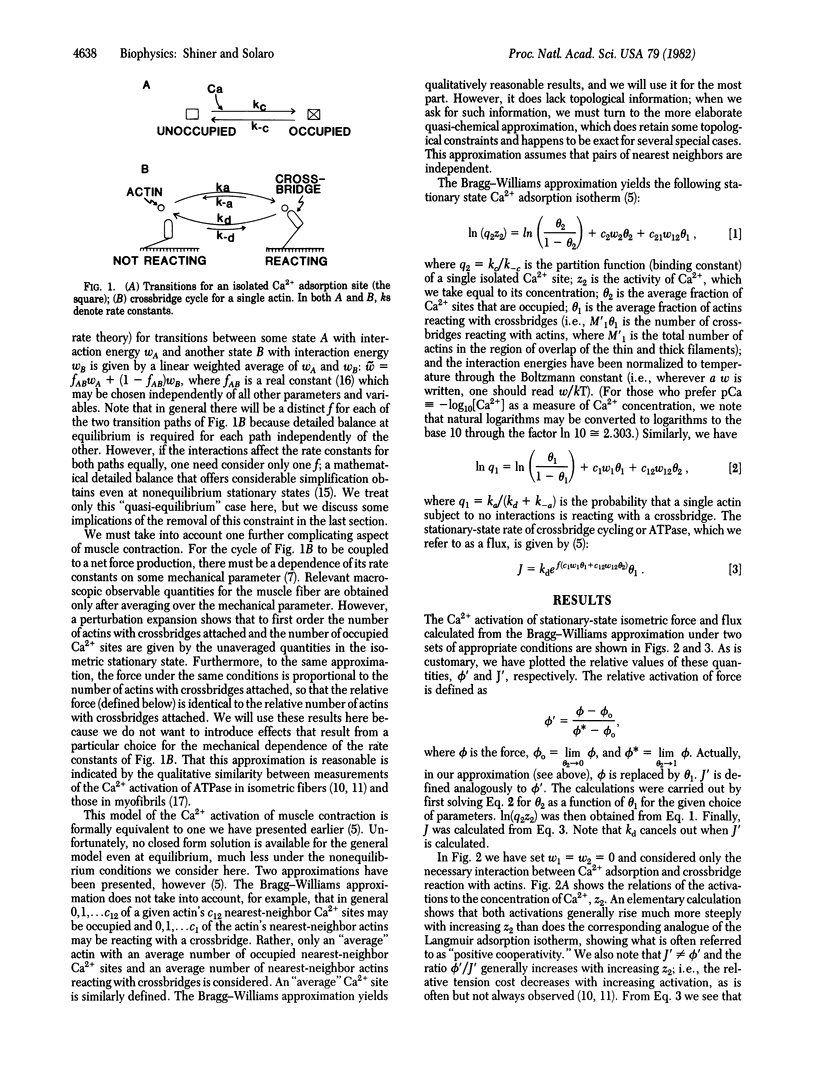
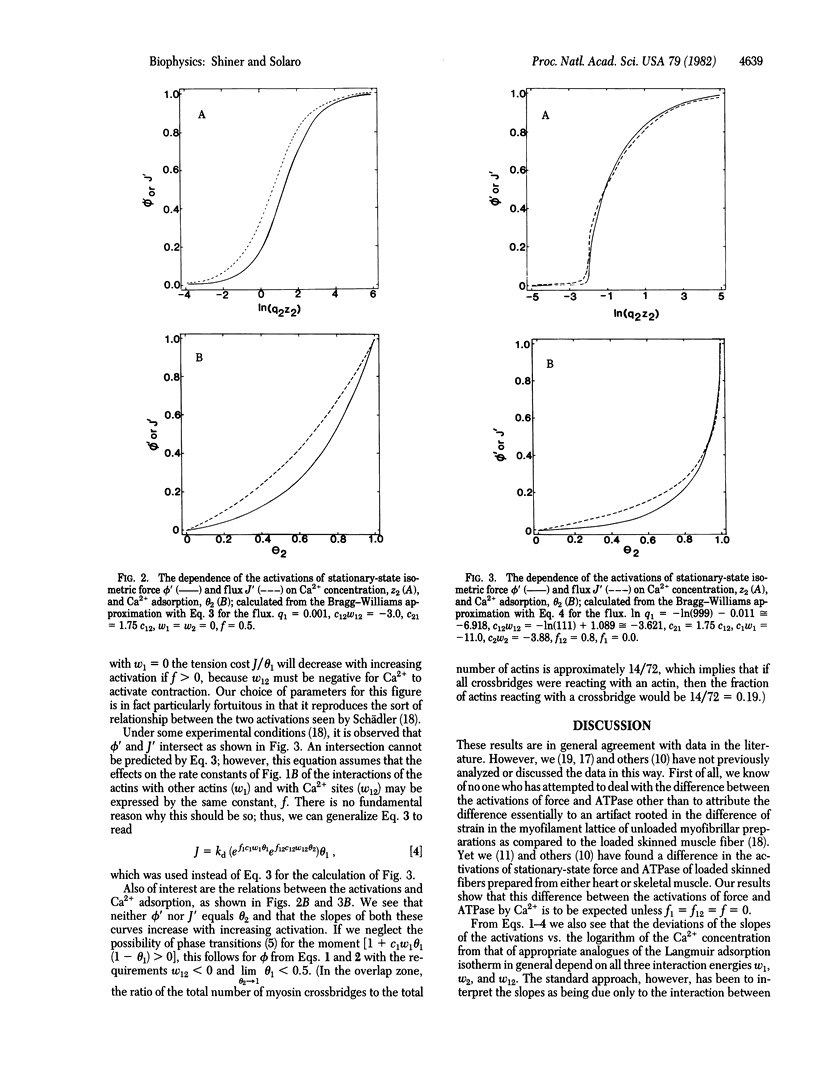
Abstract
We discuss the activation of thin-filament-regulated muscles by calcium ion in terms of a qualitative model based on nearest-neighbor lattice statistics. For the most part, the model takes into account only the essential features of the phenomenon--that there must be an interaction between calcium adsorption to troponin and crossbridge reaction with actin for calcium ion to activate contraction and that the relevant stationary states are nonequilibrium ones. Even so, the model predicts the following features which are seen experimentally but have generally not been considered in previous models: (i) the relative activations of stationary-state isometric force and ATPase are not equal; (ii) in general, neither activation of force nor that of ATPase is proportional to calcium adsorption to the activating sites; and (iii) the slopes of the relations between the activations and the logarithm of the calcium ion concentration generally depend on the necessary interaction between calcium ion adsorption and crossbridge reaction with actin. Thus, these relations show cooperative effects even if these is no interaction between calcium adsorption sites.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. C., Moisescu D. G. Model for the action of calcium in muscle. Nat New Biol. 1972 Jun 14;237(76):208–211. doi: 10.1038/newbio237208a0. [DOI] [PubMed] [Google Scholar]
- Brandt P. W., Cox R. N., Kawai M. Can the binding of Ca2+ to two regulatory sites on troponin C determine the steep pCa/tension relationship of skeletal muscle? Proc Natl Acad Sci U S A. 1980 Aug;77(8):4717–4720. doi: 10.1073/pnas.77.8.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremel R. D., Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nat New Biol. 1972 Jul 26;238(82):97–101. doi: 10.1038/newbio238097a0. [DOI] [PubMed] [Google Scholar]
- Chen Y. D., Hill T. L. Analysis of a simple prototypal muscle model near to and far from equilibrium. Proc Natl Acad Sci U S A. 1974 May;71(5):1982–1986. doi: 10.1073/pnas.71.5.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks R., Cooke R. Tension generation by threads of contractile proteins. J Gen Physiol. 1977 Jan;69(1):37–55. doi: 10.1085/jgp.69.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson S. K., Kerrick W. G. Characterization of the effects of Mg2+ on Ca2+- and Sr2+-activated tension generation of skinned skeletal muscle fibers. J Gen Physiol. 1975 Oct;66(4):427–444. doi: 10.1085/jgp.66.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton B. L. Tropomyosin binding to F-actin induced by myosin heads. Science. 1976 Jun 25;192(4246):1337–1339. doi: 10.1126/science.131972. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Fuchs F., Black B. The effect of magnesium ions on the binding of calcium ions to glycerinated rabbit psoas muscle fibers. Biochim Biophys Acta. 1980 Mar 26;622(1):52–62. doi: 10.1016/0005-2795(80)90157-9. [DOI] [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. Cooperative binding of myosin subfragment-1 to the actin-troponin-tropomyosin complex. Proc Natl Acad Sci U S A. 1980 May;77(5):2616–2620. doi: 10.1073/pnas.77.5.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Heins M., Hendricks J., Martindale L., Smock S., Stein M., Jacobs J. Attitudes of women and men physicians. Am J Public Health. 1979 Nov;69(11):1132–1139. doi: 10.2105/ajph.69.11.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig J. W., Peterson J. W., Rüegg J. C., Solaro R. J. Vanadate and phosphate ions reduce tension and increase cross-bridge kinetics in chemically skinned heart muscle. Biochim Biophys Acta. 1981 Jan 21;672(2):191–196. doi: 10.1016/0304-4165(81)90392-5. [DOI] [PubMed] [Google Scholar]
- Hill T. L. Effect of enzyme-enzyme interactions on steady-state enzyme kinetics. IV. "Strictly steady-state" examples. J Theor Biol. 1978 Dec 21;75(4):391–416. doi: 10.1016/0022-5193(78)90352-1. [DOI] [PubMed] [Google Scholar]
- Hill T. L. Theoretical formalism for the sliding filament model of contraction of striated muscle. Part I. Prog Biophys Mol Biol. 1974;28:267–340. doi: 10.1016/0079-6107(74)90020-0. [DOI] [PubMed] [Google Scholar]
- Hill T. L. Theoretical study of the effect of enzyme-enzyme interactions on steady-state enzyme kinetics. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3632–3636. doi: 10.1073/pnas.74.9.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Levy R. M., Umazume Y., Kushmerick M. J. Ca2+ dependence of tension and ADP production in segments of chemically skinned muscle fibers. Biochim Biophys Acta. 1976 May 14;430(2):352–365. doi: 10.1016/0005-2728(76)90091-8. [DOI] [PubMed] [Google Scholar]
- Marston S., Weber A. The dissociation constant of the actin-heavy meromyosin subfragment-1 complex. Biochemistry. 1975 Aug 26;14(17):3868–3873. doi: 10.1021/bi00688a021. [DOI] [PubMed] [Google Scholar]
- Moisescu D. G. Kinetics of reaction in calcium-activated skinned muscle fibres. Nature. 1976 Aug 12;262(5569):610–613. doi: 10.1038/262610a0. [DOI] [PubMed] [Google Scholar]
- Potter J. D., Gergely J. The calcium and magnesium binding sites on troponin and their role in the regulation of myofibrillar adenosine triphosphatase. J Biol Chem. 1975 Jun 25;250(12):4628–4633. [PubMed] [Google Scholar]
- Robertson S. P., Johnson J. D., Potter J. D. The time-course of Ca2+ exchange with calmodulin, troponin, parvalbumin, and myosin in response to transient increases in Ca2+. Biophys J. 1981 Jun;34(3):559–569. doi: 10.1016/S0006-3495(81)84868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schädler M. Proportional Aktivierung von ATPase-Aktivität und Kontraktionsspannung durch Calciumionen in isolierten contractilen Strukturen verschiedener Muskelarten. Pflugers Arch Gesamte Physiol Menschen Tiere. 1967;296(1):70–90. [PubMed] [Google Scholar]
- Shimizu H. Dynamic cooperativity of molecular processes in active streaming, muscle contraction, and subcellular dynamics: the molecular mechanism of self-organization at the subcellular level. Adv Biophys. 1979;13:195–278. [PubMed] [Google Scholar]
- Shiner J. S., Solaro R. J. The effects of modifiers on enzyme catalysis: a non-classical nearest neighbor approach. Biophys Chem. 1981 Aug;13(4):291–306. doi: 10.1016/0301-4622(81)85003-x. [DOI] [PubMed] [Google Scholar]
- Solaro R. J., Shiner J. S. Modulation of Ca2+ control of dog and rabbit cardiac myofibrils by Mg2+. Comparison with rabbit skeletal myofibrils. Circ Res. 1976 Jul;39(1):8–14. doi: 10.1161/01.res.39.1.8. [DOI] [PubMed] [Google Scholar]
- Solaro R. J., Wise R. M., Shiner J. S., Briggs F. N. Calcium requirements for cardiac myofibrillar activation. Circ Res. 1974 Apr;34(4):525–530. doi: 10.1161/01.res.34.4.525. [DOI] [PubMed] [Google Scholar]
- Thompson C. J. Models for hemoglobin and allosteric enzymes. Biopolymers. 1968;6(8):1101–1118. doi: 10.1002/bip.1968.360060806. [DOI] [PubMed] [Google Scholar]
- Weber A., Murray J. M. Molecular control mechanisms in muscle contraction. Physiol Rev. 1973 Jul;53(3):612–673. doi: 10.1152/physrev.1973.53.3.612. [DOI] [PubMed] [Google Scholar]



