Abstract
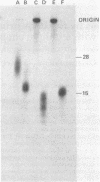
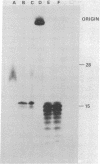
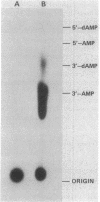
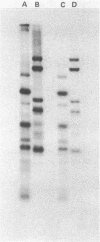
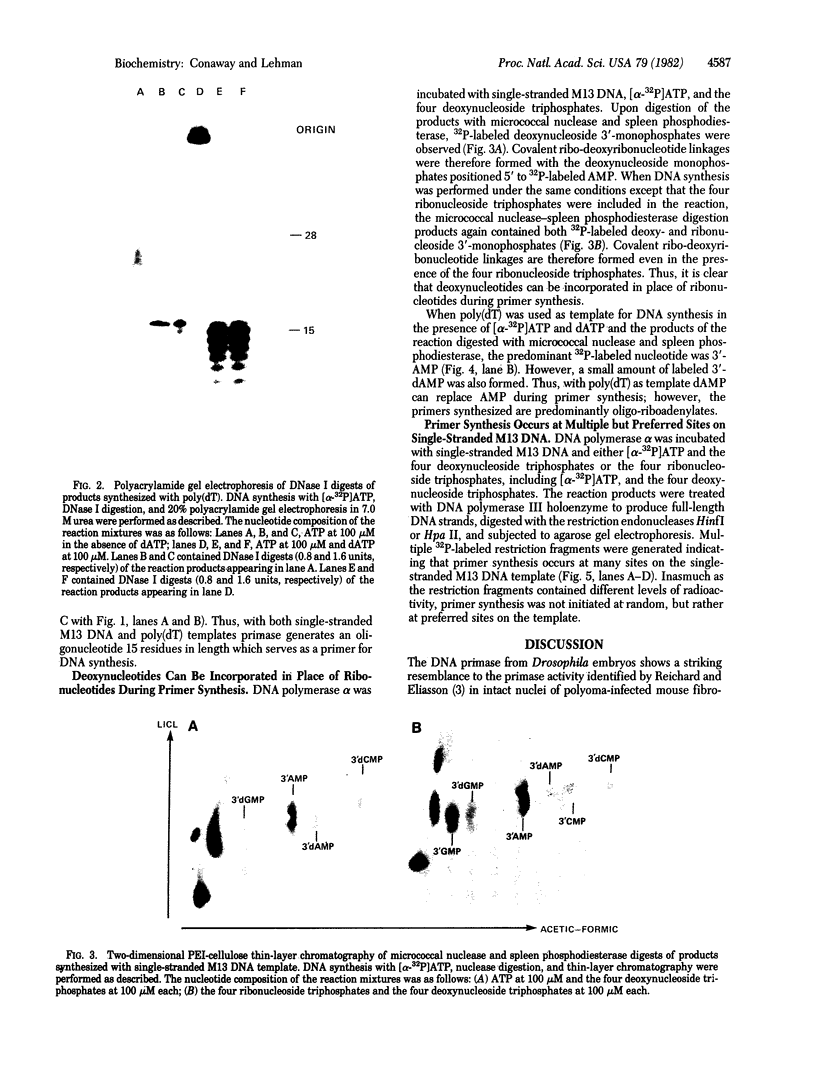
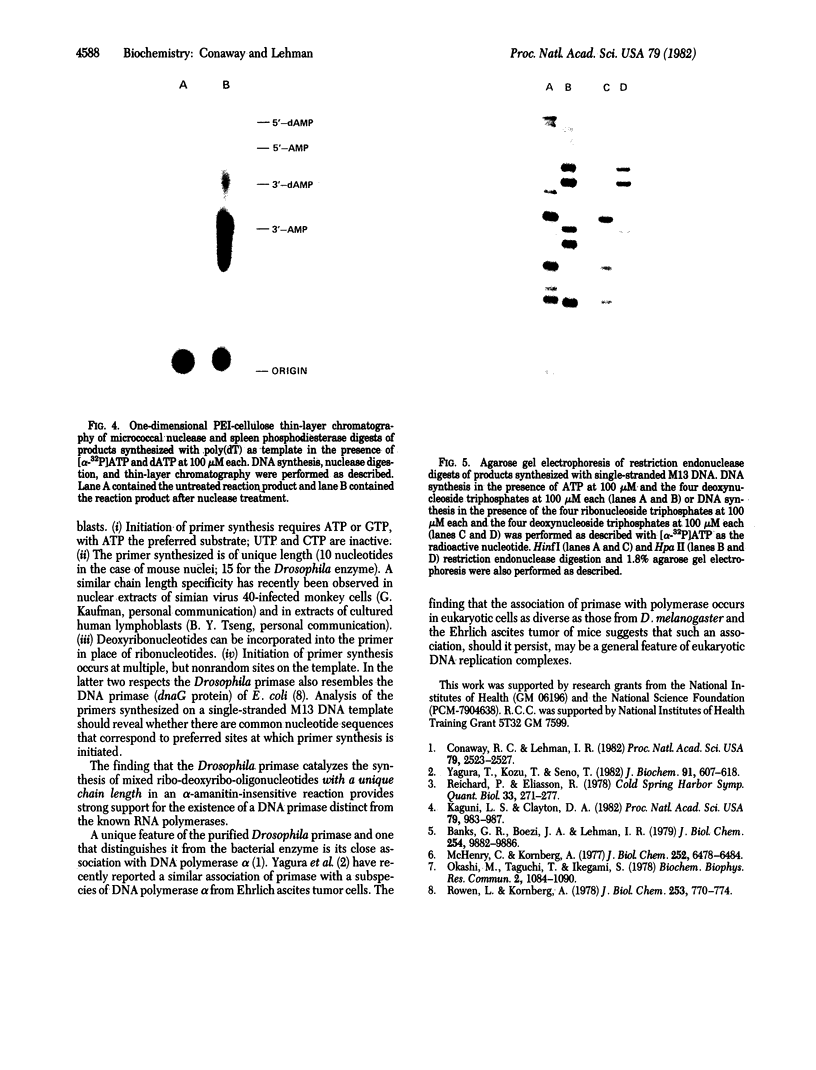
The primase associated with the DNA polymerase alpha from embryos of Drosophila melanogaster catalyzes the synthesis of ribo-oligonucleotide primers on single-stranded M13 DNA or polydeoxythymidylate templates, which can be elongated by DNA polymerase action [Conaway, R. C. & Lehman, I. R. (1982) Proc, Natl. Acad. Sci, USA 79, 2523--2527]. The primers synthesized in a coupled primase-DNA polymerase alpha reaction with an M13 DNA template are of a unique size (15 residues); those synthesized with poly(dT) range from 8 to 15 nucleotides. Primer synthesis is initiated at multiple but nonrandom sites. Like the DNA primase of Escherichia coli and the comparable activity in intact nuclei of polyoma-infected mouse cells, the DNA primase of D. melanogaster can substitute deoxynucleotides for ribonucleotides during primer synthesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks G. R., Boezi J. A., Lehman I. R. A high molecular weight DNA polymerase from Drosophila melanogaster embryos. Purification, structure, and partial characterization. J Biol Chem. 1979 Oct 10;254(19):9886–9892. [PubMed] [Google Scholar]
- Conaway R. C., Lehman I. R. A DNA primase activity associated with DNA polymerase alpha from Drosophila melanogaster embryos. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2523–2527. doi: 10.1073/pnas.79.8.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaguni L. S., Clayton D. A. Template-directed pausing in in vitro DNA synthesis by DNA polymerase a from Drosophila melanogaster embryos. Proc Natl Acad Sci U S A. 1982 Feb;79(4):983–987. doi: 10.1073/pnas.79.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry C., Kornberg A. DNA polymerase III holoenzyme of Escherichia coli. Purification and resolution into subunits. J Biol Chem. 1977 Sep 25;252(18):6478–6484. [PubMed] [Google Scholar]
- Ohashi M., Taguchi T., Ikegami S. Aphidicolin: a specific inhibitor of DNA polymerases in the cytosol of rat liver. Biochem Biophys Res Commun. 1978 Jun 29;82(4):1084–1090. doi: 10.1016/0006-291x(78)90298-x. [DOI] [PubMed] [Google Scholar]
- Rowen L., Kornberg A. A ribo-deoxyribonucleotide primer synthesized by primase. J Biol Chem. 1978 Feb 10;253(3):770–774. [PubMed] [Google Scholar]
- Yagura T., Kozu T., Seno T. Mouse DNA polymerase accompanied by a novel RNA polymerase activity: purification and partial characterization. J Biochem. 1982 Feb;91(2):607–618. doi: 10.1093/oxfordjournals.jbchem.a133732. [DOI] [PubMed] [Google Scholar]