Abstract
Study Objective:
To determine the hormonal effects of reducing sleep duration under controlled feeding conditions.
Design:
Randomized, crossover study.
Setting:
Inpatient.
Participants:
Twenty-seven normal weight, 30- to 45-yr-old men and women habitually sleeping 7-9 hr/night.
Intervention:
Participants were studied under two sleep conditions: short (4 hr in bed) or habitual (9 hr in bed) sleep. A controlled diet was provided for each 4-day study period.
Measurements and Results:
Fasting blood samples were obtained daily and frequent blood samples were obtained throughout day 4. The main outcomes measures included glucose, insulin, leptin, ghrelin, adiponectin, total glucagon-like peptide 1 (GLP-1) and peptide YY3-36 (PYY3-36) concentrations. Body weights were reduced by 2.2 ± 0.4 lb and 1.7 ± 0.4 lb during the habitual and short sleep phases, respectively (both P < 0.0001). There was no effect of sleep duration on glucose, insulin, and leptin profiles (all P > 0.05). Ghrelin and GLP-1 responses differed by sex. Short sleep increased fasting (P = 0.054) and morning (08:00-12:00) (P = 0.042) total ghrelin in men but not women. The reverse was observed for GLP-1: afternoon levels (12:30-19:00) were lower (P = 0.016) after short sleep compared with habitual sleep in women but not men.
Conclusions:
These data suggest that, in the context of negative energy balance, short sleep does not lead to a state of increased insulin resistance, but may predispose to overeating via separate mechanisms in men and women.
Clinical Trial Information:
Trial registration on http://www.clinicaltrials.gov. #NCT00935402.
Citation:
St-Onge MP; O'Keeffe M; Roberts AL; RoyChoudhury A; Laferrère B. Short sleep duration, glucose dysregulation and hormonal regulation of appetite in men and women. SLEEP 2012;35(11):1503-1510.
Keywords: Ghrelin, hormonal regulation, leptin, sleep
INTRODUCTION
Epidemiologic studies report an association between self-reported sleep duration and obesity.1 In fact, obesity and self-reported sleep duration show inverse trends with obesity prevalence increasing over the past decades and sleep duration decreasing.2 Moreover, there is increasing evidence from clinical studies showing that reducing sleep leads to greater food intake relative to habitual sleep.3–6 Data on the role of physical activity and energy expenditure on the relationship between sleep duration and obesity are controversial. We5 and others4 have found no effect of reducing sleep on energy expenditure, whereas Schmid et al.7 found that sleep restriction decreased physical activity and Jung et al.8 found greater energy expenditure during total sleep deprivation relative to habitual sleep.
Hormone and metabolite data have also supported the view that reducing sleep duration leads to a metabolic profile that would predispose to overeating. Studies by Spiegel et al.9,10 have shown that reducing sleep to 4 hr/night in normal-weight men increases ghrelin and decreases leptin, but others have not observed a change in leptin4,11 or ghrelin.4 Short sleep furthermore leads to a state of insulin resistance.10,12 The reasons for the discrepant results between studies may lie in differences in sex of the study populations and study design. Studies finding a significant effect of sleep duration on hormones and metabolites have mostly been in men. The study by Nedeltcheva et al.4 finding no effect of sleep on leptin and ghrelin levels included both men and women. A study by Bosy-Westphal et al.6 conducted in women also found no effect of sleep restriction on ghrelin levels. Also, the hormones and metabolites measured are sensitive to food intake and energy balance. The effect of sleep on hormones and metabolites could therefore be due to differences in food intake and energy balance, rather than sleep itself, if participants are not consuming the same foods or are not in identical states of energy balance during each sleep phase.
The goal of our study was to test the effects of sleep duration on hormones and metabolites involved in energy balance regulation. We enrolled both men and women and conducted our study under controlled feeding conditions to ensure that energy balance was equal between periods of short and habitual sleep. We hypothesized that a short sleep duration period of 4 hr/night in bed, for 3 consecutive nights, would lead to a hormonal profile predictive of greater energy intakes and glucose dysregulation compared with a habitual sleep duration period of 9 hr/night in bed for 3 nights.
METHODS
Participants
This study was a controlled inpatient study with two sleep periods of 6 days each. The details of participant recruitment and inclusion/exclusion criteria are described in detail elsewhere.5 Briefly, normal-weight men and women with body mass index (BMI) 22-26 kg/m2, age 30-45 yr, sleeping 7-9 hr/night, and without any sleep or metabolic/endocrine disturbances were eligible for participation. Individuals with excessive caffeine intake, smokers, shift workers, or those expecting to be traveling across time zones during the study conduct were excluded. The study was approved by the Institutional Review Boards of St. Luke's/Roosevelt Hospital Center and Columbia University (New York, NY) and all participants provided informed consent prior to enrollment.
Intervention
Upon entry into the study, participants were randomized in pairs to either short sleep or habitual sleep. They returned for the alternate sleep period after approximately 3 wk (23 days). One male participant returned for the alternate sleep period after 16 days due to personal reasons. During the short sleep period, participants went to bed at 01:00 and woke up at 05:00; during the habitual sleep period, bedtimes were 22:00-07:00. Naps were not permitted and study personnel ensured that participants remained awake throughout the planned wake time. Sleep duration was determined using polysomnography (PSG) as described previously.5
During the first 4 days of each sleep period, participants consumed a controlled diet with fixed meal times. Energy requirements were estimated using the Harris-Benedict equation with an activity factor of 1.3.13 Diets contained 30% of energy from fat, 55% from carbohydrates, and 15% from protein. Meals each provided 30% of daily energy requirements and were served at 08:00, 12:00, and 19:00. A snack providing 10% of the daily energy requirements was given at 16:00.
Fasting blood samples were obtained daily at 07:30 except on day 4, when the fasting sample was taken at 08:00. Additionally, on day 4, blood samples were taken at 08:15, 08:30, 09:00, 09:30, 10:00, 12:00, 14:00, 16:00, 18:00, 20:00, 22:00, 24:00 (day 5), 02:00, 04:00, and 06:00. During that day, participants continued to consume their usual meals at the specified time but were required to consume lunch and dinner in ≤ 15 min and consume breakfast and snack in ≤ 5 min. Blood samples were analyzed for glucose, insulin, leptin, ghrelin (total and active), adiponectin, total glucagon-like peptide 1 (GLP-1), and peptide YY3-36 (PYY3-36).
Hormone and Metabolite Measurements
All hormones and metabolites were analyzed in the New York Obesity Nutrition Research Center Hormone and Metabolite Core Laboratory (St. Luke's/Roosevelt Hospital, NY). Glucose was measured in duplicate by the glucose oxidase method (Analox Instruments, Lunenburg, MA) with a coefficient of variation of < 2%. Plasma insulin was measured by radioimmunoassay (RIA, Millipore, Billerica, MA) with a limited sensitivity of 2 μU/mL and an intra-assay coefficient of variation of 1.79-8.57%. Plasma leptin and adiponectin were determined by RIA, with detection limits of 0.5 ng/mL and 1 ng/mL and coefficients of variation 3.25-6.85% and 1.79-8.57%, respectively. RIA was also used to determine total and active ghrelin. Prior to analysis, samples collected for active ghrelin were acidified with 1 N HCl. Sensitivities for both assays were 93 pg/mL and 7.8 pg/mL and coefficients of variation were 3.34-6.12% and 3.16-6.87% for total and active ghrelin, respectively. The 3-36 fragment of plasma PYY (PYY3-36) was also assayed by RIA with sensitivity levels 20 pg/mL and coefficient of variation 2.83-7.10%. Total GLP-1 was determined by RIA, after extraction with 95% ethyl alcohol. The assay measures all forms of GLP-1 [GLP-1(7-36) amide, GLP-1(7-37), GLP-1(9-36) amide, GLP-1(9-37), GLP-1(1-36) amide and GLP-1(1-37)] in plasma. GLP-1 assay sensitivity was 3 pM and intra-assay coefficient of variation was 3.97-6.27%.
Statistical Analysis
Correlation coefficients between the variables were computed and checked for linear relationship. Then, for each hormone measure, we performed an analysis with a linear mixed model that takes into account repeated measures. Subject was treated as a random effect variable; sleep (short vs. habitual) was treated as a fixed effect; weight and sex were treated as covariates. For fasting hormone levels, day was also treated as a fixed effect variable. Fasting data from days 1 through 5 were used in the linear mixed model analyses. Data from day 6 were not used because participants were no longer consuming a controlled diet from day 5 and therefore day 6 data were subject to between-phase differences.
For the analyses with hourly hormone measurements taken on day 4, day was replaced by categorized time period of the day (as a fixed effect): morning (08:00-12:00, fasting morning sample until before lunch); afternoon (12:30-19:00, postlunch until before dinner); and night (19:30-06:00, after dinner and through the night). For hourly measures, we also performed separate analyses with a linear mixed model for each time period category, with sex and weight as covariates, sleep as fixed effect, and subject as a random effect. Additionally, all of the above analyses were done separately for men and women.
Phase/period effect and carryover effect were tested by adding a phase/period effect (fixed effect) and an interaction term between phase and sleep in our linear mixed model analysis. The P values for these terms were more than 0.2 in all cases. Thus, we did not observe any phase/period effect or carryover effect. Thereafter, we dropped the phase/period and interaction term from the linear mixed model and redid the analysis (as described in the previous paragraphs).
The hormone measures used in this study do not follow a normal distribution. However, according to statistical large sample theory, some normal-based tests can be performed for large samples, even if the data are not normal. In particular, we can perform the F-test, which is the basis of regression/analysis of variance/linear model analyses for large samples even when the data are not normal. As we have a large number (≥ 30) of data points for each linear mixed model analysis, the normality assumption is not required for these analyses. We also do not need to use any transformations (such as a log-transformation) to fit the data to normal, as the normality assumption is not needed.
The software packages R (http://cran.r-project.org) and SAS (version 9.2, SAS Institute, Cary, NC) were used for our analyses. Data are presented as regression coefficients or means ± standard error of the mean (SEM). Raw data are presented in tables and figures. Statistical significance was set at P < 0.05.
RESULTS
Baseline Measures
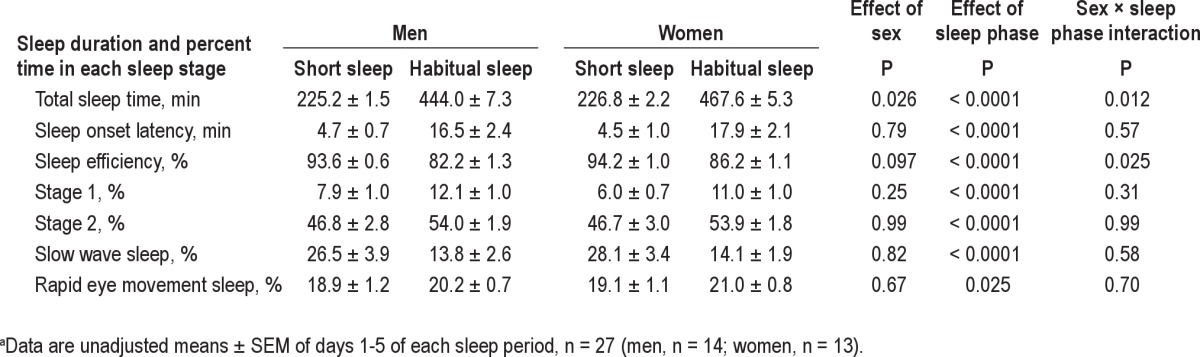
A total of 15 men and 15 women were recruited to participate in this study and 27 successfully completed both study periods. One man was excluded after the first study period due to discovery of periodic limb movement disorder, an exclusion factor diagnosed by PSG. One woman discontinued her participation after the first period for personal reasons and one woman was dismissed on the first morning of the first period on disclosure that she took antidepressant medication, an exclusion criterion for this study. Study completers were on average 35.3 ± 1.0 yr of age and had a BMI of 23.6 ± 0.2 kg/m2. Of the participants, 13 were white, 5 were black, 6 were Hispanic, 1 was Asian, and 2 were of mixed ethnicity. Sleep duration, as determined by PSG during the 5 nights of short sleep, was 226.4 ± 1.4 min (approximately 3 hr 46 min) compared with 470.4 ± 4.1 min (approximately 7 hr 50 min) for the habitual sleep phase (Table 1). Average daily energy intakes, as provided during the controlled feeding portion of the study, were 2,310 ± 194 kcal/day for men and 1,805 ± 129 kcal/d for women.5 This level of energy intake was, by design, the same during each sleep period.
Table 1.
Sleep duration during a 5-night period of short or habitual sleep in normal weight men and womena
Glucose and Insulin
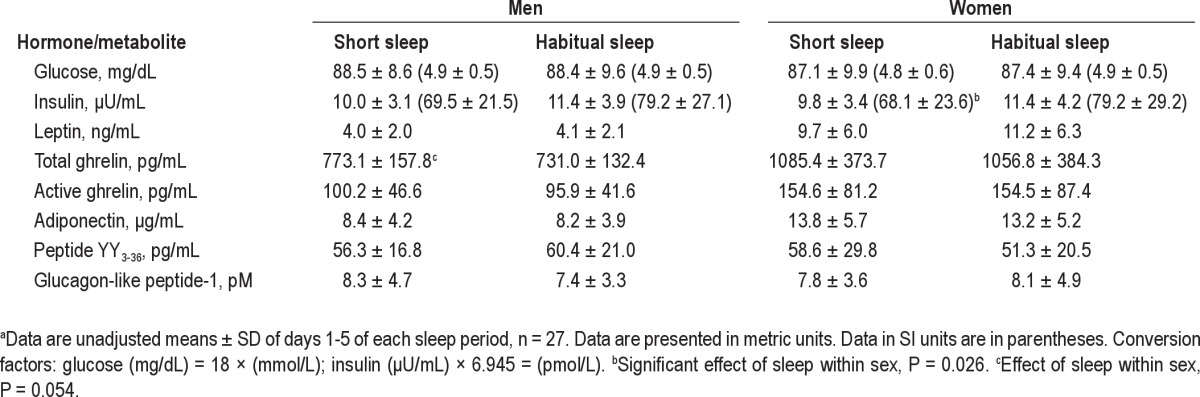
When data from all participants were analyzed together, there was no effect of sleep duration on fasting glucose (P = 0.94) and insulin (P = 0.33) (Table 2). In women, fasting glucose concentrations were not affected by sleep duration (P = 0.64) but, contrary to our hypothesis, fasting insulin was lower during the period of short sleep relative to habitual sleep (regression coefficient ± SEM: −1.39 ± 0.62, P = 0.026) (Table 3). In men, neither fasting glucose (P = 0.72) nor fasting insulin (P = 0.94) were affected by sleep duration. There was no difference between sleep periods in insulin resistance measured by the homeostatic model (P = 0.58).
Table 2.
Fasting hormone and metabolite concentrations during periods of short and habitual sleep in normal-weight men and womena
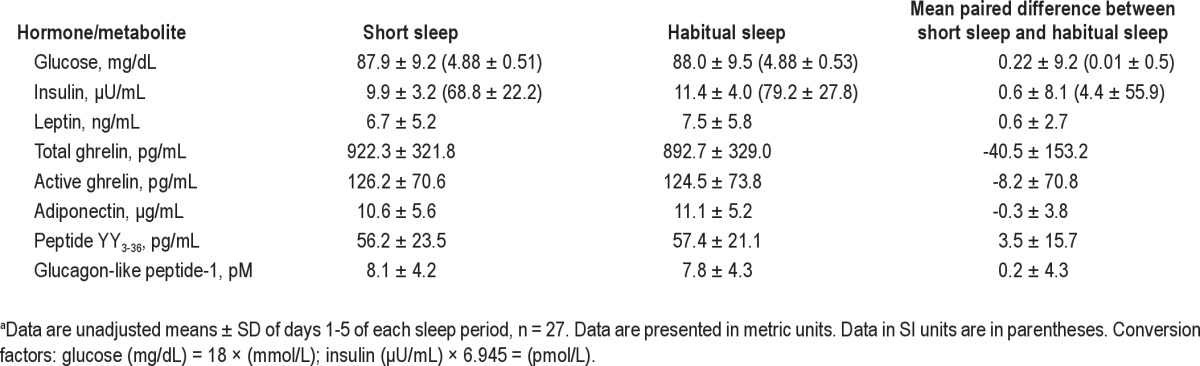
Table 3.
Fasting hormone and metabolite concentrations during periods of short and habitual sleep in normal weight men and womena
Data from day 4 were divided into three distinct periods: morning (08:00-12:00), afternoon (12:30-19:00), and night (19:30-06:00). In all participants together, overall mean glucose was not affected by sleep duration for any of the time periods (all P > 0.30). Similar results were obtained for average insulin (morning and afternoon, P > 0.80; night, P = 0.14). In women, neither glucose nor insulin was affected by sleep duration at all three time periods (all P > 0.20). Similar results were observed in men (all P > 0.5 except nighttime insulin, P = 0.17).
Leptin and Adiponectin
In all participants, fasting leptin was not affected by short sleep duration relative to habitual sleep (-0.66 ± 0.47 ng/mL, P = 0.16) (Table 2) and results were similar when men and women were analyzed separately (P = 0.34 and 0.23 for men and women, respectively) (Table 3). Data from day 4 showed similar results whereby morning, afternoon, and nighttime leptin concentrations were not affected by short sleep relative to habitual sleep in all participants analyzed together (P = 0.39, 0.75, and 0.22, respectively) or women (all P > 0.25) and men (all P > 0.40) alone. Peak leptin values between midnight and 04:00 were not different between short sleep and habitual sleep (P = 0.55).
Fasting adiponectin was not affected by sleep duration in the sample as a whole (244.5 ± 525.8, P = 0.64) (Table 2) with similar results obtained when men and women were analyzed separately (P = 0.93 and 0.58, respectively) (Table 3). Data from day 4 showed similar results when men and women were analyzed together (all P > 0.30). However, we found differences when men and women were analyzed separately. Short sleep led to generally lower 24-hr adiponectin levels in men (P = 0.0061), but not in women (P = 0.56). This was mostly due to lower nighttime adiponectin during the period of short sleep relative to habitual sleep (-2453.4 ± 882.2 ng/mL, P = 0.0064).
Gut Peptide Hormones: Ghrelin, PYY3-36, and GLP-1
Fasting total ghrelin levels were not affected by sleep when men and women were grouped together (P = 0.23) (Table 2) but showed a strong sex effect (P < 0.0001). In women, sleep duration did not affect fasting total ghrelin levels (P = 0.65) whereas fasting total ghrelin levels were higher during short sleep relative to habitual sleep in men (47.4 ± 24.4 pg/mL, P = 0.054) (Table 3). Similar results were obtained from the 24-hr data: no difference in average total ghrelin levels was observed between sleep phases for morning, afternoon, or night periods in men and women together (all P > 0.4) and women alone (all P > 0.5) but higher overall morning total ghrelin levels were observed after short sleep relative to habitual sleep in men (42.5 ± 20.8 pg/mL, P = 0.042) (Table 4, Figure 1). Afternoon and nighttime levels of total ghrelin were not affected by sleep duration in men (both P > 0.4).
Table 4.
Results of the regression analysis for total ghrelin and glucagon-like peptide 1 concentrations during morning, afternoon, and evening after 3 nights of short relative to habitual sleep in normal weight men and womena
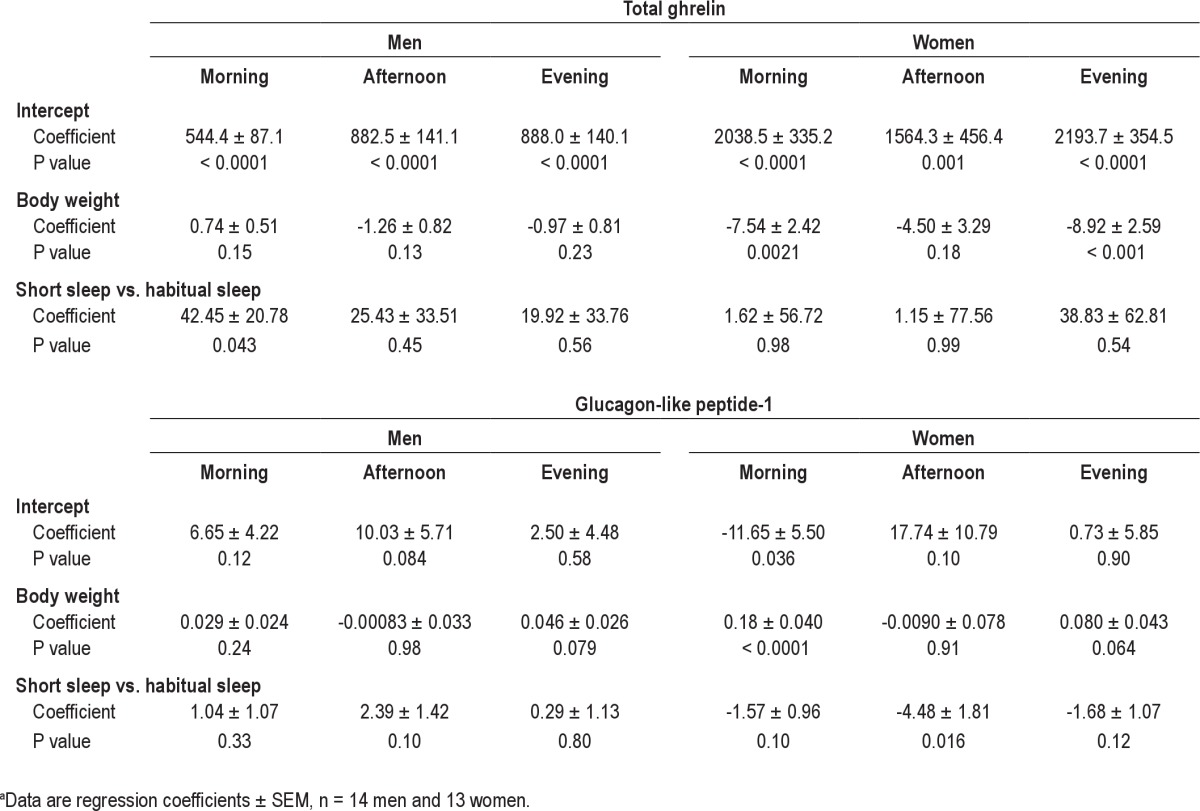
Figure 1.
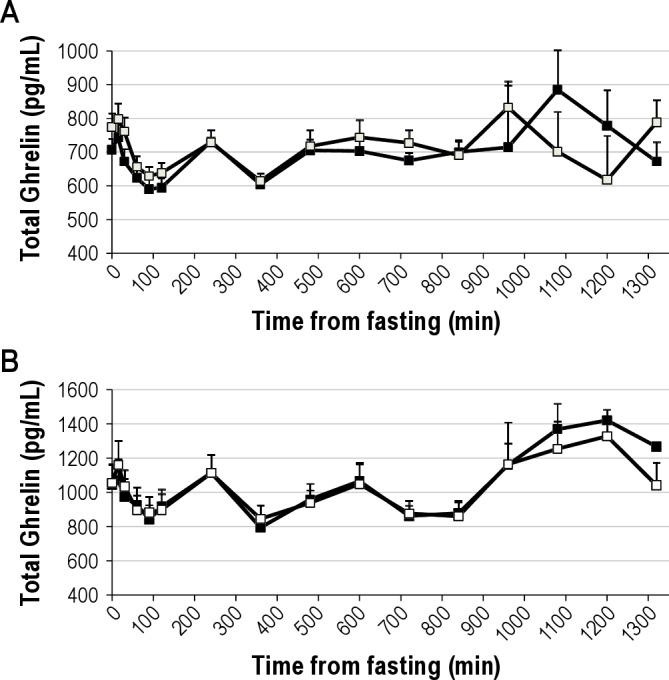
Total ghrelin concentration during a day of frequent blood sampling after 3 nights of either habitual (9 hr in bed, black squares) or short (4 hr in bed, white squares) sleep in men (panel A) and women (panel B). Time is presented as min from fasting sample. Fasting sample was taken at 08:00. Meals and snack were served after the 0, 240, and 480 min blood draw and at 660 min. Bedtimes were at 840 min (habitual sleep) and 1,020 min (short sleep) relative to the fasting blood draw (equivalent to 22:00 and 01:00 for habitual and short sleep, respectively). Higher morning ghrelin levels were observed after short sleep relative to habitual sleep in men; no differences between sleep periods were observed in women. Data are unadjusted means and SEM, n = 14 men or 13 women.
Fasting active ghrelin levels were not affected by sleep duration in the group as a whole (P = 0.32) (Table 2) or as analyzed separately for men (P = 0.20) and women (P = 0.77) (Table 3). Similarly for the 24-hr data, sleep duration had no effect on active ghrelin levels in the group as a whole (P = 0.35). In men, morning, afternoon, and nighttime active ghrelin levels were not affected by sleep duration (P = 0.92, 0.84, and 0.14, respectively). In women, morning and afternoon active ghrelin were not affected by sleep duration (P = 0.52 and 0.48, respectively) but nighttime levels tended to be higher during short sleep compared with habitual sleep (82.0 ± 45.6 pg/mL, P = 0.079).
Fasting PYY3-36 concentrations and PYY3-36 concentrations during morning, afternoon, and night periods on day 4 were not affected by sleep duration in all participants together (Table 2) or in women and men separately (Table 3). Fasting total GLP-1 concentrations were not affected by short sleep relative to habitual sleep in all participants (P = 0.80) or women (P = 0.73) and men (P = 0.49) separately (Table 3), despite a significant effect of sex on fasting total GLP-1 (P = 0.020). In all participants together, day 4 total GLP-1 concentrations were not affected by sleep duration for any of the time periods (all P > 0.3). However, we found a strong effect of sex on 24-hr GLP-1 (P < 0.001) and analyzed our data separately for men and women (Table 4, Figure 2). In women, there were trends for lower morning (P = 0.10) and nighttime (P = 0.12) GLP-1 and lower afternoon (P = 0.016) concentrations after short sleep relative to habitual sleep. In men, afternoon GLP-1 concentrations tended to be higher after short sleep than habitual sleep (P = 0.10) but morning and nighttime values were not affected by sleep duration (both P > 0.30).
Figure 2.
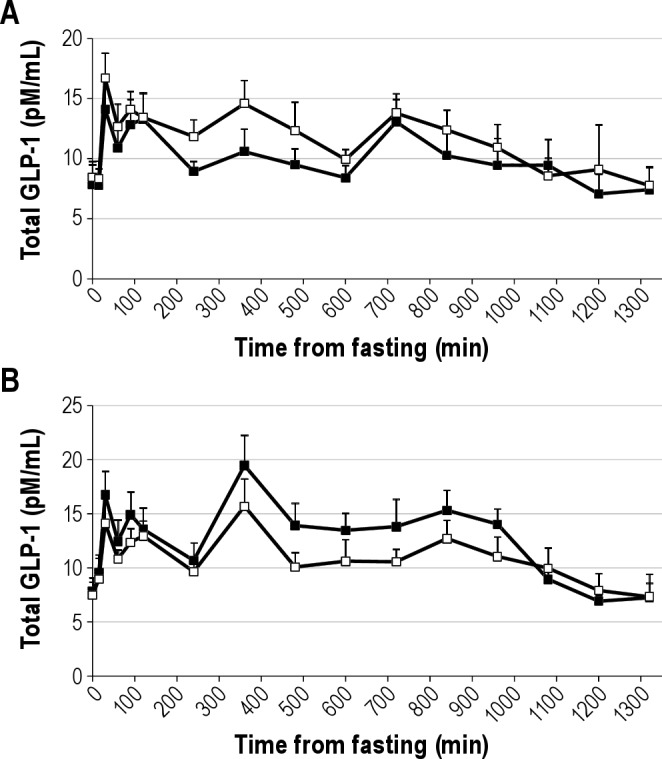
Total GLP-1 concentration during a day of frequent blood sampling after 3 nights of either habitual (9 hr in bed, black squares) or short (4 hr in bed, white squares) sleep in men (panel A) and women (panel B). Time is presented as mins from fasting sample. Fasting sample was taken at 08:00. Meals and snack were served after the 0, 240, and 480 min blood draw and at 660 min. Bedtimes were at 840 min (habitual sleep) and 1,020 min (short sleep) relative to the fasting blood draw (equivalent to 22:00 and 01:00 for habitual and short sleep, respectively). Morning (P = 0.10) and night time levels (P = 0.12) tended to be lower and afternoon levels were significantly lower (P = 0.016) during short sleep compared with habitual sleep in women whereas in men, afternoon GLP-1 concentrations tended to be higher after short sleep than habitual sleep (P = 0.10). Data are unadjusted means and SEM, n = 14 men or 13 women.
DISCUSSION
To our knowledge, our study is the largest controlled clinical investigation of the effects of sleep reduction on hormonal regulation of food intake. We strictly controlled food intake for the 4 days during which we assessed hormonal and metabolic profiles. In the context of equal food and caloric intakes, we found no evidence of glucose dysregulation or changes in leptin concentrations, as previously suggested.10,12 However, we found some interesting sex differences related to ghrelin and GLP-1 concentrations: short sleep increased total ghrelin levels in men but not women and reduced GLP-1 levels in women but not in men. These data, if replicated, could represent separate mechanisms by which sleep duration affects food intake regulation in men and women.
Our data, showing no effect of sleep duration on glucose concentrations and a slight reduction in insulin relative to habitual sleep, were not expected. Based on studies by Spiegel et al.10,12 and others,14–16 we anticipated that short sleep would lead to a state of relative insulin resistance with a rise in insulin and glucose concentrations. However, others have also reported no effect of short sleep duration on glucose and insulin levels6,17 and Donga et al.18 reported no effect of sleep duration on fasting glucose and insulin despite a reduction in insulin sensitivity measured by the hyperinsulinemic-euglycemic clamp. In these studies, food intake was not controlled and either not reported17,18 or increased by short sleep compared with habitual sleep.6 A few authors have suggested that differences between study results may be due to a threshold effect whereby more pronounced sleep reductions lead to a more adverse metabolic profile.7,10,18 In the current study, participants slept approximately 4 hr less during the short sleep phase relative to the habitual sleep phase. This is a more pronounced sleep restriction than exerted by Bosy-Westphal et al.,6 who found no effect of progressively reducing sleep from 8 hr to 4 hr/night over a 4-night period on glucose and insulin and Nedeltcheva et al.,15 who found higher 2-hr glucose and glucose area under the curve after an oral glucose tolerance test taken after 14 days of sleep restriction to 5.5 hr/night. However, our sleep restriction protocol involved milder10,12,14 or similar18 reduction in sleep to others who found significant effects of sleep duration on glucose and insulin.
In addition to a possible sleep restriction dose effect, we propose that energy balance may play a role in the effects of sleep duration on glucose regulation. In the current study, participants were fed the same meals in the same quantities and at the same time of day for both short and habitual sleep phases. No other study has been done with such rigorous control over feeding patterns. Most other studies have permitted participants to self-select their intakes for at least part of the time over the days preceding the measurement period.12,14–16,18 We and others have shown that short sleep duration leads to overeating relative to habitual sleep.3–5 Knowing that glucose and insulin concentrations are sensitive to meal intakes and energy balance, it is possible that this may explain some of the discrepancies in study results. Moreover, in the current study, the use of the Harris-Benedict equation to estimate energy requirements led to an underestimation of weight maintenance requirements and participants lost approximately 0.9 kg (2 lb) over the controlled feeding period in both sleep phases. Therefore, the effect of sleep deprivation was assessed under mild negative energy balance conditions, which may have contributed to the lack of effect of sleep restriction on glucose tolerance and insulin resistance. However, there was no statistical difference in starting body weight between the two sleep phases.
Our study showed no effect of sleep duration on leptin levels after controlling for sex and body weight. Again, previous literature highlights controversy with respect to the effect of sleep duration on leptin levels with some studies showing lower, higher, or no difference between short and habitual or longer sleep. 9,10,17,19 Such study differences may be due to differences in sleep reduction, energy balance, and feeding conditions, including diet composition.
We found a strong sex effect for ghrelin levels and analyzed our data separately for men and women. These analyses revealed that sleep duration affected total ghrelin levels in men but not in women. These results are in agreement with other studies that have been conducted in men.9,11,20 Interestingly, studies that have found no effect of sleep duration on ghrelin levels4,6 included women in their participant population. Unlike those two studies, our study was large enough to allow separate analyses by sex. It may be that men and women have different responses to sleep restriction for food intake regulation. It has also been proposed that ghrelin concentrations only increase with short sleep relative to habitual sleep under conditions of negative energy balance.21 In the studies by Nedeltcheva et al.,4,22 when participants overate during short sleep relative to habitual sleep,4 ghrelin was not affected, whereas ghrelin levels were higher during negative energy balance.22 This is a hypothesis that deserves further attention.
This study is the first to examine the effects of sleep duration on other gut hormones, such as PYY3-36 and total GLP-1. Although PYY3-36 and GLP-1 are secreted by the same L cells in the intestine,23 we found that sleep duration affected each hormone differently. GLP-1 concentrations, but not PYY3-36 concentrations, were affected by sleep duration in women but not in men. This sex difference has not been reported before. These observations further support the notion that a different mechanism of action exists for sleep duration to affect food intake regulation between men and women. Men may be more susceptible to overeating during short sleep than during habitual sleep because of increases in the orexigenic hormone ghrelin, whereas women may be more susceptible to overeating as a result of reduced concentration of the satiety hormone GLP-1. Therefore, men would respond to short sleep with enhanced appetite whereas women would experience lesser satiety signals. However, hunger and appetite ratings obtained on day 4 were not different between periods of short and habitual sleep.5 Nevertheless, despite not reporting differences in hunger and appetite, participants ate approximately 300 kcal more on day 5, during a day of ad libitum feeding, during short sleep compared with habitual sleep.5 Our hypothesis of enhanced appetite in men and lessened satiety in women is based on the available objective data derived from this study. The associated subjective feelings reported by our participants do not, unfortunately, add to this argument.
Although our study has various strengths, including large numbers of measurements and equal numbers of men and women, controlled feeding and sleep conditions, and numerous hormone measurements related to food intake regulation, it has some limitations. First, our participants were in a state of mild negative energy balance during the controlled feeding period. Due to the short duration of this study, we could not adjust intakes in time to compensate for the loss in body weight. Future studies should assess weight maintenance energy requirements more precisely with resting metabolic rate measurements. Nevertheless, this raises interesting questions surrounding the role of energy balance in the effects of sleep duration on glucose and insulin regulation. Our data suggest that sleep restriction does not seem to affect glucose homeostasis detrimentally in a state of negative energy balance. Second, we only measured fasting hormones and metabolites and postprandial levels in response to regular meals. We did not assess insulin sensitivity using an intravenous glucose tolerance test or hyperinsulinemic-euglycemic clamp and therefore do not have an assessment of insulin sensitivity. Our participants were all healthy, normal-weight individuals. We do not know if similar results would be observed in overweight participants. Only one study to date enrolled all overweight individuals15 and they also suggest that energy balance may be instrumental in the role of sleep in modulating glucose concentrations. Also, we did not measure cortisol levels. However, although some studies suggest that cortisol is elevated during short sleep compared with habitual sleep,10,24,25 others do not find differences between short and habitual sleep duration.15,16,18,26 In this study, we found trends for higher morning systolic blood pressure (P = 0.07) and resting heart rate (P = 0.11) during a period of short sleep but no effect on diastolic blood pressure (P = 0.50) (data submitted elsewhere). Finally, although the 3-wk washout period ensured that participants were tested exactly 28 days apart, we did not control for menstrual phase and women may have been in either the luteal or follicular phase of their menstrual cycle during both phases of our study. However, this is unlikely to be a major factor in our results because each person acted as their own control and each woman would have been in the same phase of her menstrual cycle for each measurement.
In conclusion, in the context of a mild energy deficit, short sleep duration does not lead to adverse glucose and insulin concentrations in healthy, normal-weight adults. It is possible that the adverse effects of short sleep on glucose regulation may only be observed in an environment that allows overeating and positive energy balance. The negative energy balance in which our participants were during the controlled feeding portion of the study may have masked the effects of sleep on our outcome variables. Such proposition is speculative and more research is necessary to address the role of energy balance on the effects of sleep duration on metabolic risk profiles. Our study also revealed sex differences in the hormones implicated in the control of feeding behavior during periods of short sleep relative to habitual sleep. Future studies should be sure to enroll adequate numbers of men and women to further explain how sleep duration can affect energy intake and body weight regulation differently in men and women. Our study suggests two separate hormonal control mechanisms for men and women: one that involves ghrelin and possibly enhanced appetite for men and one that implicates GLP-1 and reduced satiety for women. Finally, these data showing enhanced levels of an appetite-promoting hormone in men and reduced levels of an appetite-suppressing hormone in women support a causal role of sleep duration on energy intake and weight control.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Michael Kelleman, MSc (St. Luke's/Roosevelt Hospital), Jennifer Ahn, MSc (Columbia University, NY), Andrew McReynolds, MSc (Columbia University, NY), Sari Tepper, MSc (Columbia University, NY), and Zalak Trivedi, MSc (Columbia University, NY), for their help in supervising research participants and collecting data, along with the staff at Clinilabs and research participants. This study was funded by the National Institutes of Health grants #1R01HL091352-01A1, 1R01HL091352-01S1, 1 UL1 RR024156-03, and P30 DK-26687. The Almond Board of California provided almonds and Cabot Cheese provided cheese for the study. The funding agencies played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Author Contributions
Drs. St-Onge and Laferrère designed research; Ms. Roberts and Dr. O'Keefe conducted research; Dr. St-Onge, Ms. Roberts, and Dr. O'Keeffe obtained data; Dr. St-Onge and Ms. Roberts analyzed data; Dr. St-Onge, Ms. Roberts, and Dr. Laferrère wrote the paper; Dr. St-Onge had primary responsibility for final content. All authors read and approved the final manuscript.
ABBREVIATIONS
- ANOVA
analysis of variance
- BMI
body mass index
- GLP-1
total glucagon-like peptide-1
- PSG
polysomnography
- PYY
peptide YY
REFERENCES
- 1.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–53. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keith SW, Redden DT, Katzmarzyk PT, et al. Int J Obes (Lond) 2006. Putative contributors to the secular increase in obesity: exploring the roads less traveled. [DOI] [PubMed] [Google Scholar]
- 3.Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr. 2010;91:1550–9. doi: 10.3945/ajcn.2009.28523. [DOI] [PubMed] [Google Scholar]
- 4.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–33. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.St-Onge M-P, Roberts A, Chen J, Kelleman M, O'Keeffe M, Jones P. Short sleep duration increases energy intakes but does not change expenditure expenditure in normal weight individuals. Am J Clin Nutr. 2011;94:410–6. doi: 10.3945/ajcn.111.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosy-Westphal A, Hinrichs S, Jauch-Chara K, et al. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obes Facts. 2008;1:266–73. doi: 10.1159/000158874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmid SM, Hallschmid M, Jauch-Chara K, et al. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr. 2009;90:1476–82. doi: 10.3945/ajcn.2009.27984. [DOI] [PubMed] [Google Scholar]
- 8.Jung CM, Melanson EL, Frydendall EJ, Perreault L, Eckel RH, Wright KP. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol. 2011;589(Pt 1):235–44. doi: 10.1113/jphysiol.2010.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 10.Spiegel K, Leproult R, L'Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–71. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 11.Schmid SM, Hallschmid M, Jauch-Chara K, Born J, Schultes B. A single night of sleep deprivation increases ghrelin levels and feelings of hunger in normal-weight healthy men. J Sleep Res. 2008;17:331–4. doi: 10.1111/j.1365-2869.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 12.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 13.Harris JA, Benedict FG. Washington, D.C: Carnegie Institution of Washington; 1919. A biometric study of basal metabolism in man. [Google Scholar]
- 14.Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59:2126–33. doi: 10.2337/db09-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nedeltcheva AV, Kessler L, Imperial J, Penev PD. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab. 2009;94:3242–50. doi: 10.1210/jc.2009-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmid SM, Hallschmid M, Jauch-Chara K, et al. Disturbed glucoregulatory response to food intake after moderate sleep restriction. Sleep. 2011;34:371–7. doi: 10.1093/sleep/34.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donga E, van Dijk M, van Dijk JG, et al. A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. J Clin Endocrinol Metab. 2010;95:2963–8. doi: 10.1210/jc.2009-2430. [DOI] [PubMed] [Google Scholar]
- 19.Chaput JP, Despres JP, Bouchard C, Tremblay A. Short sleep duration is associated with reduced leptin levels and increased adiposity: Results from the Quebec family study. Obesity (Silver Spring) 2007;15:253–61. doi: 10.1038/oby.2007.512. [DOI] [PubMed] [Google Scholar]
- 20.Dzaja A, Dalal MA, Himmerich H, Uhr M, Pollmacher T, Schuld A. Sleep enhances nocturnal plasma ghrelin levels in healthy subjects. Am J Physiol Endocrinol Metab. 2004;286:E963–7. doi: 10.1152/ajpendo.00527.2003. [DOI] [PubMed] [Google Scholar]
- 21.Kilkus JM, Booth JN, Bromley LE, Darukhanavala AP, Imperial JG, Penev PD. Sleep and eating behavior in adults at risk for type 2 diabetes. Obesity (Silver Spring) 2012;20:112–7. doi: 10.1038/oby.2011.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nedeltcheva AV, Kilkus JM, Imperial J, Schoeller DA, Penev PD. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153:435–41. doi: 10.1059/0003-4819-153-7-201010050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinert RE, Poller B, Castelli MC, Drewe J, Beglinger C. Oral administration of glucagon-like peptide 1 or peptide YY 3-36 affects food intake in healthy male subjects. Am J Clin Nutr. 2010;92:810–7. doi: 10.3945/ajcn.2010.29663. [DOI] [PubMed] [Google Scholar]
- 24.Benedict C, Hallschmid M, Lassen A, et al. Acute sleep deprivation reduces energy expenditure in healthy men. Am J Clin Nutr. 2011;93:1229–36. doi: 10.3945/ajcn.110.006460. [DOI] [PubMed] [Google Scholar]
- 25.Omisade A, Buxton OM, Rusak B. Impact of acute sleep restriction on cortisol and leptin levels in young women. Physiol Behav. 2010;99:651–6. doi: 10.1016/j.physbeh.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 26.Pejovic S, Vgontzas AN, Basta M, et al. Leptin and hunger levels in young healthy adults after one night of sleep loss. J Sleep Res. 2010;19:552–8. doi: 10.1111/j.1365-2869.2010.00844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


