Abstract
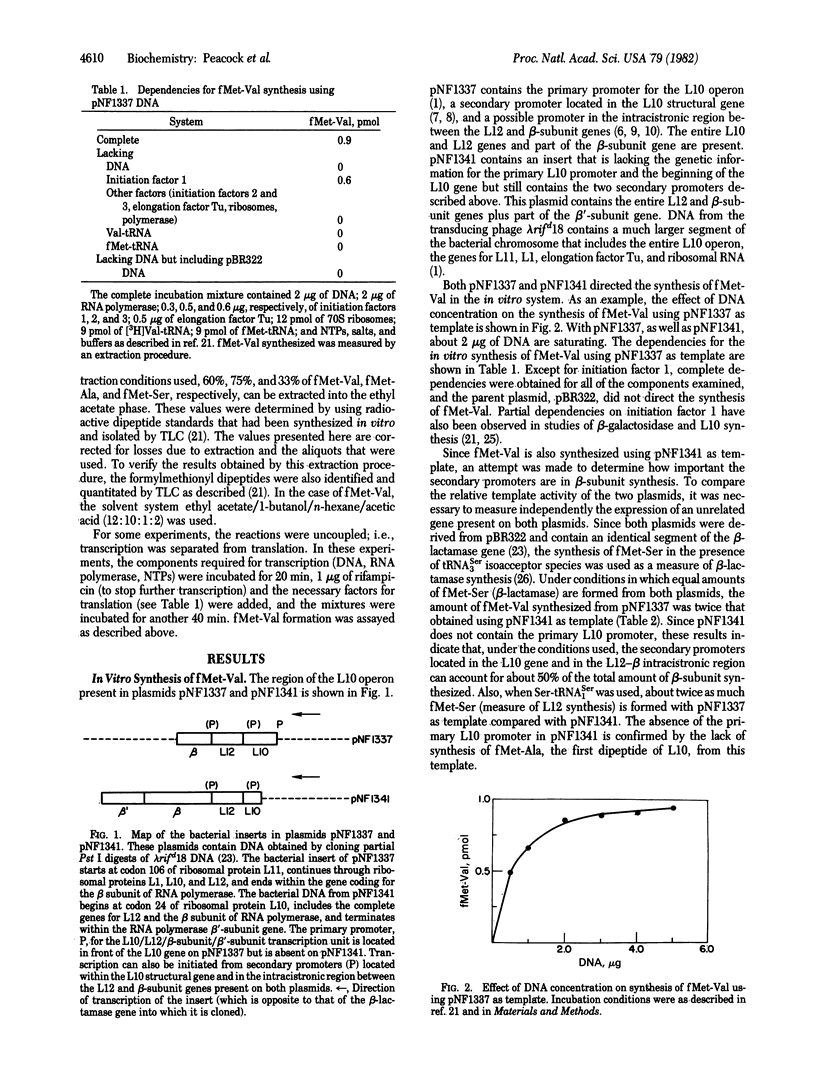
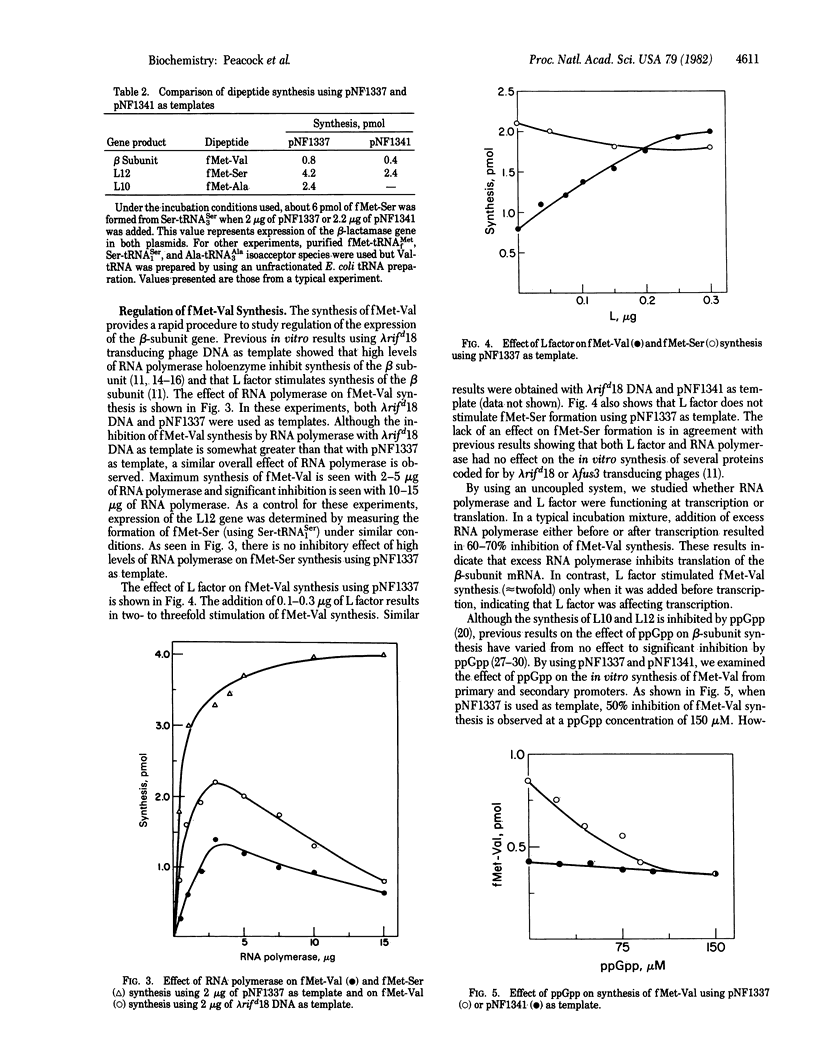
Plasmids pNF1337 and pNF1341, which contain part of the L10 operon including the RNA polymerase beta-subunit gene, have been used as templates in vitro to investigate expression of the beta-subunit gene. For these studies, the synthesis of the first dipeptide of the beta subunit, fMet-Val, was measured instead of that of the entire protein. By using this dipeptide system, we studied the effects of RNA polymerase holoenzyme and L factor (nus A gene product) on fMET-Val synthesis and compared the relative effects of the primary and secondary promoters in the L10 operon on expression of the beta-subunit gene. The results show that the inhibitory effect of RNA polymerase on beta-subunit synthesis and the stimulatory effect of L factor occur before formation of the first dipeptide bond. In this in vitro system, the secondary promoters account for about 50% of the total fMet-Val synthesized. Although the primary promoter is sensitive to guanosine 5'-diphosphate 3'-diphosphate in vitro, the secondary promoters are not affected by this nucleotide.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry G., Squires C. L., Squires C. Control features within the rplJL-rpoBC transcription unit of Escherichia coli. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4922–4926. doi: 10.1073/pnas.76.10.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry G., Squires C., Squires C. L. Attenuation and processing of RNA from the rplJL--rpoBC transcription unit of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3331–3335. doi: 10.1073/pnas.77.6.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal R. M., Lemaux P. G., Neidhardt F. C., Dennis P. P. The effects of the relA gene on the synthesis of aminoacyl-tRNA synthetases and other transcription and translation proteins in Escherichia coli A. Mol Gen Genet. 1976 Dec 22;149(3):291–296. doi: 10.1007/BF00268530. [DOI] [PubMed] [Google Scholar]
- Boyle S. M., Chu F., Brot N., Sells B. H. The relationship between the spoT gene, the synthesis of stable RNA, ribosomal proteins, and the beta beta' subunits of RNA polymerase following a nutritional shiftup of Escherichia coli. Can J Biochem. 1978 Jun;56(6):528–533. doi: 10.1139/o78-081. [DOI] [PubMed] [Google Scholar]
- Brot N., Caldwell P., Weissbach H. Autogenous control of Escherichia coli ribosomal protein L10 synthesis in vitro. Proc Natl Acad Sci U S A. 1980 May;77(5):2592–2595. doi: 10.1073/pnas.77.5.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brückner R., Matzura H. In vivo synthesis of a polycistronic messenger RNA for the ribosomal proteins L11, L1, L10 and L7/12 in Escherichia coli. Mol Gen Genet. 1981;183(2):277–282. doi: 10.1007/BF00270629. [DOI] [PubMed] [Google Scholar]
- Cenatiempo Y., Robakis N., Meza-Basso L., Brot N., Weissbach H., Reid B. R. Use of different tRNASer isoacceptor species in vitro to discriminate between the expression of plasmid genes. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1466–1468. doi: 10.1073/pnas.79.5.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu F., Kung H. F., Caldwell P., Weissbach H., Brot N. DNA dependent synthesis of protein L12 from escherichia coli ribosomes, in vitro. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3156–3159. doi: 10.1073/pnas.73.9.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerman H. W., Steers E., Jr, Redfield B. G., Weissbach H. Methionyl soluble ribonucleic acid transformylase. I. Purification and partial characterization. J Biol Chem. 1967 Apr 10;242(7):1522–1525. [PubMed] [Google Scholar]
- Errington L., Glass R. E., Hayward R. S., Scaife J. G. Structure and orientation of an RNA polymerase operon in Escherichia coli. Nature. 1974 Jun 7;249(457):519–522. doi: 10.1038/249519a0. [DOI] [PubMed] [Google Scholar]
- Fiil N. P., Bendiak D., Collins J., Friesen J. D. Expression of Escherichia coli ribosomal protein and RNA polymerase genes cloned on plasmids. Mol Gen Genet. 1979 May 23;173(1):39–50. doi: 10.1007/BF00267689. [DOI] [PubMed] [Google Scholar]
- Fukuda R., Taketo M., Ishihama A. Autogenous regulation of RNA polymerase beta subunit synthesis in vitro. J Biol Chem. 1978 Jul 10;253(13):4501–4504. [PubMed] [Google Scholar]
- Goldberg G., Caldwell P., Weissbach H., Brot N. In vitro regulation of DNA-dependent synthesis of Escherichia coli ribosomal protein L12. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1716–1720. doi: 10.1073/pnas.76.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg G., Zarucki-Schulz T., Caldwell P., Weissbach H., Brot N. Regulation of the in vitro synthesis of E. coli ribosomal protein L12. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1453–1461. doi: 10.1016/0006-291x(79)91229-4. [DOI] [PubMed] [Google Scholar]
- Greenblatt J., Li J., Adhya S., Friedman D. I., Baron L. S., Redfield B., Kung H. F., Weissbach H. L factor that is required for beta-galactosidase synthesis is the nusA gene product involved in transcription termination. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1991–1994. doi: 10.1073/pnas.77.4.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowachuk E. W., Friesen J. D., Fiil N. P. Bacteriophage lambda vehicle for the direct cloning of Escherichia coli promoter DNA sequences: feedback regulation of the rplJL-rpoBC operon. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2124–2128. doi: 10.1073/pnas.77.4.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajitani M., Fukuda R., Ishihama A. Autogenous and post-transcriptional regulation of Escherichia coli RNA polymerase synthesis in vitro. Mol Gen Genet. 1980;179(3):489–496. doi: 10.1007/BF00271738. [DOI] [PubMed] [Google Scholar]
- Kassavetis G. A., Chamberlin M. J. Pausing and termination of transcription within the early region of bacteriophage T7 DNA in vitro. J Biol Chem. 1981 Mar 25;256(6):2777–2786. [PubMed] [Google Scholar]
- Kirschbaum J. B., Scaife J. Evidence for a lambda transducing phage carrying the genes for the beta and beta' subunits of Escherichia coli RNA polymerase. Mol Gen Genet. 1974;132(3):193–201. doi: 10.1007/BF00269392. [DOI] [PubMed] [Google Scholar]
- Kung H., Spears C., Weissbach H. Purification and properties of a soluble factor required for the deoxyribonucleic acid-directed in vitro synthesis of beta-galactosidase. J Biol Chem. 1975 Feb 25;250(4):1556–1562. [PubMed] [Google Scholar]
- Lang-Yang H., Zubay G. Negative regulation of beta and beta' synthesis by RNA polymerase. Mol Gen Genet. 1981;183(3):514–517. doi: 10.1007/BF00268773. [DOI] [PubMed] [Google Scholar]
- Linn T., Scaife J. Identification of a single promoter in E. coli for rplJ, rplL and rpoBC. Nature. 1978 Nov 2;276(5683):33–37. doi: 10.1038/276033a0. [DOI] [PubMed] [Google Scholar]
- Maher D. L., Dennis P. P. In vivo transcription of E. coli genes coding for rRNA, ribosomal proteins and subunits of RNA polymerase: influence of the stringent control system. Mol Gen Genet. 1977 Oct 20;155(2):203–211. doi: 10.1007/BF00393161. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Monastyrskaya G. S., Gubanov V. V., Guryev S. O., Chertov OYu, Modyanov N. N., Grinkevich V. A., Makarova I. A., Marchenko T. V., Polovnikova I. N. The primary structure of Escherichia coli RNA polymerase. Nucleotide sequence of the rpoB gene and amino-acid sequence of the beta-subunit. Eur J Biochem. 1981 Jun 1;116(3):621–629. doi: 10.1111/j.1432-1033.1981.tb05381.x. [DOI] [PubMed] [Google Scholar]
- Post L. E., Strycharz G. D., Nomura M., Lewis H., Dennis P. P. Nucleotide sequence of the ribosomal protein gene cluster adjacent to the gene for RNA polymerase subunit beta in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1697–1701. doi: 10.1073/pnas.76.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeh S., Pedersen S., Friesen J. D. Biosynthetic regulation of individual proteins in relA+ and relA strains of Escherichia coli during amino acid starvation. Mol Gen Genet. 1976 Dec 22;149(3):279–289. doi: 10.1007/BF00268529. [DOI] [PubMed] [Google Scholar]
- Robakis N., Meza-Basso L., Brot N., Weissbach H. Translational control of ribosomal protein L10 synthesis occurs prior to formation of first peptide bond. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4261–4264. doi: 10.1073/pnas.78.7.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Nomura M. Contranscription of genes for RNA polymerase subunits beta and beta' with genes for ribosomal proteins in Escherichia coli. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3891–3895. doi: 10.1073/pnas.75.8.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. L., Dean D., Strycharz W. A., Nomura M. E. coli ribosomal protein L10 inhibits translation of L10 and L7/L12 mRNAs by acting at a single site. Nature. 1981 Nov 12;294(5837):190–192. doi: 10.1038/294190a0. [DOI] [PubMed] [Google Scholar]
- Zarucki-Schulz T., Jerez C., Goldberg G., Kung H. F., Huang K. H., Brot N., Weissbach H. DNA-directed in vitro synthesis of proteins involved in bacterial transcription and translation. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6115–6119. doi: 10.1073/pnas.76.12.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]


