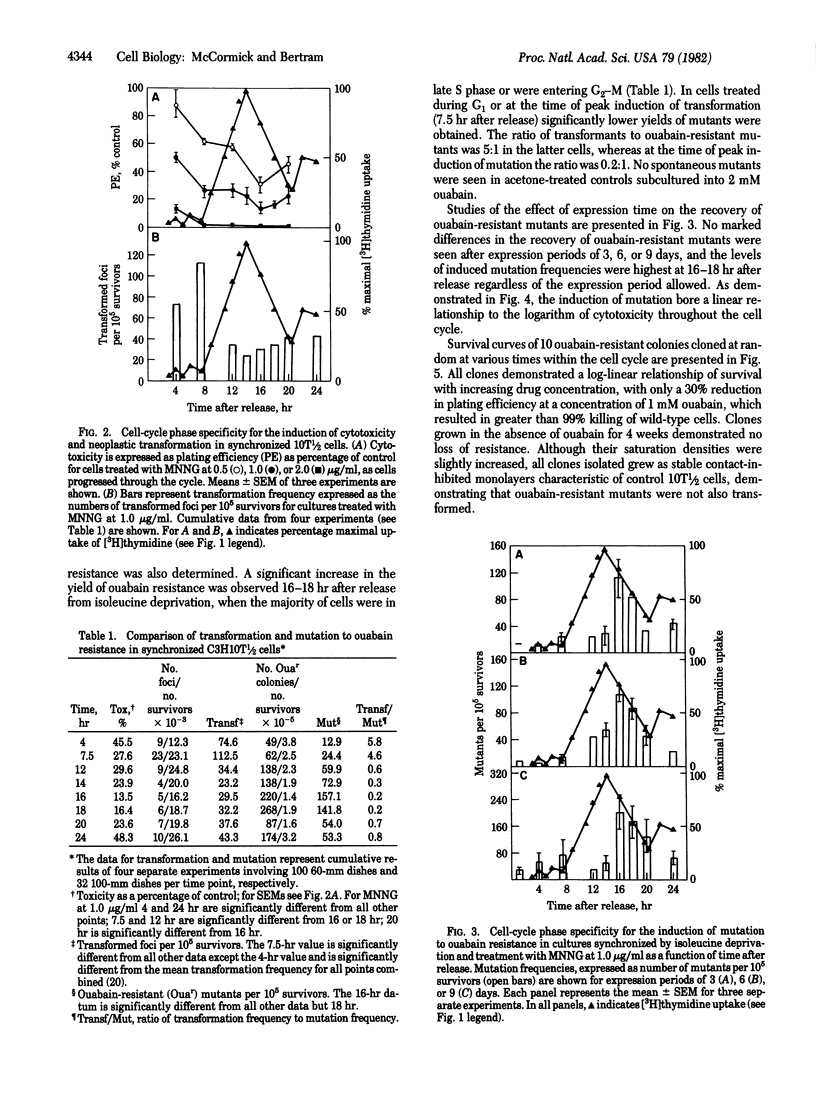
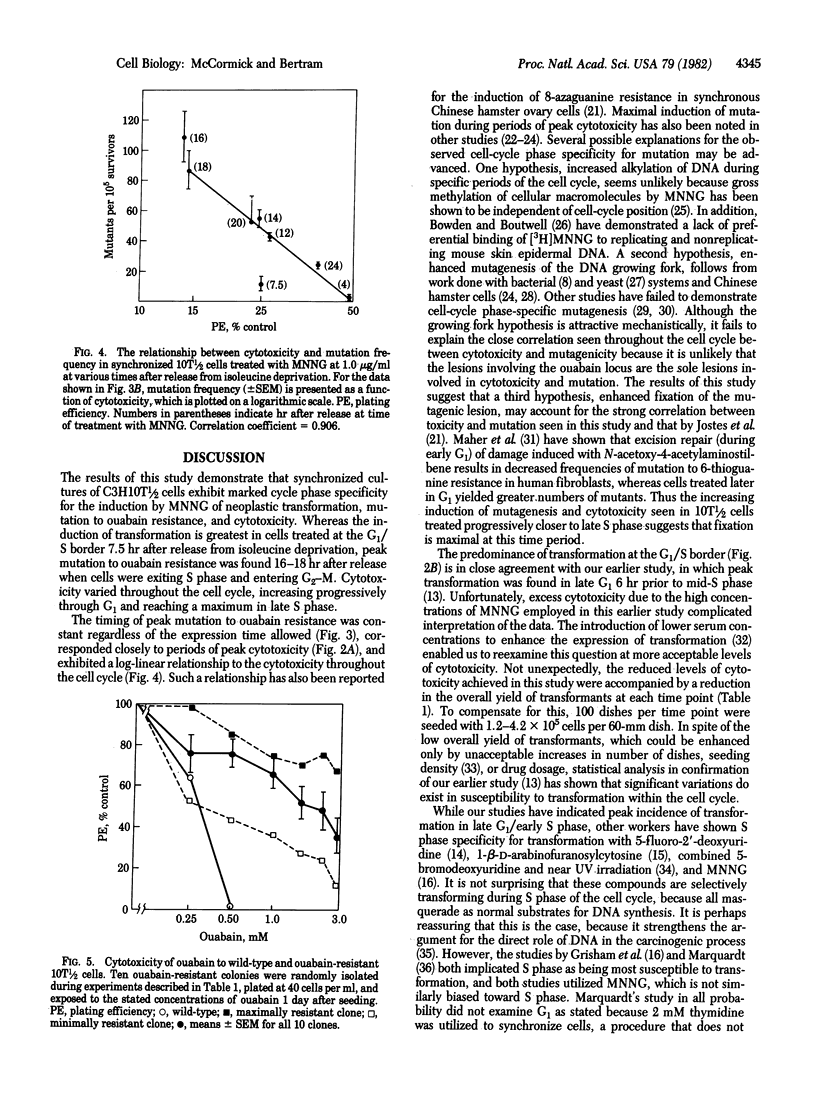
The transformable mouse embryo fibroblast cell line C3H10T 1/2 C18 has been employed to study the induction by the carcinogen N-methyl-N'-nitro-N-nitrosoguanidine (MNNG) of morphological transformation and mutation to ouabain resistance throughout the cell cycle. Cells were synchronized by means of isoleucine deprivation for 24 hr and initiated DNA synthesis with a high degree of synchrony 7.5 hr after release of the isoleucine block. At various intervals throughout the cell cycle cultures were treated with MNNG at 1.0 microgram/ml and the induction of cytotoxicity, morphological transformation, and ouabain-resistant colonies was determined. All three phenomena exhibited marked cell-cycle phase dependency. Maximal induction of transformation occurred in cultured treated 7.5 hr after release from isoleucine deprivation, when the cells were at the G1/S boundary. In contrast, induction of ouabain-resistant colonies was at a minimum at the time of maximal induction of transformation, and peak induction of ouabain resistance did not occur until 16-18 hr after release from the isoleucine block, when cells were in late S phase. A close correlation was observed between the induction of cytotoxicity and of ouabain-resistant mutants. The results suggest that differences exist in the production or cellular processing of the various early lesions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebersold P. M. Mutagenic mechanism of 5-bromodeoxyuridine in Chinese hamster cells. Mutat Res. 1976 Sep;36(3):357–362. doi: 10.1016/0027-5107(76)90245-1. [DOI] [PubMed] [Google Scholar]
- Aebersold P. M. Mutation induction by 5-fluorodeoxyuridine in synchronous Chinese hamster cells. Cancer Res. 1979 Mar;39(3):808–810. [PubMed] [Google Scholar]
- Ames B. N., Durston W. E., Yamasaki E., Lee F. D. Carcinogens are mutagens: a simple test system combining liver homogenates for activation and bacteria for detection. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2281–2285. doi: 10.1073/pnas.70.8.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. C., Ts'o P. O. Relationship between somatic mutation and neoplastic transformation. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3297–3301. doi: 10.1073/pnas.75.7.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. C., Tsutsui T., Ts'o P. O. Neoplastic transformation induced by a direct perturbation of DNA. Nature. 1978 Jul 20;274(5668):229–232. doi: 10.1038/274229a0. [DOI] [PubMed] [Google Scholar]
- Bertram J. S. Effects of serum concentration on the expression of carcinogen-induced transformation in the C3H/10T1/2 CL8 cell line. Cancer Res. 1977 Feb;37(2):514–523. [PubMed] [Google Scholar]
- Bertram J. S., Heidelberger C. Cell cycle dependency of oncogenic transformation induced by N-methyl-N'-nitro-N-nitrosoquanidine in culture. Cancer Res. 1974 Mar;34(3):526–537. [PubMed] [Google Scholar]
- Bertram J. S. Reduction in the formation of carcinogen-induced transformed foci by pencillin G sodium in the C3H/10T1/2 CL8 cell lines. Cancer Lett. 1979 Sep;7(5):289–298. doi: 10.1016/s0304-3835(79)80056-7. [DOI] [PubMed] [Google Scholar]
- Bowden G. T., Boutwell R. K. Studies on the role of stimulated epidermal DNA synthesis in the initiation of skin tumors in mice by N-methyl-N'-nitro-N-nitrosoguanidine. Cancer Res. 1974 Jul;34(7):1552–1563. [PubMed] [Google Scholar]
- Cerdá-Olmedo E., Hanawalt P. C., Guerola N. Mutagenesis of the replication point by nitrosoguanidine: map and pattern of replication of the Escherichia coli chromosome. J Mol Biol. 1968 May 14;33(3):705–719. doi: 10.1016/0022-2836(68)90315-x. [DOI] [PubMed] [Google Scholar]
- Chan G. L., Little J. B. Induction of ouabain-resistant mutations in C3H 10T1/2 mouse cells by ultraviolet light. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3363–3366. doi: 10.1073/pnas.75.7.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez A., Mondal S., Heidelberger C. Probabilistic view of the transformation of cultured C3H/10T1/2 mouse embryo fibroblasts by 3-methylcholanthrene. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7272–7276. doi: 10.1073/pnas.77.12.7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goth-Goldstein R., Burki H. J. Ethylnitrosourea-induced mutagenesis in asynchronous and synchronous Chinese hamster ovary cells. Mutat Res. 1980 Jan;69(1):127–137. doi: 10.1016/0027-5107(80)90182-7. [DOI] [PubMed] [Google Scholar]
- Grisham J. W., Greenberg D. S., Kaufman D. G., Smith G. J. Cycle-related toxicity and transformation in 10T1/2 cells treated with N-methyl-N'-nitro-N-nitrosoguanidine. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4813–4817. doi: 10.1073/pnas.77.8.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger C. Chemical carcinogenesis. Annu Rev Biochem. 1975;44:79–121. doi: 10.1146/annurev.bi.44.070175.000455. [DOI] [PubMed] [Google Scholar]
- Huberman E., Mager R., Sachs L. Mutagenesis and transformation of normal cells by chemical carcinogens. Nature. 1976 Nov 25;264(5584):360–361. doi: 10.1038/264360a0. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Baker M. S., Bertram J. S., Benedict W. F. Cell cycle-specific oncogenic transformation of C3H/10T1/2 clone 8 mouse embryo cells by 1-beta-D-arabinofuranosylcytosine. Cancer Res. 1977 Jul;37(7 Pt 1):2214–2217. [PubMed] [Google Scholar]
- Jones P. A., Benedict W. F., Baker M. S., Mondal S., Rapp U., Heidelberger C. Oncogenic transformation of C3H/10T1/2 clone 8 mouse embryo cells by halogenated pyrimidine nucleosides. Cancer Res. 1976 Jan;36(1):101–107. [PubMed] [Google Scholar]
- Jostes R. F., Bushnell K. M., Dewey W. C. X-ray induction of 8-azaguanine-resistant mutants in synchronous Chinese hamster ovary cells. Radiat Res. 1980 Jul;83(1):146–161. [PubMed] [Google Scholar]
- Kaneko M., Cerutti P. A. Excision of N-acetoxy-2-acetylaminofluorene-induced DNA adducts from chromatin fractions of human fibroblasts. Cancer Res. 1980 Nov;40(11):4313–4319. [PubMed] [Google Scholar]
- Kee S. G., Haber J. E. Cell cycle-dependent induction of mutations along a yeast chromosome. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1179–1183. doi: 10.1073/pnas.72.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy A. R., Fox M., Murphy G., Little J. B. Relationship between x-ray exposure and malignant transformation in C3H 10T1/2 cells. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7262–7266. doi: 10.1073/pnas.77.12.7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolph J. R., Heidelberger C. Chemical carcinogens produce mutations to ouabain resistance in transformable C3H/10T1/2 Cl 8 mouse fibroblasts. Proc Natl Acad Sci U S A. 1979 Feb;76(2):930–934. doi: 10.1073/pnas.76.2.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolph J. R., Telfer N., Heidelberger C. Further evidence that ouabain-resistant variants induced by chemical carcinogens in transformable C3H/10T1/2 Cl 8 mouse fibroblasts are mutants. Mutat Res. 1980 Sep;72(2):295–310. doi: 10.1016/0027-5107(80)90044-5. [DOI] [PubMed] [Google Scholar]
- Marquardt H. Cell cycle dependence of chemically induced malignant transformation in vitro. Cancer Res. 1974 Jul;34(7):1612–1615. [PubMed] [Google Scholar]
- Pederson T. Chromatin structure and the cell cycle. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2224–2228. doi: 10.1073/pnas.69.8.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier L. A., de Serres F. J. Initial National Cancer Institute studies on mutagenesis as a prescreen for chemical carcinogens: an appraisal. J Natl Cancer Inst. 1979 Apr;62(4):919–926. [PubMed] [Google Scholar]
- Reznikoff C. A., Bertram J. S., Brankow D. W., Heidelberger C. Quantitative and qualitative studies of chemical transformation of cloned C3H mouse embryo cells sensitive to postconfluence inhibition of cell division. Cancer Res. 1973 Dec;33(12):3239–3249. [PubMed] [Google Scholar]
- Reznikoff C. A., Brankow D. W., Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973 Dec;33(12):3231–3238. [PubMed] [Google Scholar]
- Riddle J. C., Hsie A. W. An effect of cell-cycle position on ultraviolet-light-induced mutagenesis in Chinese hamster ovary cells. Mutat Res. 1978 Dec;52(3):409–420. doi: 10.1016/0027-5107(78)90179-3. [DOI] [PubMed] [Google Scholar]
- Rubin H. Is somatic mutation the major mechanism of malignant transformation? J Natl Cancer Inst. 1980 May;64(5):995–1000. [PubMed] [Google Scholar]
- Studzinski G. P., Lambert W. C. Thymidine as a synchronizing agent. I. Nucleic acid and protein formation in synchronous HeLa cultures treated with excess thymidine. J Cell Physiol. 1969 Apr;73(2):109–117. doi: 10.1002/jcp.1040730204. [DOI] [PubMed] [Google Scholar]
- Terzaghi M., Little J. B. X-radiation-induced transformation in a C3H mouse embryo-derived cell line. Cancer Res. 1976 Apr;36(4):1367–1374. [PubMed] [Google Scholar]
- Thompson L. H. Mutant isolation. Methods Enzymol. 1979;58:308–322. doi: 10.1016/s0076-6879(79)58147-6. [DOI] [PubMed] [Google Scholar]
- Tsutsui T., Barrett J. C., Ts'o P. O. Morphological transformation, DNA damage, and chromosomal aberrations induced by a direct DNA perturbation of synchronized Syrian hamster embryo cells. Cancer Res. 1979 Jul;39(7 Pt 1):2356–2365. [PubMed] [Google Scholar]
- Watanabe M., Horikawa M. Analyses of differential sensitivities of synchronized HeLa S3 cells to radiations and chemical carcinogens during the cell cycle. Par V. Radiation- and chemical carcinogen-induced mutagenesis. Mutat Res. 1980 Jul;71(2):219–231. doi: 10.1016/0027-5107(80)90074-3. [DOI] [PubMed] [Google Scholar]


