Abstract
Objective:
To investigate the long-term outcome of patients with spinal cord infarct (SCI) and identify prognostic predictors.
Methods:
We reviewed 115 patients with SCI treated between 1990 and 2007. Severity of impairment was defined using the American Spinal Injury Association (ASIA) scoring. Functional outcome endpoints were ambulatory status, need for bladder catheterization, and pain.
Results:
Mean age was 64 years; 72 (62.6%) patients were men. A total of 45% of infarcts were perioperative (69% aortic surgeries). A total of 68% reached maximal deficit within 1 hour (mean = 5 hours). Impairment at nadir was ASIA A 23%, B 26%, C 14%, and D 37%. A total of 75/93 (81%) patients studied with MRI had cord signal abnormality. At nadir, 81% required wheelchair, 86% required catheterization, and 32% had pain. At last follow-up (mean = 3 years), 23% had died. Among survivors, 42% required a wheelchair, 54% required catheterization, and 29% had pain upon last follow-up. Of 74 patients using a wheelchair at hospital dismissal, 41% were walking by final follow-up. Of 83 patients catheterized at dismissal, 33% were catheter-free at last follow-up. Older age (p < 0.0001), increased severity of impairment at nadir (p = 0.02), and peripheral vascular disease (p = 0.003) were independent risk factors for mortality. Severe impairment (ASIA A/B) at nadir predicted wheelchair use (p < 0.0001) and bladder catheterization (p < 0.0001) at last follow-up.
Conclusions:
Gradual improvement in not uncommon after spinal cord infarction and it may continue long after hospital dismissal. While severe impairment at nadir is the strongest predictor of poor functional outcome, meaningful recovery is also possible in a substantial minority of these patients.
Clinicians typically think of spinal cord infarction (SCI) as a devastating complication of aortic surgery. However, neurologists can encounter this disease in other clinical settings.1–3 It remains a clinical diagnosis and presents most often as an abrupt onset anterior spinal artery syndrome.4–8 MRI can confirm the presence, location, and extension of the infarction, but its diagnostic yield depends on the timing and quality (i.e., absence of artifact) of imaging.3,7,9,10
Outcome is often considered poor but the literature assessing long-term outcome in SCI is actually very limited and insufficient to guide prognostication. Previous studies on long-term outcome after SCI have assessed relatively small populations (varying from 8 to 57 patients)4,5,11,12 and efforts to identify clinical and radiologic prognostic factors have led to inconsistent and sometimes contradictory results.
The purpose of this study was to determine the causes, characteristics of clinical presentation, functional outcome, and prognostic indicators in a large cohort of patients with SCI.
METHODS
Identification of patients and diagnostic criteria.
We first performed a search to identify the terms “anterior spinal artery syndrome,” “spinal cord infarct,” “spinal cord ischemia,” “ischemic myelopathy,” “spinal cord hemorrhage,” and “hematomyelia” in the electronic database of patients treated at Mayo Clinic (Rochester, MN) between January 1990 and August 2007. A total of 461 patients were found using these terms and the comprehensive medical record was reviewed by one neurologist (C.E.R.). Patients were included if they had an acute onset spinal cord syndrome (anterior, posterior, central cord) with no alternative explanation. Patients were excluded from the study if their symptoms were related to a vascular malformation (n = 33), hemorrhage (n = 53), transient symptoms (spinal cord TIA) (n = 16), if there was any uncertainty about the diagnosis of infarct (question of tumor, compression, myelitis, vasculitis, or some other diagnosis) (n = 160), or if the patient had come from an outside hospital without enough information available regarding their examination and assessment (n = 84). When the diagnostic certainty or stroke mechanism was in question, the case was reviewed by a second neurologist (A.A.R.). After all exclusions, 115 patients were entered into our study.
Data collection and evaluation.
We categorized the cases into etiologic groups: 1) perioperative spinal cord infarcts (aortic and nonaortic surgeries), 2) aortic dissection without surgery, 3) aortic aneurysm or atheroma, 4) hypoperfusion, based on marked hypotension (systolic blood pressure ≤70 mm Hg) at the onset, 5) idiopathic, and 6) other, subdivided into embolic, vertebrobasilar disease, and vasospasm.
For each patient, we collected information regarding clinical manifestations at presentation, comorbid medical conditions, imaging and other testing, and interventions. Impairment at maximal deficit (i.e., the nadir) was measured using the American Spinal Injury Association impairment scale (table e-1 on the Neurology® Web site at www.neurology.org).13 All neuroradiologic reports of MRI scans were collected and the actual images were reviewed when available. We tabulated the timing of the scan with respect to the onset of deficits, presence of intrinsic spinal cord lesion on fluid-attenuated inversion recovery, T2-weighted sequence, and diffusion-weighted imaging, the precise location of the lesion, and the number of spine levels involved.
Functional status was assessed by the level of ambulation (normal, gait aid, or wheelchair), the need for catheterization, and the presence of pain recorded at the time of maximal deficit, at hospital dismissal, and at intermediate (<6 months) and long-term follow-up (>6 months after symptom onset). All known deaths were recorded, as well as the cause of death when available.
Statistical analysis.
For the individual outcome measures related to final functional outcome, univariate analysis was performed using Fisher exact test for nominal variables and nonparametric Wilcoxon rank sum test for quantitative variables. For “final follow-up,” the last known functional status was used for all patients who did not die in the hospital. To improve power, American Spinal Injury Association (ASIA) impairment scores were dichotomized into severely impaired (A+B) and mildly to moderately impaired (C+D).
Variables with p values <0.1 on univariate analysis were then analyzed using multivariate logistic regression. Multivariate models also adjusted for length of follow-up. All known deaths were analyzed using Kaplan-Meier survival analysis. Variables that might affect mortality were analyzed using Cox proportional hazards regression. A multivariate model was created in a manner similar to that described for functional outcome. p Values <0.05 were considered statistically significant. Analysis was performed using JMP statistical software (version 8, SAS Institute, Cary, NC).
Standard protocol approvals, registration, and patient consents.
The Mayo Clinic institutional review board approved the study design. All patients included had provided consent to allow the use of their medical records for research purposes.
RESULTS
Mean age was 64 years (median 69 years; range 9–93). There were 72 male and 43 female patients. A total of 45% of the infarcts occurred perioperatively (69% aortic surgery and 31% nonaortic surgery), 7% with aortic aneurysm/atheroma, 7% with aortic dissection, 7% with hypotension, 13% with other mechanisms, and 21% idiopathic (figure e-1). The evaluation of idiopathic cases, patients' comorbidities, and the tempo of symptoms at presentation are presented in the e-Appendix.
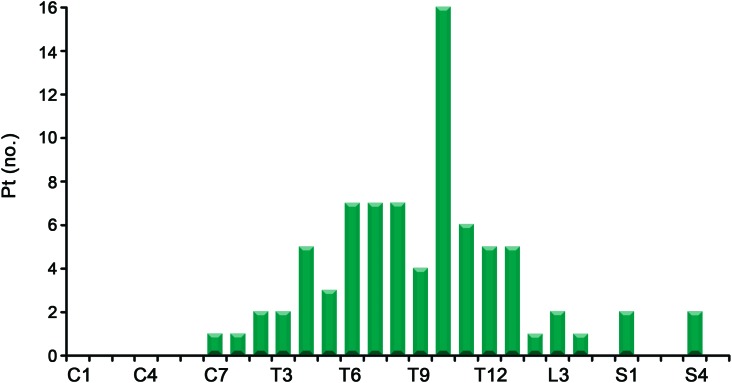
On initial evaluation, the severity of impairment was graded as ASIA A in 26 (23%), ASIA B in 30 (26%), ASIA C in 16 (14%), and ASIA D in 43 (37%) patients. At their nadir, 93 (81%) patients were requiring a wheelchair for mobility and 99 (86%) were catheterized. A total of 37 (32%) patients complained of pain on their initial examination: 13 patients had pain isolated to their back, 20 patients had pain involving the legs, and 4 had pain in other areas, including perianal pain and arm pain. On examination, 80 patients had documented sensory levels (figure 1). Unilateral or bilateral Babinski sign was present in 34 (31%) of the 109 patients in whom plantar stimulation was documented.
Figure 1. Sensory level available for 80 patients (1 listed as “upper thoracic” not shown).
Twenty-five patients had no sensory level found; 10 were not examined for level.
CSF.
CSF was examined in 27 patients. It was normal in the majority of cases. Twelve patients had modestly elevated protein (median 58, range 49–163), but a normal cell count. Two patients had both elevated protein and cells (1 postsubarachnoid hemorrhage and 1 with meningococcemia).
MRI.
MRI was performed in 93 patients and 75 (81%) of those had cord abnormalities (increased T2 signal or atrophy) on initial or repeat MRI. Twenty-six patients had a normal MRI (mean of 15.4 days from symptom onset), 13 of whom underwent repeat imaging; 8 of these patients had evidence of ischemia on repeat MRI. In the cases with an initial negative MRI and diagnostic findings later, the average time to initial MRI was 3.1 days (median 1 day), and the average time to repeat MRI was 13.2 days. Among the 18 patients with no MRI abnormalities ever documented, 11 had a defined mechanism (9 postoperative, 1 with aortic aneurysm, 1 with atrial fibrillation and an aortic valve mass) and all but 1 of the other 7 had extensive diagnostic evaluations (e-Appendix).
The majority of patients (64%) had their highest MRI abnormality in the thoracic region, followed by cervical (10%) and lumbar/conus (6%). Thoracic lesions often extended down to the conus (30/57 [53%]) as illustrated in figure 2. Patients with severe impairment at nadir (ASIA A or B) were more likely to have MRI evidence of infarction than patients with less severe deficits (91% vs 71%; p = 0.018). In fact, 78% of the negative MRIs corresponded to patients whose deficits were mild to moderate (ASIA C and D). Diffusion-weighted images were obtained in a very small minority of cases and therefore these sequences were not analyzed further.
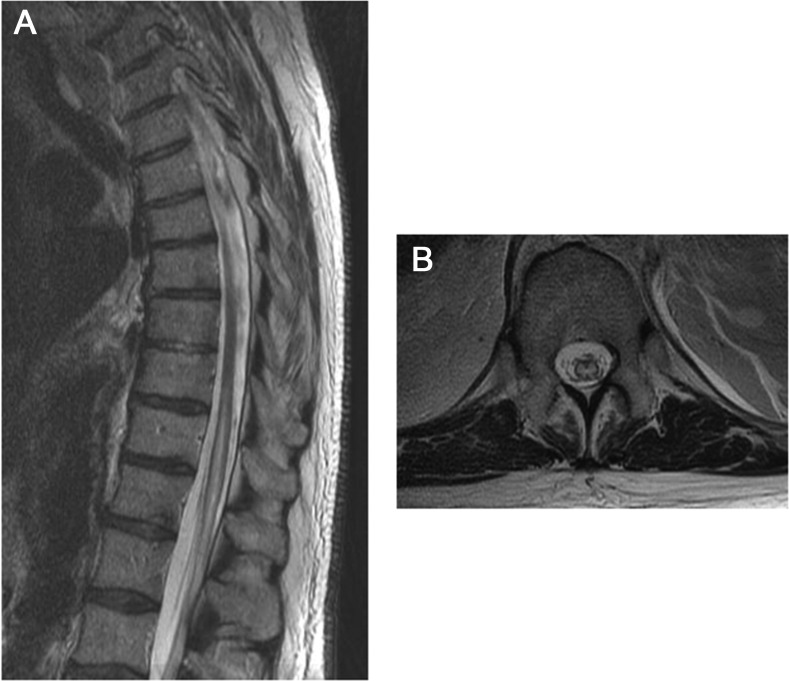
Figure 2. MRI.
The sagittal MRI (A) shows abnormal increased T2 signal within the central gray matter of the thoracic cord, extending from mid-T10 through the conus. There is associated cord expansion. The axial MRI (B) shows an axial view of the same infarct, demonstrating the classic “owl's eye” pattern of increased T2 signal within the gray matter.
Prognosis.
Patients were in the hospital for an average of 18 days (median 13 days, range 1–118 days) following symptom onset. Long-term follow-up data were available in 70% of survivors (mean 3 years, range 2 days–22 years). Upon last follow-up, 26 (23%) patients had died. Among survivors, at their last follow-up 37 (42%) were using a wheelchair, 23 (26%) were using a gait aid (cane or walker), 29 (33%) walked unaided, 48 (54%) needed a bladder catheter, and 26 (29%) patients still had significant pain.
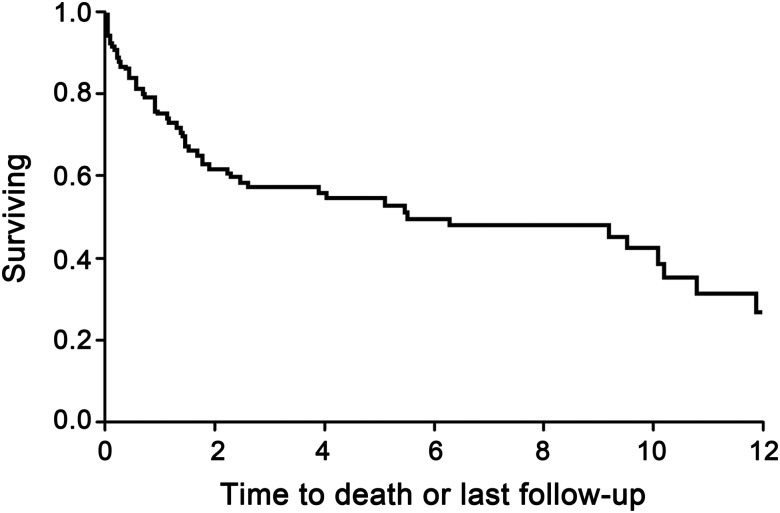
Information on functional status at nadir, hospital dismissal, intermediate follow-up (mean 3.4 months), and long-term follow-up (mean 4.8 years) is outlined in table 1. Long-term follow-up was available for 71 patients, with 14 others having died prior to 6 months, and 30 lost to follow-up. We are aware of the deaths of 57 patients to date, including those patients who were known to have died by last follow-up, and those who died years after their last follow-up. Using a Kaplan-Meier survival curve, there was a median survival of 6.1 years (95% confidence interval [CI] 2.3–10.1), with a 5-year estimated survival of 55%, and a 10-year estimated survival of 42.5% (figure 3).
Table 1.
Functional status at different intervals from symptom onseta
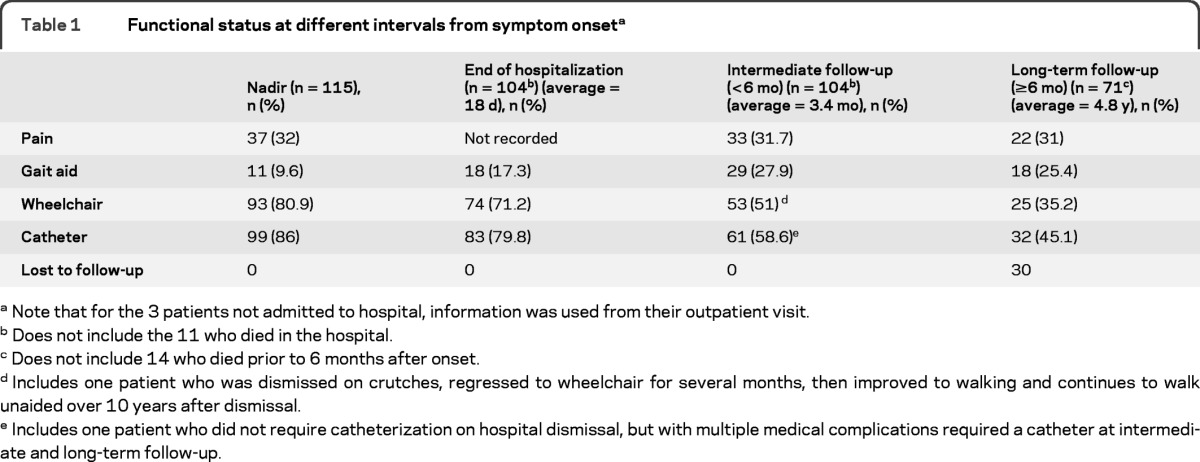
Note that for the 3 patients not admitted to hospital, information was used from their outpatient visit.
Does not include the 11 who died in the hospital.
Does not include 14 who died prior to 6 months after onset.
Includes one patient who was dismissed on crutches, regressed to wheelchair for several months, then improved to walking and continues to walk unaided over 10 years after dismissal.
Includes one patient who did not require catheterization on hospital dismissal, but with multiple medical complications required a catheter at intermediate and long-term follow-up.
Figure 3. Kaplan-Meier survival curve.
We also examined the functional outcome of a subset of the more severely impaired patients. Of the 83 patients who required a catheter at dismissal, 23 (28%) no longer required a catheter at intermediate follow-up. By final follow-up, 4 more patients no longer required a catheter (using scheduled voiding and Valsalva instead), though one of them still had incontinence. Similarly, of the 74 patients in wheelchairs at dismissal, 4 (5%) were walking unaided and 18 (24%) walked with an aid at intermediate follow-up. By final follow-up, 7 more were walking with an aid, and 1 more was walking unaided. Pain had a less clear pattern. Of the 37 patients who had pain initially, only 17 had pain at intermediate follow-up. However, 16 previously pain-free patients reported pain at that visit. In fact, of the 22 who had pain at final follow-up, 11 had not complained of pain initially.
Predictors of poor outcome.
The results from univariate analysis are presented in table 2. Severe impairment (ASIA A or B) on initial examination, absence of Babinski sign, presence of sensory level, longitudinally extensive MRI lesions, and MRI lesions with highest level in the thoracic region were associated with wheelchair and catheter use at final follow-up. Age, gender, and comorbidities were not associated with functional outcome. When we adjusted for time to last follow-up using multivariate logistic regression, severity of impairment was the only variable associated with requiring wheelchair (odds ratio [OR] = 13.4, 95% CI 5.1–39.0, p < 0.0001) or bladder catheter (OR = 15.7, 95% CI 5.9–48.5, p < 0.0001) at final follow-up. There were no significant predictors for pain.
Table 2.
Univariate analysis of predictors of long-term outcomes following spinal cord infarcta
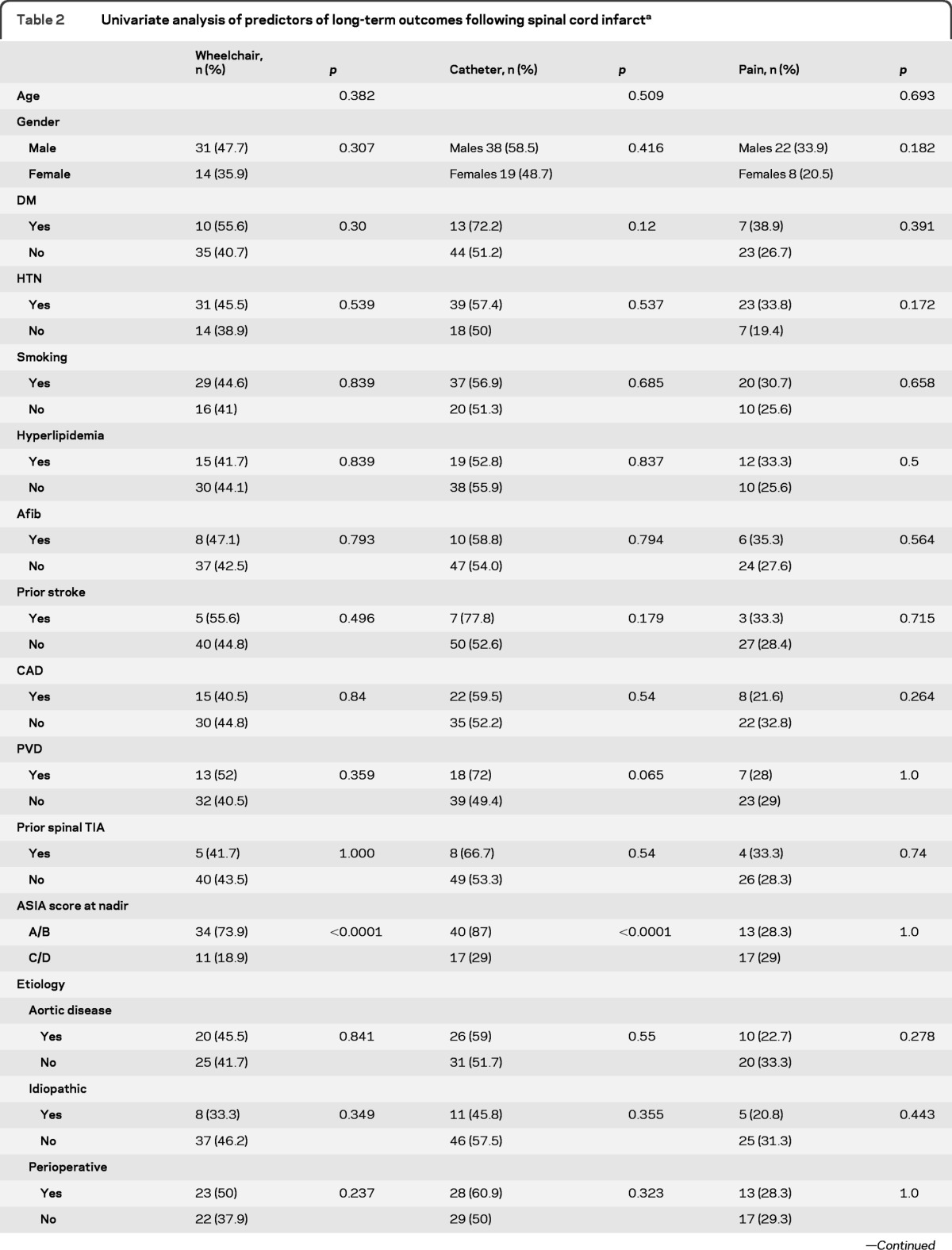
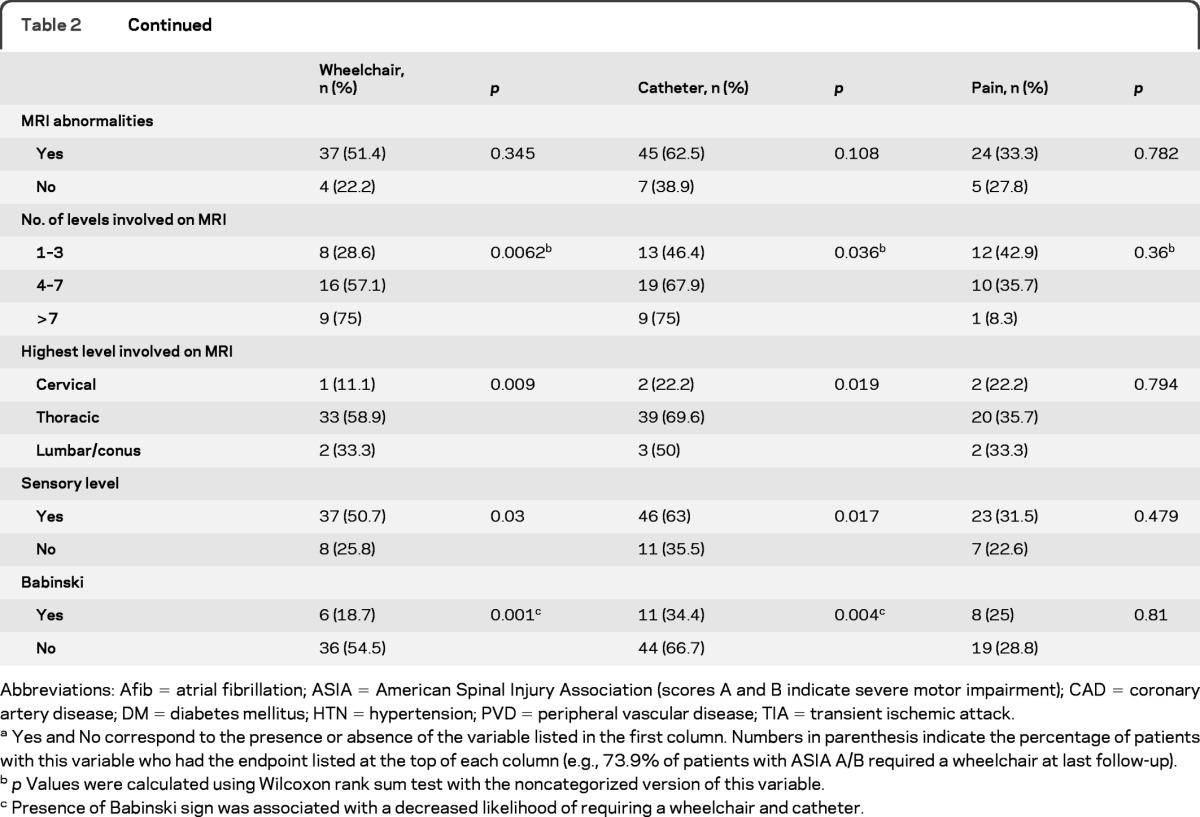
Abbreviations: Afib = atrial fibrillation; ASIA = American Spinal Injury Association (scores A and B indicate severe motor impairment); CAD = coronary artery disease; DM = diabetes mellitus; HTN = hypertension; PVD = peripheral vascular disease; TIA = transient ischemic attack.
Yes and No correspond to the presence or absence of the variable listed in the first column. Numbers in parenthesis indicate the percentage of patients with this variable who had the endpoint listed at the top of each column (e.g., 73.9% of patients with ASIA A/B required a wheelchair at last follow-up).
p Values were calculated using Wilcoxon rank sum test with the noncategorized version of this variable.
Presence of Babinski sign was associated with a decreased likelihood of requiring a wheelchair and catheter.
Older age and diabetes mellitus were independently associated with mortality by final follow-up. Because we had information on many deaths that took place years after final follow-up, we used Cox proportional hazard regression to further analyze risk factors associated with death while taking into account those lost to follow-up. On univariate analysis, cardiovascular risk factors (coronary disease, hypertension, and smoking), peripheral vascular disease, older age, and severity of impairment were all associated with an increased risk of death. On multivariate analysis, age (hazard ratio [HR] 1.1, 95% CI 1.0–1.1, p < 0.0001), increased severity of impairment by ASIA scoring (HR 2.0, 95% CI 1.1–3.6, p = 0.02), and the presence of peripheral vascular disease (HR 2.4, 95% CI 1.4–4.3, p = 0.003) remained independently associated with death.
DISCUSSION
Neurologists are often asked to prognosticate after a SCI and often express little hope for recovery. However, there is surprisingly scant published information on the long-term prognosis of SCI. The detailed analysis of our large series of patients with SCI allowed us to quantify the chances of recovery and to identify prognostic indicators. Long-term outcome can remain poor in patients with complete or nearly complete syndromes, but our most remarkable and relatively unexpected finding is that delayed functional recovery is possible and, in fact, not infrequent. Although initially severe impairment is the strongest predictor of poor outcome, substantial functional recovery may occur over time even in patients with very severe deficits upon hospital discharge and at early follow-up.
Nearly half of our patients had severe impairment (ASIA grades A and B) at their worst. This is comparable to 30%–47% noted by previous larger studies that used the ASIA scoring.4,11 MRI abnormalities were most prevalent below T7 (77%) and often involved the conus (51%).7,10,14 Of note, we had 18 patients with no abnormalities on MRI, performed at a median of 3 days (range 1–120) following infarct. Five of these had repeat imaging that was also negative. This finding is similar to what was reported in a prospective study of MRI in patients with SCI, where 4 (14%) of their patients had negative MRI studies, even with repeat imaging.10 Thus, although MRI is very useful in evaluating spinal cord infarcts, it should not be used as the sole criterion for diagnosis.
The most frequent mechanism of infarction in our patients was surgical manipulation of the aorta or its branches. Thoracic aortic surgery appears to be associated with the highest risk, as rates of SCI of 5%–21% have been reported after open thoracic aortic aneurysm surgery and 3%–12% after endovascular repair.15,16 We also had 16 patients with SCI following nonaortic surgery, including vertebral laminectomy/fusion,17 coronary artery bypass graft,18–20 and femoral-arterial bypass.21,22 It is unlikely that the higher rate of postoperative cases in our cohort compared with previously published series had a major impact on outcome because etiology was not associated with prognosis in our analysis. Our rate of idiopathic cases was lower than in some previous studies.3,11
Our study provides novel and clinically useful data on long-term outcome. At their final follow-up visit, 58% of the survivors were ambulating with or without a gait aid. This is a better outcome than the 25%–47% reported by most previous studies with fewer patients and a shorter follow-up.4,5,14,23 Only one study of 57 patients has reported a better long-term outcome than our series; in that study, 78% of survivors were ambulating after a mean follow-up of 4.5 years.11 The difference in outcome may relate to patient selection, as we had a greater percentage of severely impaired patients in our cohort (49% ASIA grade A and B vs 30% in that previous series).11 Our study reports on a much larger population of SCI patients and therefore it is more likely to be a reliable representation of the outcome of this disease. It also provides a measure of functional improvement over time by presenting data at different time points and offers a more detailed analysis of clinical and radiologic prognosticators of outcome.
Chances of recovery in our patients were adversely affected by the presence of more severe deficits at nadir, but not invariably so. The pace of functional recovery varied. While in most patients who regained function the improvement was already noticed in the first 6 months, gradual recovery often continued to occur over a much longer time.
The severity of impairment on initial examination was the best prognostic indicator of poor functional outcome.4,10,11,14,23 Unlike some previous studies, however, we did not find age4,14 or gender11 to be predictors of functional outcome. We also did not find evidence for different prognosis depending on the cause of ischemia. Presence of Babinski sign on initial examination was associated with an improved functional outcome on univariate analysis. Possibly the presence of Babinski indicates less complete involvement of the anterior horns, and therefore a less complete cord lesion. Previous studies6,22 have noted that patients with greater lower motor neuron involvement (anterior horn dysfunction) had a tendency to fare worse than those with an upper motor neuron, incomplete spastic paraplegia. In our series, patients with longitudinally extensive ischemic changes on MRI had worse outcome, but these findings were typically seen in patients with severe neurologic deficits and their association with poor outcome was no longer significant when the analysis controlled for clinical severity.
Our study confirms that the mortality rate after SCI is high (23% cumulative mortality at last follow-up), as observed in previous smaller series (9%–22%).4,11 Most deaths occurred early after the SCI. Although our cohort is the largest on record, it is still insufficient to assess whether there was a change in mortality rate over the study period. Older age, severe neurologic impairment, and peripheral vascular disease were independently associated with increased mortality in our cohort. The negative impact of age and severity of initial neurologic deficits on mortality in patients with SCI parallels what has been reported in patients with traumatic spinal cord injury.24
The main strength of this study is the size of the population analyzed, which is essentially twice as large as any previously reported. The majority of our patients had long-term follow-up (mean 4.8 years) thus supporting the validity of our assessment of functional outcome. We exclusively included patients with confirmed ischemic SCI. Several previous studies included hemorrhagic infarcts, vascular malformations, transient ischemic attacks, and vasculitis. We excluded these cases to optimize the homogeneity of our cohort and avoid confounding our analysis. We also excluded any case in which the clinician questioned the certainty of the diagnosis of SCI.
Our study also has several limitations, mostly inherent to the retrospective design. We did not have data on the patients' functional status before the SCI. The timing and duration of follow-up was not uniform and we cannot rule out the possibility of bias among patients lost to follow-up (i.e., these patients could have had a different outcome than the average patient with longer follow-up). The timing of MRI scanning was variable and diffusion-weighted sequences were not routinely obtained during the study period. We did not have electrophysiologic data in the great majority of our patients and thus could not examine their prognostic value. The value of therapeutic interventions could not be evaluated because of the lack of treatment standardization. Our population is representative of SCI treated at a tertiary referral center, but we cannot be certain that our findings can be extrapolated to patients with SCI treated in a different type of hospital. Finally, it is possible that a mechanism other than ischemia could have caused some of the deficits/lesions noted, but this is very unlikely because of our strict inclusion criteria and detailed analysis of each case.
Our data show that patients with SCI can recover function over time. The prognosis is better for patients with less severe deficits at onset, but even patients with complete paraplegia and sensory loss at nadir can improve substantially over months to years. Therefore, when evaluating patients with SCI for prognostication, clinicians should avoid categorical pessimistic statements and emphasize the possibility of functional recovery to encourage patients to establish a long-term commitment toward their rehabilitation.
Supplementary Material
GLOSSARY
- ASIA
American Spinal Injury Association
- CI
confidence interval
- HR
hazard ratio
- OR
odds ratio
- SCI
spinal cord infarct
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Robertson: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, statistical analysis. Dr. Brown: drafting/revising the manuscript, analysis or interpretation of data, study supervision. Dr. Wijdicks: drafting/revising the manuscript, study supervision. Dr. Rabinstein: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, study supervision.
DISCLOSURE
Dr. Robertson reports no disclosures. Dr. Brown receives publishing royalties for Handbook of Stroke (Lippincott Williams & Wilkins, 2005); serves on the Board of Directors of the Neilsen Foundation and on the Medical Advisory Board of the Brain Aneurysm Foundation; and receives research support from the NIH/NINDS. Dr. Wijdicks receives honoraria from Springer for his role as the editor-in-chief of Neurocritical Care and receives royalties from books published with Oxford University Press. Dr. Rabinstein serves as Section Editor for Year Book Neurology and Neurosurgery Section Editor for Neurocritical Care; has received research support from CardioNet and Boston Scientific; and receives royalties for books published with Elsevier.
REFERENCES
- 1. Sandson TA, Friedman JH. Spinal cord infarction: report of 8 cases and review of the literature. Medicine 1989;68:282–292 [PubMed] [Google Scholar]
- 2. de Seze J, Stojkovic T, Breteau G, et al. Acute myelopathies: clinical, laboratory and outcome profiles in 79 cases. Brain 2001;124:1509–1521 [DOI] [PubMed] [Google Scholar]
- 3. Novy J, Carruzzo A, Maeder P, Bogousslavsky J. Spinal cord ischemia: clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Arch Neurol 2006;63:1113–1120 [DOI] [PubMed] [Google Scholar]
- 4. Salvador de la Barrera S, Barca-Buyo A, Montoto-Marques A, et al. Spinal cord infarction: prognosis and recovery in a series of 36 patients. Spinal Cord 2001;39:520–525 [DOI] [PubMed] [Google Scholar]
- 5. Cheshire WP, Santos CC, Massey EW, Howard JF., Jr Spinal cord infarction: etiology and outcome. Neurology 1996;47:321–330 [DOI] [PubMed] [Google Scholar]
- 6. Kim SW, Kim RC, Choi BH, Gordon SK. Non-traumatic ischaemic myelopathy: a review of 25 cases. Paraplegia 1988;26:262–272 [DOI] [PubMed] [Google Scholar]
- 7. Weidauer S, Nichtweiss M, Lanfermann H, Zanella FE. Spinal cord infarction: MR imaging and clinical features in 16 cases. Neuroradiology 2002;44:851–857 [DOI] [PubMed] [Google Scholar]
- 8. Tator CH, Koyanagi I. Vascular mechanisms in the pathophysiology of human spinal cord injury. J Neurosurg 1997;86:483–492 [DOI] [PubMed] [Google Scholar]
- 9. Krings T, Lasjaunias PL, Hans FJ, et al. Imaging in spinal vascular disease. Neuroimaging Clin N Am 2007;17: 57–72 [DOI] [PubMed] [Google Scholar]
- 10. Masson C, Pruvo JP, Meder JF, et al. Spinal cord infarction: clinical and magnetic resonance imaging findings and short term outcome. J Neurol Neurosurg Psychiatry 2004;75:1431–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nedeltchev K, Loher TJ, Stepper F, et al. Long-term outcome of acute spinal cord ischemia syndrome. Stroke 2004;35:560–565 [DOI] [PubMed] [Google Scholar]
- 12. Pelser H, van Gijn J. Spinal infarction: a follow-up study. Stroke 1993;24:896–898 [DOI] [PubMed] [Google Scholar]
- 13. Maynard FM, Jr, Bracken MB, Creasey G, et al. International Standards for Neurological and Functional Classification of Spinal Cord Injury: American Spinal Injury Association. Spinal Cord 1997;35:266–274 [DOI] [PubMed] [Google Scholar]
- 14. Cheng MY, Lyu RK, Chang YJ, et al. Spinal cord infarction in chinese patients: clinical features, risk factors, imaging and prognosis. Cerebrovasc Dis 2008;26:502–508 [DOI] [PubMed] [Google Scholar]
- 15. Iseli E, Cavigelli A, Dietz V, Curt A. Prognosis and recovery in ischaemic and traumatic spinal cord injury: clinical and electrophysiological evaluation. J Neurol Neurosurg Psychiatry 1999;67:567–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kawaharada N, Morishita K, Kurimoto Y, et al. Spinal cord ischemia after elective endovascular stent-graft repair of the thoracic aorta. Eur J Cardiothorac Surg 2007;31:998–1003 [DOI] [PubMed] [Google Scholar]
- 17. Gravereaux EC, Faries PL, Burks JA, et al. Risk of spinal cord ischemia after endograft repair of thoracic aortic aneurysms. J Vasc Surg 2001;34:997–1003 [DOI] [PubMed] [Google Scholar]
- 18. Hobai IA, Bittner EA, Grecu L. Perioperative spinal cord infarction in nonaortic surgery: report of three cases and review of the literature. J Clin Anesth 2008;20:307–312 [DOI] [PubMed] [Google Scholar]
- 19. Rossi D, Goodwin D, Cruzzavala J. Spinal cord infarction following coronary artery bypass grafting surgery. W V Med J 2008;104:24–25 [PubMed] [Google Scholar]
- 20. Harris RE, Reimer KA, Crain BJ, et al. Spinal cord infarction following intraaortic balloon support. Ann Thorac Surg 1986;42:206–207 [DOI] [PubMed] [Google Scholar]
- 21. Geyer TE, Naik MJ, Pillai R. Anterior spinal artery syndrome after elective coronary artery bypass grafting. Ann Thorac Surg 2002;73:1971–1973 [DOI] [PubMed] [Google Scholar]
- 22. Pashkovskoya I, Smith CE. Spinal cord infarction and paraplegia after peripheral vascular surgery with spinal anesthesia. J Clin Anesth 2004;16:440–444 [DOI] [PubMed] [Google Scholar]
- 23. Little JW, Goldstein B, Gitter A, Haselkorn JK. Spinal cord infarction: varying degrees of upper and lower motoneuron dysfunction. J Spinal Cord Med 1996;19:242–248 [DOI] [PubMed] [Google Scholar]
- 24. Furlan JC, Fehlings MG. The impact of age on mortality, impairment, and disability among adults with acute traumatic spinal cord injury. J Neurotrauma 2009;26:1707–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.