Abstract
Study Objectives:
Investigate the relationship between gestational age and weight for gestational age and sleep apnea diagnosis in a cohort of children aged up to 6 years old.
Design:
A cohort study, using record linked population health data.
Setting:
New South Wales, Australia.
Participants:
398,961 children, born between 2000 and 2004, aged 2.5 to 6 years.
Measurements:
The primary outcome was sleep apnea diagnosis in childhood, first diagnosed between 1 and 6 years of age. Children with sleep apnea were identified from hospital records with the ICD-10 code G47.3: sleep apnea, central or obstructive.
Results:
A total of 4,145 (1.0%) children with a first diagnosis of sleep apnea were identified. Mean age at first diagnosis was 44.2 months (SD 13.9). Adenoidectomy, tonsillectomy, or both were common among the children diagnosed with sleep apnea (85.6%). Children born preterm compared to term were significantly more likely to be diagnosed with sleep apnea (< 32 weeks versus term hazard ratio 2.74 [95% CI: 2.16, 3.49]) this remained even after adjustment for known confounding variables. Children born small for gestational age were not at increased risk of sleep apnea compared to children born appropriate for gestational age, hazard ratio 0.95 (95% CI 0.86-1.06).
Conclusions:
This is the largest study investigating preterm birth and sleep apnea diagnosis and suggests that diagnosis of sleep disordered breathing is more prevalent in children born preterm, but not those who are small for gestational age.
Citation:
Raynes-Greenow CH; Hadfield RM; Cistulli PA; Bowen J; Allen H; Roberts CL. Sleep apnea in early childhood associated with preterm birth but not small for gestational age: a population-based record linkage study. SLEEP 2012;35(11):1475-1480.
Keywords: Premature infant, apnea, record linkage, cohort study
INTRODUCTION
There has been growing interest in the relationship between perinatal risk factors for childhood sleep disordered breathing and the subsequent child and adult health outcomes. Sleep disordered breathing (SDB), or more specifically obstructive sleep apnea (OSA), is a condition of recurrent upper-airway obstruction with intermittent nocturnal hypoxia and may occur in both children and adults. In children, OSA has been associated with long-term effects including impaired daytime functioning, behavioral problems, lower educational attainment, hypertension, and growth failure.1,2 Symptoms may include snoring or oral breathing in children and frequent night waking, thus causing excessive daytime sleepiness and poor functioning. However, OSA often remains undiagnosed, especially in children. The population burden estimates vary between 0.7% to 13.0%.3 The most common treatment for OSA for children is surgery—tonsillectomy, adenoidectomy, or adenotonsillectomy.4 Although these procedures are associated with some risks, in many cases the OSA discontinues and the related symptoms improve or resolve completely.3 Hence identification of children with OSA can have both an immediate impact on their life and the life of their families and can reduce the risk of poor health outcomes in adulthood.
Preterm birth and being small for gestational age have been associated with a wide range of short and long term health outcomes.5 Early investigations of preterm birth and OSA found associations between children who have been born preterm, small for gestational age, or to mothers with pregnancy-related morbidity and sleep disorders,6 with effect sizes between 2 and 3. Most of these studies have been small7,8 or retrospective9 with wide confidence intervals. More recently, Calhoun proposed that lower socioeconomic status should be considered a risk factor for screening for SDB especially among children who were born preterm.6 Current recommendations by the American Academy of Pediatrics include screening children for snoring during usual healthcare visits.10
The underlying association between preterm birth and childhood OSA is not well understood.3 The suggested mechanisms include dento-facial development and dental arch morphology4 that result in airway dimension changes11 and hence obstruction. This is evident in the high prevalence of sleep apnea and congenital anomalies associated with upper airway abnormalities12 such as cleft-lip/palate defects and associated syndromes. The association between environmental and birth-related factors also have been investigated; childhood metabolic disease resulting in anatomical structural changes may also underlie childhood OSA.3
The primary aim of this study was to investigate the relationship between gestational age and weight for gestational age and sleep apnea in children aged between one and 6 years. The secondary aim was to examine other perinatal risk factors for the development of sleep apnea in childhood.
METHODS
New South Wales (NSW) is the most populous state of Australia with a current population of > 7.0 million and > 90,000 births per annum. Probabilistic record linkage enables the identification of multiple records from a variety of sources for one individual over time. For example, hospital admissions relating to an infant can be longitudinally linked from birth to all subsequent infant and child hospitalizations.
Study Design and Population
This was a longitudinal, population-based study including all live births in NSW during the period 2000 to 2004, with follow-up of infant hospitalizations for up to a maximum of 6 years of life; flow diagram is shown in Figure 1.
Figure 1.
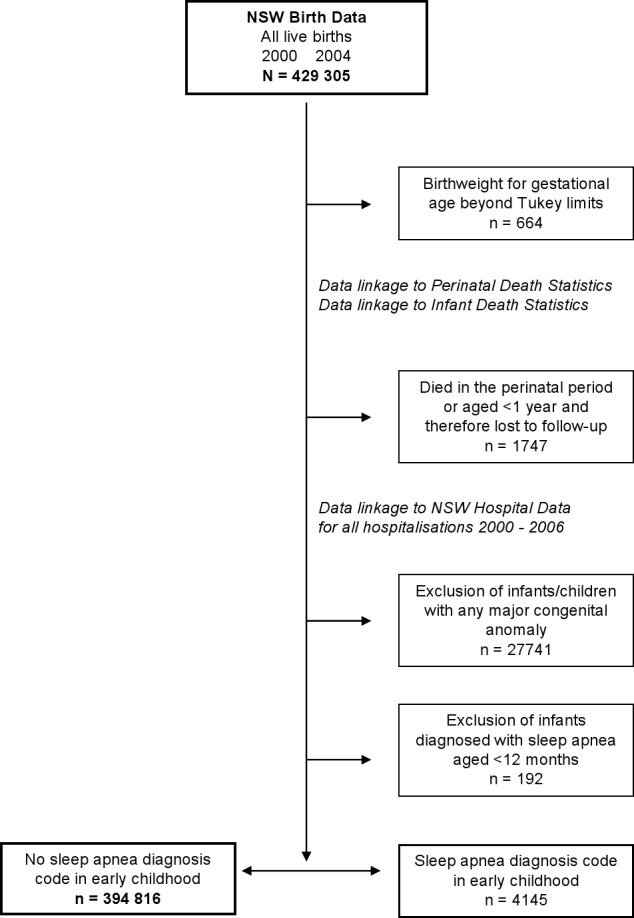
Schema showing data linkage and exclusion criteria.
Data Sources and Data Linkage
Data from births from 2000–2004 were obtained via the NSW Midwives Data Collection, a legislated population-based surveillance system that includes information on all babies born at ≥ 20 weeks gestation or weighing ≥ 400 g. Data from hospital admissions from 1 July 2000–30 June 2007 were obtained from the NSW Department of Health Admitted Patient Data Collection, an administrative dataset of all hospitalizations and day surgeries in NSW. Infant deaths were identified by linkage to the Australian Bureau of Statistics perinatal mortality data. Length of follow-up varied from 2.5 years to a maximum of 6 years according to the year of birth.
Data linkage was carried out at the Centre for Health Record Linkage13 (New South Wales, Australia using probabilistic record linkage through record linkage software called ChoiceMaker14). The validity of the probabilistic record linkage is extremely high, with < 3 in 1,000 false positive links and < 5 in 1,000 missed links.15 Personal identifiers are separated from health information to preserve privacy. Researchers receive only health information and a linkage key from the data custodians. Records for patients transferred to hospitals outside the State of NSW were not available.
Outcome Variables
The primary outcome was sleep apnea diagnosis in childhood, first diagnosed between 1 and 6 years of age. Those babies with sleep apnea diagnosed before 12 months of age (n = 213) were not included because of the likelihood of their diagnosis being related to another underlying condition.
In the hospital data, a maximum of 55 separate fields for principal diagnosis and comorbidities were recorded for each patient discharge record and coded according to the International Classification of Diseases and Related Health Problems 10th revision – Australian Modification (ICD-10-AM). Children with sleep apnea were identified from those hospital records with the ICD-10 code G47.3: sleep apnea, central or obstructive. Minor and major congenital anomalies were distinguished according to the list given by Macintosh et al.16 Birthweight was adjusted for gestational age and sex using national birthweight centile charts.17 Polysomnography was identified using the Australian Classification of Health Interventions18 procedure code 12203-00. Adenotonsillectomy, adenoidectomy, and tonsillectomy were identified by the procedure codes 41789-01, 41801-00, and 41789-00, respectively.
Exclusions
Birthweight outliers were identified for each gestational age using the Tukey method.19 Those babies with birthweight lying 3 interquartile ranges greater than the 75th percentile or less than the 25th percentile were removed from the analysis. Babies who died in the perinatal period were excluded, as were any infants who died < 12 months. Any infant/child with a major identified congenital anomaly (n = 27,741) was excluded. The prevalence of sleep apnea among these children was 2.1% (581/27,741). Anomalies seen among children with a subsequent sleep apnea diagnosis included congenital malformation syndromes affecting facial appearance and associated with short stature, cleft palate, congenital laryngomalacia, Down syndrome, tracheomalacia, Hirschsprung disease, and achondroplasia.
Analysis
Contingency tables and Fisher exact test were used to analyze the crude relationship between childhood sleep apnea and perinatal factors, by maternal characteristics, baby characteristics, and birth characteristics. Cox proportional hazard model was used to investigate the association between childhood sleep apnea and gestation at birth (weeks), year of birth, baby's sex, maternal age, smoking status during pregnancy, mode of delivery, hypertension in pregnancy and number of previous pregnancies, and adjust for the differential follow-up. Crude odds ratios (ORs) with 95% confidence intervals were estimated for the explanatory variables. Adjusted HRs were calculated by entering the proposed explanatory variables into the hazard model and retaining only variables for which the hazard ratio changed by ~10% or more when the factor was fitted were retained in the models. Subgroup analysis was carried out on those births that were at term (> 36 weeks gestation). Analyses were carried out using SAS, version 9 (SAS Institute, Cary NC, USA). The study had approval from The NSW Department of Health Ethics Committee.
RESULTS
A total of 4,145 (1.0%) children, with a first diagnosis of sleep apnea after 12 months of age, were identified and compared to a group of 394,816 children with no sleep apnea diagnosis (Figure 1). Longitudinal follow-up of children ranged from 2.5 to 6 years. The mean length of follow-up was 5.04 years (SD 1.3) for children with sleep apnea compared to 5.02 years (SD 1.5) for children with no sleep apnea. The mean age at first diagnosis for sleep apnea was 44.2 months (SD 13.9). In only those children with ≥ 5 years follow up (n = 2,121), the mean age at first diagnosis was 47.4 months (SD 14.8).
Adenoidectomy, tonsillectomy, or both were common among the children diagnosed with sleep apnea, with 3,548/4,145 (85.6%) having had these procedures. In comparison, among children without sleep apnea, only 10,954/362,375 (3.0%) had had these procedures. Similarly, procedure codes for polysomnography were present for 1,505/4,145 (36.3%) children with sleep apnea, compared to 791/362,675 (0.22%) children with no sleep apnea diagnosis.
Adjusted hazard ratios (aHR) for neonatal, maternal and birth characteristics are presented in Table 1. Children born preterm were significantly more likely to be diagnosed with sleep apnea than those born at term; babies born at < 32 weeks gestation were significantly more likely to have the diagnosis than those born at term (aHR 2.74; 95% CI 2.16–3.49). There was a dose-response relationship between preterm birth and sleep apnea diagnosis: as gestation increased, diagnosis of sleep apnea in childhood decreased (Figure 2). For infants born at < 27 weeks, the hazard raio (HR) was 3.77 (95% CI; 2.06, 5.48); for 27–29 weeks, HR was 2.74 (95% CI; 1.76, 3.72); 30–32 weeks, HR was 2.30 (95% CI; 1.74, 2.86); 33–35 weeks HR was 1.30 (95% CI; 1.08, 1.52); 36–38 weeks HR was 1.15 (95% CI; 1.08, 1.22); > 38 weeks HR 0.97 (95% CI; 0.94, 1.01) (Figure 2).
Table 1.
Adjusted hazard ratios (aHR) for sleep apnea diagnosis in early childhood by perinatal characteristics (birth cohort 2000–2004, follow-up to 2007)*
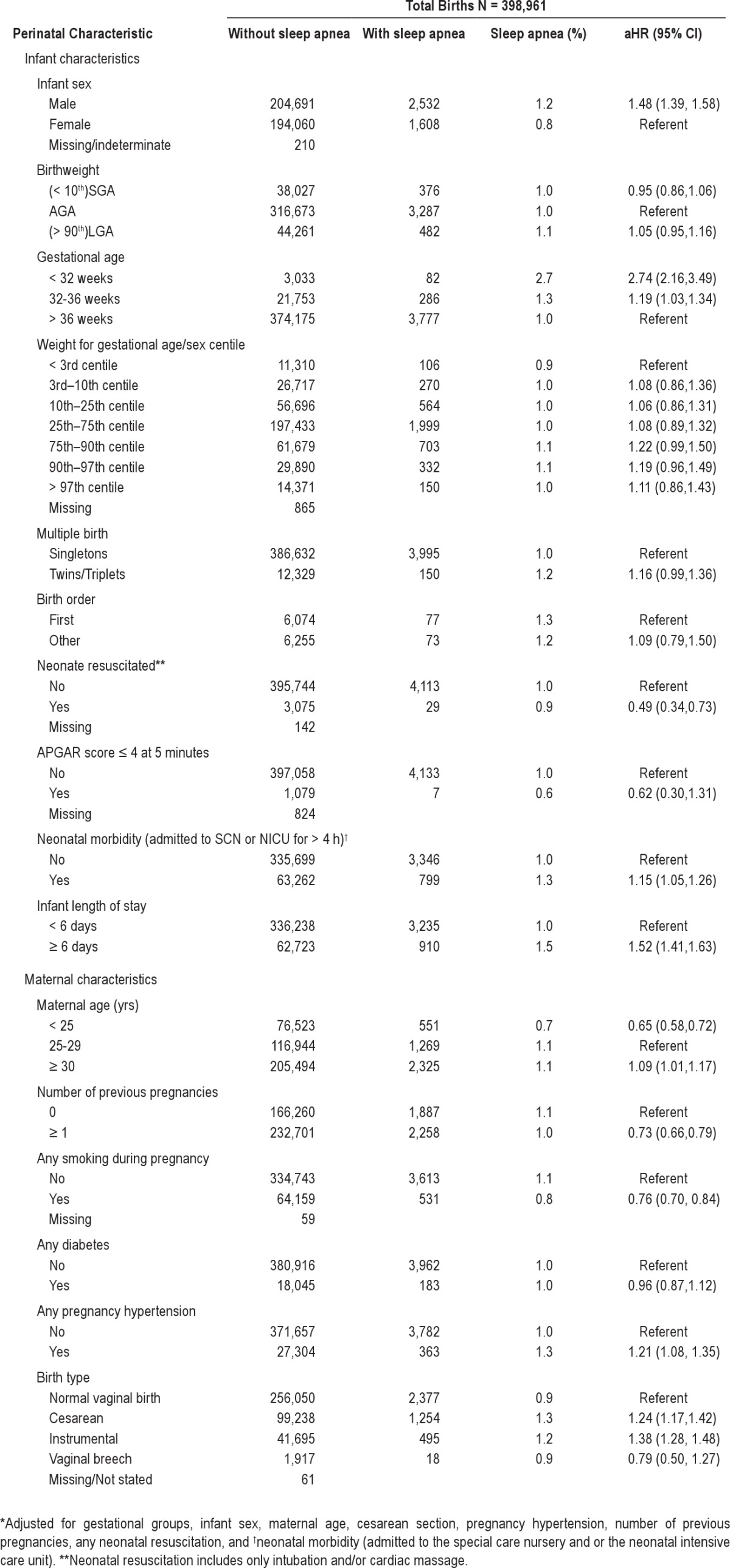
Figure 2.
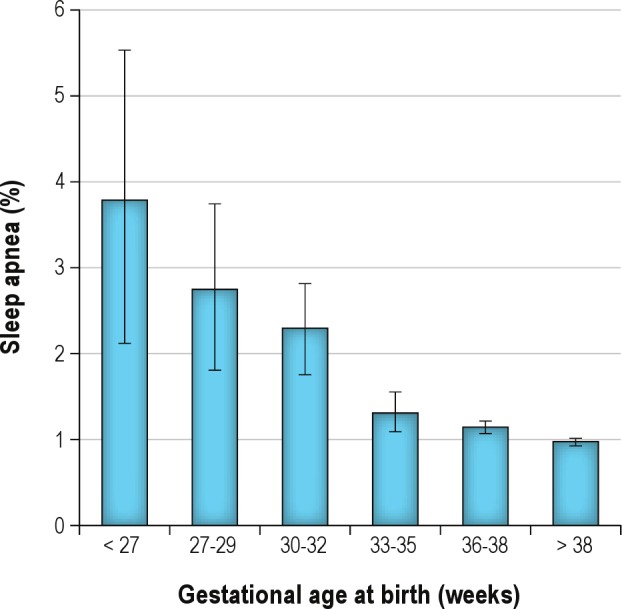
Relationship between gestational age at birth and the prevalence of sleep apnea diagnosis in early childhood (HR with 95% confidence intervals).
Children who were born small for gestational age ([SGA] < 10th percentile) or large for gestational age ([LGA], > 90th percentile) were not at increased risk of developing sleep apnea compared to those born at the appropriate weight for their gestational age (AGA) and sex (Table 1). Further analysis showed no significant difference in the risk of childhood sleep apnea for babies born between the 3rd and the 10th centile (HR 1.08; 95% CI 0.86, 1.36) or > 97th centile (HR 1.11; 95% CI 0.86, 1.43) for their sex and gestational age compared to those born < 3rd centile.
After adjusting for gestation at birth, year of birth, baby's sex, maternal age, smoking status during pregnancy, mode of delivery, and number of previous pregnancies, the association between sleep apnea and any hypertension during pregnancy remained significant, aHR 1.21 (95% CI 1.08, 1.12). Further adjusted analyses also remained significant; the association between sleep apnea and cesarean birth HR was 1.24 (95% CI 1.17, 1.42), and the HR for preterm < 32 weeks compared to term also remained significant aHR 2.74 (95% CI: 2.16, 3.49). A subgroup analysis limited to term babies did not change the associations.
DISCUSSION
We report an increased risk of sleep apnea diagnosis in children born preterm compared to children born at term, with a stronger association for those born before < 32 weeks, which remained after adjustment for known confounders. This risk did not appear to be related to birthweight, as analysis of gestationally adjusted birthweight did not show any differences in risk, even among those at extremes of birthweight. We further found an increased risk of childhood diagnosis of sleep apnea in children of mothers with hypertension during pregnancy and cesarean birth, which remained after adjusting for potential confounders and regardless of subgroup analysis of preterm or term birth.
Our findings concord with previous research in this area that found an increased association between preterm birth and OSA diagnosis in childhood7,8; however, our results did not support other research that also found a similar association between SGA and OSA.9 A small study of 40 cases of children born preterm found an increased risk of sleep disordered breathing in children aged 8–11 years (OR 3.0, 95% CI 1.5, 6.5).7 Another study of 48 cases found an increased risk OR 2.2; (95% CI 1.5, 6.2), of chronic snoring in young adults who were very low birthweight.9 Our findings suggest that childhood OSA may be related to maturation at birth, specifically development of the lungs, facial symmetry, airways, and/or nervous system, and not necessarily size, and that the causal pathway between preterm birth and childhood OSA are not necessarily those that influence size at birth. Previous research has described associations between prematurity and facial asymmetry resulting in airway dimensional changes that support this hypothesis.11
Sleep apnea is not routinely investigated and in Australia requires referral to tertiary care and a hospital admission for polysomnography. Children born preterm have increased contact with medical professionals and therefore may receive increased health surveillance and consequent intervention. This may account for our findings.
The association between cesarean and instrumental delivery and sleep apnea in children is likely to be related to the indication for the cesarean section. Cesarean section at term may also be due to maternal and fetal morbidities that are related to subsequent childhood OSA, and it is not the cesarean section per se that causes the OSA.20 Bourjeily found that among pregnant women with sleep disordered breathing and or symptoms of sleep disordered breathing, there were higher rates of unplanned cesarean section aOR 1.7, (95% CI 1.1, 3.3) and higher rates of preterm birth (aOR 1.9, 95% CI 1.1–3.3).21 These and other poor outcomes were related to hypertensive disorders. Further investigation is needed to understand this relationship and the subsequent relationship with childhood SDB. Hibbs suggested that an underlying genetic predisposition of both mothers and their children to upper airway obstruction or to obesity-related diseases or the metabolic syndrome may account for these associations.8 Unfortunately our results are not able to further explain this association but do suggest that the effect of hypertension in pregnancy may be related to longer term outcomes for children of these women. The implications of SDB and birth outcomes and subsequent childhood health require further elucidation.
In previous studies, maternal smoking in pregnancy has been associated with chronic snoring in young adults.9 Our results are in the opposite direction, and this is likely to be related to the exclusions in the selection of the population. We excluded deaths up to the first year of life and babies with major congenital anomalies, both of which are strongly correlated to maternal smoking.22 These selection issues may also account for the maternal age and neonatal resuscitation findings, whereby babies with the poorest outcomes have already been excluded, thus leaving well babies with these exposure variables.
To our knowledge this is the largest study investigating preterm birth and sleep apnea diagnosis in a population with relatively long follow-up. Strengths of this study include the prospective and independent nature of the data collection, the population base of the study, the objective measurement of both the exposure and study factor, and the relatively long follow-up. In previous work we found good validation of data in perinatal databases.23,24 Sim et al. gives positive predictive value of 88% for a validation study of ICD-9 codes for sleep apnea diagnosis, albeit in an adult population.25 We are confident that we have correctly identified all cases of childhood OSA in this cohort. Huang et al. found a bi-modal distribution of OSA in the NSW population, including a peak between 0–4 years of age, similar to our data, thus validating this cohort.26 There is also good biological evidence for high prevalence in a cohort of children in this age group, as the difference in the size of the tonsils/adenoids and the diameter of the airways is greatest at this age.4 We originally hypothesized that children in our study identified with OSA may have higher socioeconomic status with increased access to health interventions. However, a recent study by Brouillette found an association between sleep apnea in children and social disadvantage12; this combined with Australia's universal public health system, suggests that our findings may not be due to increased health surveillance.
We were able to exclude infants with reported congenital anomalies which are known to have a high prevalence of childhood OSA. The underlying association between congenital anomaly and sleep apnea is related to those malformations that are associated with anatomical abnormalities which narrow the upper airway12 such as cleft-lip/palate defects and associated syndromes. Less severe congenital anomalies tend to be underreported in this dataset, and therefore we may have included some children with congenital anomalies. However this is likely to be non-differential misclassification, as not all babies with a congenital anomaly are born preterm, thus biasing the estimators towards the null.
There are some limitations to acknowledge when interpreting our results. These findings are based on routinely collected population data and therefore do not include all risk factors (e.g., BMI and breastfeeding) which may affect the findings. Secondly, we were not able to adjust for the underlying cause of preterm birth, which may also be confounding the findings. Similarly we were also not able to adjust for severity of OSA or tonsil/adenoid size; however, a recent study reported that tonsil size was not necessarily an indicator of OSA severity.3
Regardless of the mechanism of how preterm birth and OSA are associated, this study provides sufficient evidence of such an association. Considering the impact that untreated OSA can have on a child's neurological development27 and their subsequent health outcomes, screening children born preterm for risk of sleep apnea should be a priority.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the NSW Department of Health for access to the population health data and the NSW Centre for Health Record Linkage for linking the data sets. C.H. Raynes-Greenow is supported by a National Health and Medical Research Council (NHMRC) Early Career Research Fellowship (#511984); R. Hadfield at the time of the study was supported by a part-time an NHMRC Early Career Research Fellowship; and C.L. Roberts is supported by a NHMRC Senior Research Fellowship (#457078).
Footnotes
A commentary on this article appears in this issue on page 1441.
REFERENCES
- 1.Montgomery-Downs H, Jones V, Molfese VJ, Gozal D. Snoring in preschoolers: associations with sleepiness, ethnicity, and learning. Clin J Pediatr. 2003;42:719–26. doi: 10.1177/000992280304200808. [DOI] [PubMed] [Google Scholar]
- 2.Calhoun DA. Obstructive sleep apnea and hypertension. Curr Hypertens Rep. 2010;12:189–95. doi: 10.1007/s11906-010-0112-8. [DOI] [PubMed] [Google Scholar]
- 3.Bixler EO, Vgontzas AN, Lin H-M, et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009;32:731–6. doi: 10.1093/sleep/32.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hultcrantz E, Lofstrand Tidestrom B. The development of sleep disordered breathing from 4 to 12 years and dental arch morphology. Int J Pediatr Otorhinolaryngol. 2009;73:1234–41. doi: 10.1016/j.ijporl.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–9. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 6.Calhoun SL, Vgontzas AN, Mayes SD, et al. Prenatal and perinatal complications: is it the link between race and SES and childhood sleep disordered breathing? J Clin Sleep Med. 2010;6:264–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Rosen C, Larkin E, Kirchner H, et al. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J Pediatr. 2003;142:383–9. doi: 10.1067/mpd.2003.28. [DOI] [PubMed] [Google Scholar]
- 8.Hibbs AM, Johnson NL, Rosen CL, et al. J Pediatr. 2008. Prenatal and neonatal risk factors for sleep disordered breathing in school-aged children born preterm; p. 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paavonen EJ, Strang-Karlsson S, Raikkonen K, et al. Very low birth weight increases risk for sleep-disordered breathing in young adulthood: the Helsinki Study of Very Low Birth Weight Adults. Pediatrics. 2007;120:778–84. doi: 10.1542/peds.2007-0540. [DOI] [PubMed] [Google Scholar]
- 10.Section on Pediatric Pulmonology; Subcommittee on Obstructive Sleep Apnea Syndrome; American Academy of Pediatrics. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrom. Pediatrics. 2002;109:704–12. doi: 10.1542/peds.109.4.704. [DOI] [PubMed] [Google Scholar]
- 11.Harila-Kaera V, Gron M, Heikkinen T, Alvesalo L. Sagittal occlusal relationships and asymmetry in prematurely born children. Eur J Orthod. 2002;24:615–25. doi: 10.1093/ejo/24.6.615. [DOI] [PubMed] [Google Scholar]
- 12.Brouillette RT, Horwood L, Constantin E, Brown K, Ross NA. Childhood sleep apnea and neighborhood disadvantage. J Pediatr. 2011;158:789–95. doi: 10.1016/j.jpeds.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence G, Dinh I, Taylor L. The Centre for Health Record Linkage: a new resource for health services research and evaluation. HIM J. 2008;37:60–2. doi: 10.1177/183335830803700208. [DOI] [PubMed] [Google Scholar]
- 14.Open Source ChoiceMaker Technology. Available from: http://oscmt.sourceforge.net.
- 15.Centre for Health Record Linkage. Available from: http://www.cherel.org.au.
- 16.MacIntosh MC, Fleming KM, Bailey JA, et al. Perinatal mortality and congenital anomalies in babies of women with type 1 or type 2 diabetes in England, Wales, and Northern Ireland: population based study. BMJ. 2006;333:177. doi: 10.1136/bmj.38856.692986.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts CL, Lancaster PA. Australian national birthweight percentiles by gestational age. Med J Aust. 1999;170:114–8. doi: 10.5694/j.1326-5377.1999.tb127678.x. [DOI] [PubMed] [Google Scholar]
- 18.National Centre for Classification in Health. The Australian Classification of Health Interventions (ACHI) - Seventh Edition - Tabular list of Interventions and Alphabetic index of Interventions. Sydney: Faculty of Health Sciences. The University of Sydney; 2009. [Google Scholar]
- 19.Tukey JW. Reading, MA: Addison-Wesley Publishing Co; 1977. Exploratory data analysis. [Google Scholar]
- 20.Louis JM, Auckley D, Sokol RJ, Mercer BM. Maternal and neonatal morbidities associated with obstructive sleep apnea complicating pregnancy. Am J Obstet Gynecol. 2010;202:261. doi: 10.1016/j.ajog.2009.10.867. e1-5. [DOI] [PubMed] [Google Scholar]
- 21.Bourjeily G, Raker CA, Chalhoub M, Miller MA. Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. Eur Respir J. 2010;36:849–55. doi: 10.1183/09031936.00021810. [DOI] [PubMed] [Google Scholar]
- 22.Simmons LE, Rubens CE, Darmstadt GL, Gravett MG. Preventing preterm birth and neonatal mortality: exploring the epidemiology, causes, and interventions. Semin Perinatol. 2010;34:408–15. doi: 10.1053/j.semperi.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Lain SJ, Hadfield RM, Raynes-Greenow CH, et al. Quality of data in perinatal population health databases: a systematic review. Med Care. 2012;50:e7–e20. doi: 10.1097/MLR.0b013e31821d2b1d. [DOI] [PubMed] [Google Scholar]
- 24.Taylor L, Travis S, Pym M, Olive E, Henderson-Smart D. How useful are hospital morbidity data for monitoring conditions occurring in the perinatal period? Aust N Z J Obstet Gynaecol. 2005;45:36–41. doi: 10.1111/j.1479-828X.2005.00339.x. [DOI] [PubMed] [Google Scholar]
- 25.Sim JJ, Rasgon SA, Derose SF. Sleep apnea and hypertension: prevalence in chronic kidney disease. J Clin Hypertens. 2007;9:837–41. doi: 10.1111/j.1524-6175.2007.07176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang QR, Qin Z, Zhang S, Chow CM. Clinical patterns of obstructive sleep apnea and its comorbid conditions: a data mining approach. J Clin Sleep Med. 2008;4:543–50. [PMC free article] [PubMed] [Google Scholar]
- 27.Beebe D. Neural and neurobehavioral dysfunction in children with obstructive sleep apnea. PLoS Med. 2006;8:e323. doi: 10.1371/journal.pmed.0030323. http://dx.doi.org/10.1371/journal.pmed.0030323. [DOI] [PMC free article] [PubMed] [Google Scholar]


