Abstract
Background
Pupil diameter is a frequently assessed objective index of the pharmacodynamic effects of opioids in adults, but to our knowledge has never been examined in infants. Such a measure could improve assessment and treatment of neonates exposed to opioids in utero. The present study examined changes in pupil diameter after opioid administration in opioid-exposed infants who required pharmacological treatment for neonatal abstinence syndrome (NAS) to test the feasibility of using pupil diameter as a measure of opioid effects in these infants.
Methods
Ten infants (2–7 days old) receiving methadone (0.4–0.5 mg every 12 hours) for the treatment of NAS participated. A picture of one of each infant's eyes was taken under controlled illumination conditions with a standard digital camera just prior to dosing and 0–1, 2–4, 5–7, and 8–10 hours after dosing. The diameters of the pupil and iris were measured and relative pupil diameter (pupil diameter expressed as a percentage of iris diameter) was analyzed.
Results
Mean (±SE) relative pupil diameter decreased significantly after dosing from 41±2% to 29±2%. After dosing, a significant increasing linear trend was observed over time, with values of 29±2%, 33±3%, 38±3%, and 41±3% at 0–1, 2–4, 5–7, and 8–10 hours after dosing.
Conclusions
Infant pupils respond to opioid administration in the same sensitive, orderly manner as is commonly observed in adults. Pupil diameter appears to be an objective, sensitive measure of neonatal response to opioids that may be a useful complement to, or perhaps at times a replacement for, observer-rated scale scores.
Keywords: Opioids, methadone, pupil diameter, neonatal abstinence syndrome
1. Introduction
Prenatal opioid exposure is a rapidly growing public health problem due to continued heroin abuse and alarming increases in prescription opioid abuse by pregnant women (Patrick et al., 2012). Both illicit and licit opioid dependence during pregnancy is associated with adverse consequences for the neonate, including a high incidence of neonatal abstinence syndrome (NAS), a disorder characterized by central nervous system hyperirritability, gastrointestinal dysfunction, respiratory distress, and autonomic dysregulation (Finnegan et al., 1975). Extended hospitalization (4 days minimum) is recommended to monitor NAS in all opioid-exposed neonates. At least 50% will require pharmacological treatment to manage their NAS (Cleary et al., 2010; Jones et al., submitted), resulting in hospital stays of 8–79 days (Kraft et al., 2011) and dramatically increasing costs. A recent report conservatively estimated that the total US hospital charges to care for infants with NAS currently exceed $700 million per year (Patrick et al., 2012).
The only tools currently available for evaluating the need for and effectiveness of pharmacological treatment for NAS are observer-rated scales. The most commonly used observer-rated scales (e.g., Finnegan Scale, Lipsitz tool) were developed and underwent rudimentary validation testing in the mid-1970s, driven by dramatic increases in the number of opioid-exposed neonates as a result of an epidemic of heroin use (Hughes and Rieche, 1995). While the development and dissemination of observer-rated scales was an important advance in the assessment and treatment of NAS, the scales have some shortcomings that limit their utility. For example, there are concerns regarding the lack of rigorous psychometric testing to establish reliability and validity as well as the lengthy training and administration times required to accurately administer most observer-rated scales (American Academy of Pediatrics, 1998; Jones, 2011; O’Brien et al., 2004). Increasing calls for the development of more objective and sensitive measures of NAS (Jones, 2011; O’Brien et al., 2004; Velez et al., 2009) prompted us to investigate pupil diameter as a potential marker of infant response to opioid administration.
In adults, pupil diameter is a frequently assessed objective index of the pharmacodynamic effects of opioids (see review by Loewenfeld, 1999). For example, opioid administration results in pupil constriction, followed by predictable, progressive dilation over time as the drug is metabolized (e.g., Fraser and Isbell, 1952a; 1952b; Fraser et al., 1954). The magnitude and time course of pupillary effects vary according to the pharmacodynamics and pharmacokinetics of the opioid. For example, milligram for milligram, methadone is more potent than morphine and has a longer duration of action. Consistent with this profile, pupil constriction is four-fold greater and lasts significantly longer after 30 mg of oral methadone vs. morphine (Fraser et al., 1954). Pupil diameter measures also (1) correlate significantly with self-report and observer-rated measures of withdrawal (e.g., Fraser et al., 1954; Higgins et al., 1985; Jasinski et al., 1978), (2) are an index of the severity of tolerance (e.g., Higgins et al., 1985), and (3) differentiate between dependent and nondependent populations (e.g., Tress et al., 1978). These results underscore the elegant, orderly relationship between opioids and pupil diameter in adults.
Although the pupillary effects of opioids are well documented in adults, to our knowledge, they have not been examined in neonates. While the pressing need for more objective and sensitive measures of NAS is perhaps the primary motivation for exploring pupil diameter in infants, it is by no means the only motivation. Opioid withdrawal also occurs in upwards of 60% of infants and children treated with opioids following surgery or other serious illnesses and recent reviews have noted the dearth of valid and reliable methods to measure opioid effects in pediatric populations (Anand et al., 2010; Ista et al., 2007). With more than 5 million pediatric surgeries performed each year in the US alone (Cullen et al., 2009; Hall et al., 2010), development of an objective, sensitive measure of withdrawal could be a significant advance in the assessment and treatment of opioid withdrawal that develops iatrogenically as well as after passive prenatal exposure.
As a first step in this direction, the primary aim of the present study was to examine pupil diameter in neonates receiving pharmacological treatment for NAS related to in utero exposure. Specifically, the time course of changes in neonatal pupil diameter in response to methadone, the first-line NAS treatment medication at our university-affiliated hospital, was measured to test whether neonatal pupils respond to opioids in an orderly, graded manner dependent on time since administration as is so well established in adults.
2. Methods
2.1 Participants
Participants were 10 neonates exposed to opioids in utero and requiring pharmacological treatment for NAS (Table 1). Infants had to have been full term at delivery (> 37 weeks estimated gestational age) since the pupillary reflex is altered in pre-term infants (Cocker et al., 2005). Infants also could not have any obvious ocular problems or any serious medical conditions (e.g., need for intubation or surgery). At the time of the present study, all neonates had been stabilized (i.e., dose had not changed for 24 hours) on methadone (0.4–0.5 mg every 12 hours). The study was approved by the University of Vermont Institutional Review Board and parental informed consent was obtained for each neonate.
Table 1.
Participant Characteristics (N=10)
| Characteristics | Methadone- treated neonates (N=10) |
|---|---|
| Sex (% male) | 70 |
| Race (% White) | 100 |
| Prenatal exposure (%) | |
| Buprenorphine maintenance | 60 |
| Methadone maintenance | 30 |
| Not stated in medical record | 10 |
| Estimated gestational age at birth (weeks) | 39.6 ± 1.3 |
| Birthweight (g) | 3235.7 ± 391.2 |
| Apgar scores at birth | |
| 1 minute | 7.9 ± 1.0 |
| 5 minutes | 9.0 ± 0.0 |
| Age at time of pupil study participation (days) | 4.0 ± 1.6 |
| Methadone dose (mg/kg) every 12 hours | 0.44 ± .05 |
Note: Values are mean (± SD) unless otherwise indicated.
2.2 Procedures
Prior to administration of the next dose of methadone, light levels were measured with a luxometer and adjusted as needed. Within each neonate, all digital photographs to measure pupil diameter were taken at the same lux level (± 2 lux) and all neonates were photographed between 20–40 lux. At least one minute was allowed to elapse between establishing light levels and taking photographs. At least 5 minutes were allowed to elapse if the neonate had just awakened (Reeves, 1920). All efforts were made to obtain pictures while the neonate was calm, generally while the infant was lying in a pram or being held by a nurse or parent. The same eye was photographed each time within individual neonates to control for anisocoria (i.e., unequal pupil sizes; Isenberg et al., 1989; Roarty and Keltner, 1990).
All photographs were taken by the second author using an Olympus Stylus 770 SW 7.1 megapixel digital camera set on macro with an automatic flash. The camera’s image capture speed was much shorter than the minimum latent period of the pupillary light reflex (<10 milliseconds vs. 180 millisecond; Lowenfeld, 1999); thus, the flash did not alter the size of the pupil in the resulting image, but did make the boundaries of the pupil and iris on the resulting image crisper, facilitating measurement. Other studies measuring pupil diameter in infants, children, and adults have used this same procedure (MacLachlan and Howland, 2002; Roarty and Keltner, 1990; Twa et al., 2004). Every attempt was made to hold the camera on the same horizontal and vertical plane as the eye that was being photographed. The minimum focal distance of the camera was 6–8 inches, so the camera was positioned approximately 8–10 inches from the eye to ensure a focused shot. To be considered a valid image, the resulting picture had to include at least a full horizontal slice of the eye consisting of the middle of the pupil, the surrounding iris and some sclera visible on both sides of the iris. If more than one photograph was required (approximately 20% of all photos), at least one minute was allowed to elapse between them to allow the pupil to recover from the flash of the camera (MacLachlan and Howland, 2002).
A second photograph was taken within the first hour after methadone administration as the adult literature suggests significant constriction should occur during this time (McCaul et al., 1982). If the neonate fell asleep before this photograph was taken, their eyelid was gently manually retracted to facilitate photography, a common practice in eye photography with infants (e.g., Cocker et al., 2005). Subsequent digital photographs were timed to coincide with routine clinical assessments of the infants every 3 hours (± 1 hour) until the next methadone dose was administered. Thus, photographs were taken 0–1, 2–4, 5–7, and 8–10 hours after dosing for a total of 4 photographs per infant post-dosing.
2.3 Photograph processing and measurement
Digital photographs were downloaded onto a computer. They were cropped using XnView (Version 1.97.8, freeware developed by P-e Gougelet) so that only the target eye was visible and rotated, if necessary, so that the corners of the eye were on the same horizontal plane. The files were then renamed with a random unique number to allow for blind coding.
The first author, who was blind to the infant photographed and time of each photo, made all pupil and iris measurements using ImageJ (Version 1.43; Rasband, 2011). The horizontal width of the pupil at its widest point was measured in pixels and then the width of the iris was measured along the same horizontal plane. The relative size of the pupil was calculated by dividing pupil width by iris width and multiplying the result by 100. Relative pupil diameter controlled for any variations in the vertex distance (i.e., the distance from the camera lens to the front of the participant’s eye), a strategy that has been used successfully in other studies of fetal and neonatal eye function (Birch and Held, 1982; Lopez, 2011; Shea et al., 1985).
2.4 Data analysis
A MANOVA was used to test for differences across time points in relative pupil diameter. A univariate repeated measures ANOVA (SAS, PROC MIXED) was also used in order to construct specific contrasts to test for a difference between pre- and post-dosing means and to evaluate the quantitative temporal trends over the four post-dosing time points (i.e. linear, quadratic, and cubic). For the evaluation of quantitative trends, the spacing associated with the post-dosing time points were based on the midpoints of the assessment intervals (0.5, 3, 6 and 9 hours). The univariate repeated measures ANOVA utilized compound symmetry as a covariance structure based on Akaike’s Information Criterion (AIC). Analyses were performed using SAS statistical software Version 9 (SAS Institute, Cary, NC). Statistical significance was determined based on α = .05.
3. Results
3.1 Pupillary response
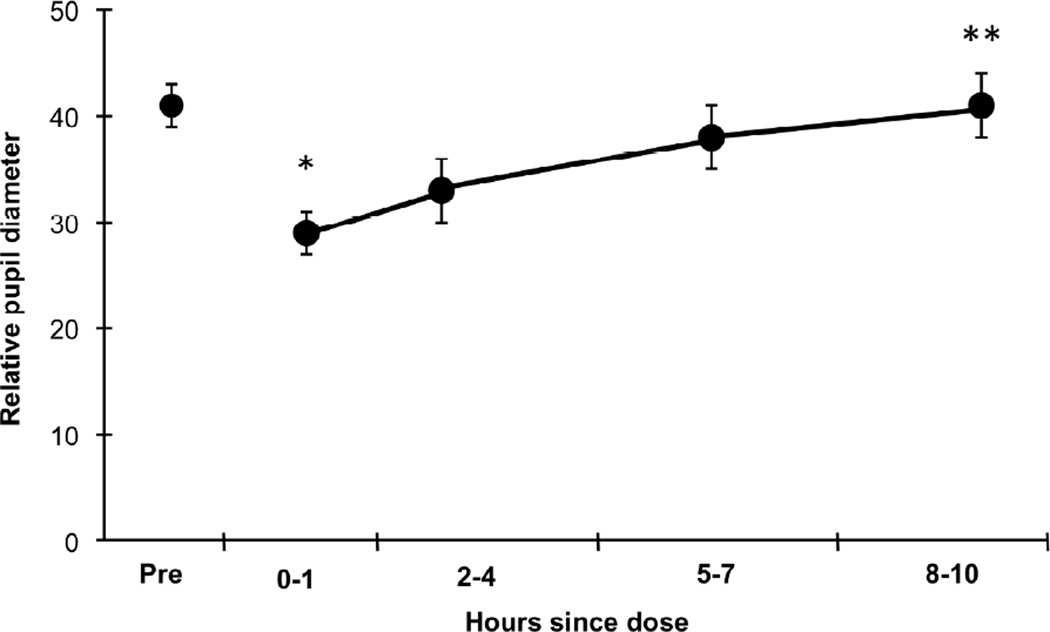
There were orderly, significant changes over time for relative pupil diameter (MANOVA F4, 6 = 15.0, p = .003) (Figure 1). Relative pupil diameter decreased significantly after dosing (F1,36 =54.9, p <.001). After dosing, a significant increasing trend was observed over time across assessments (linear trend, F1,36 =58.3, p <.001) with no significant higher order trends (quadratic, F1,36 =3.16, p =.08 and cubic, F1,36 =0.07, p=.78).
Figure 1.
Mean (± SE) relative pupil diameter for 10 neonates exposed to opioids in utero who required pharmacological treatment for NAS postnatally. Neonates were stabilized on methadone (0.4–0.5 mg every 12 hours) and relative pupil diameter measurements were calculated from pictures taken just prior to dosing (Pre) and 0–1, 2–4, 5–7, and 8–10 hours after dosing. * Indicates the 0–1 time point is significantly different from the Pre time point (p <.001). ** Indicates a significant linear time trend across the 4 post-dosing time points (p <.001).
4. Discussion
The primary aim of this study was to examine the sensitivity of neonatal pupil diameter in response to methadone dosing to test whether neonatal pupils respond to opioids in an orderly, graded manner that is comparable to the well-characterized changes observed in adults. The results provide strong evidence that infant pupillary response is indeed highly sensitive to opioid agonist medications. Neonatal pupil diameter decreased significantly following methadone administration, then gradually increased over time, returning to pre-treatment levels by 8–10 hours after dosing. These results demonstrate the feasibility of using pupil diameter as an objective, sensitive measure of neonatal response to opioids.
Having demonstrated that neonatal pupils respond to opioid administration in an orderly, time-sensitive manner comparable to that seen in adults, the rich adult literature suggests other studies that could be done to investigate the utility of this measure in managing neonatal opioid exposure. One brief example is the observation that pupillary response differentiates between opioid-dependent and non-dependent adults (e.g., Tress and El-Sobky, 1979). Demonstrating that opioid-exposed neonates exhibit greater pupil dilation after delivery compared to non-exposed neonates would provide additional evidence of the validity of pupil diameter as a measure of NAS, but could also have clinical applicability, as it is often difficult to differentiate signs of common neonatal problems such as colic, infection, and metabolic disorders from signs of opioid withdrawal when exposure status is unknown (American Academy of Pediatrics, 1998).
Limitations of the present study include the small sample size and lack of information about neonatal exposure to other licit and illicit drugs. Despite these limitations, results of this feasibility study suggest that pupil diameter is an objective, sensitive measure of opioid exposure. Further research is warranted to examine whether pupillary response is a useful complement to, or perhaps at times a replacement for, observer-rated scale scores when treating the growing number of neonates exposed to opioids in utero as well as infants and children receiving chronic opioid treatment as part of routine medical care.
Acknowledgments
Role of Funding Source
This study was supported in part by grant T32 DA007242 from the National Institute on Drug Abuse (NIDA). NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Drs. Heil and Gaalema designed the study and wrote the protocol with input from Drs. Higgins, Johnston, and Sigmon. Dr. Gaalema collected the data. Mr. Badger conducted the statistical analyses. Dr. Heil wrote the first draft of the manuscript and all authors contributed to and have approved the final manuscript.
Conflict of Interest
The authors have no conflict of interest to declare.
References
- Anand KJ, Willson DF, Berger J, Harrison R, Meert KL, Zimmerman J, Carcillo J, Newth CJ, Prodhan P, Dean JM, Nicholson C Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network. Tolerance and withdrawal from prolonged opioid use in critically ill children. Pediatrics. 2010;125:e1208–e1225. doi: 10.1542/peds.2009-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Pediatrics Committee on Drugs. Neonatal drug withdrawal. Pediatrics. 1998;101:1079–1088. [PubMed] [Google Scholar]
- Birch EE, Held R. The development of binocular summation in human infants. Invest Ophthalmol. Vis. Sci. 1982;24:1103–1107. [PubMed] [Google Scholar]
- Cleary BJ, Donnelly J, Strawbridge J, Gallagher PJ, Fahey T, Clarke M, Murphy DJ. Methadone dose and neonatal abstinence syndrome-systematic review and meta-analysis. Addiction. 2010;105:2071–2084. doi: 10.1111/j.1360-0443.2010.03120.x. [DOI] [PubMed] [Google Scholar]
- Cocker KD, Fielder AR, Moseley MJ, Edwards AD. Measurements of pupillary responses to light in term and preterm infants. Neuroophthalmology. 2005;29:95–101. [Google Scholar]
- Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl. Health Stat. Report. 2009;11:1–25. [PubMed] [Google Scholar]
- Finnegan LP, Connaughton JF, Kron RE, Emich JP. Neonatal abstinence syndrome: Assessment and management. Addict. Dis. 1975;2:141–158. [PubMed] [Google Scholar]
- Fraser HF, Isbell H. Actions and addiction liabilities of alpha-acetylmethadols in man. J. Pharmacol. Exp. Ther. 1952a;105:458–465. [PubMed] [Google Scholar]
- Fraser HF, Isbell H. Comparative effects of 20 mgm of morphine sulfate on non-addicts and former morphine addicts. J. Pharmacol. Exp. Ther. 1952b;105:498–502. [PubMed] [Google Scholar]
- Fraser HF, Nash TL, Vanhorn GD, Isbell H. Use of miotic effect in evaluating analgesic drugs in man. Arch. Int. Pharmacodyn. Thér. 1954;98:443–451. [PubMed] [Google Scholar]
- Hall MJ, DeFrances CJ, Williams SN, Golosinskiy A, Schwartzman A. National Hospital Discharge Survey: 2007 summary. Natl. Health Stat. Report. 2010;29:1–20. [PubMed] [Google Scholar]
- Higgins ST, Stitzer ML, McCaul ME, Bigelow GE, Liebson IA. Pupillary response to methadone challenge in heroin users. Clin. Pharmacol. Ther. 1985;37:460–463. doi: 10.1038/clpt.1985.71. [DOI] [PubMed] [Google Scholar]
- Hughes PH, Rieche O. Heroin epidemics revisited. Epidemiol. Rev. 1995;17:66–73. doi: 10.1093/oxfordjournals.epirev.a036186. [DOI] [PubMed] [Google Scholar]
- Isenberg SJ, Dang Y, Jotterand V. The pupils of term and preterm infants. Am. J. Ophthalmol. 1989;108:75–79. doi: 10.1016/s0002-9394(14)73264-7. [DOI] [PubMed] [Google Scholar]
- Ista E, van Dijk M, Gamel C, Tibboel D, de Hoog M. Withdrawal symptoms in children after longer-term administration of sedatives and/or analgesics: a literature review. “Assessment remains troublesome.”. Intensive Care Med. 2007;33:1396–1406. doi: 10.1007/s00134-007-0696-x. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Pevnick JS, Griffith JD. Human pharmacology and abuse potential of the analgesic buprenorphine: a potential agent for treating narcotic addiction. Arch. Gen. Psychiatry. 1978;35:501–516. doi: 10.1001/archpsyc.1978.01770280111012. [DOI] [PubMed] [Google Scholar]
- Jones HE. Commentary on Kraft 2011. Treatment of neonatal abstinence syndrome – morphine, buprenorphine and beyond. Addiction. 2011;106:775–781. doi: 10.1111/j.1360-0443.2010.03236.x. [DOI] [PubMed] [Google Scholar]
- Jones HE, Arria AM, Baewert A, Heil SH, Kaltenbach K, Martin PR, Coyle MG, Selby P, Stine SM. Methadone or buprenorphine treatment of opioid-dependent pregnant women: an overview of current issues. Addiction. doi: 10.1111/j.1360-0443.2012.04035.x. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft WK, Dysart K, Greenspan JS, Gibson E, Kaltenbach K, Ehrlich ME. Revised dose schema of sublingual buprenorphine in the treatment of the neonatal opioid abstinence syndrome. Addiction. 2011;106:574–580. doi: 10.1111/j.1360-0443.2010.03170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenfeld IE. The Pupil: Anatomy, Physiology and Clinical Applications. Boston: Butterworth-Heinemann; 1999. [Google Scholar]
- Lopez RYCC. Response of the foetal pupil to vibro-acoustic stimulation: a foetal attention test. Early Hum. Dev. 2011;87:199–204. doi: 10.1016/j.earlhumdev.2010.12.007. [DOI] [PubMed] [Google Scholar]
- MacLachlan C, Howland HC. Normal values and standard deviations for pupil diameter and interpupillary distance in subjects aged 1 month to 19 years. Ophthal. Physiol. Opt. 2002;22:175–182. doi: 10.1046/j.1475-1313.2002.00023.x. [DOI] [PubMed] [Google Scholar]
- McCaul ME, Bigelow GE, Stitzer ML, Liebson I. Short-term effects of oral methadone in methadone maintenance subjects. Clin. Pharmacol. Ther. 1982;31:753–761. doi: 10.1038/clpt.1982.106. [DOI] [PubMed] [Google Scholar]
- O’Brien C, Hunt R, Jeffery HE. Measurement of movement is an objective method to assist in assessment of opiate withdrawal in newborns. Arch. Dis. Child Fetal Neonatal Ed. 2004;89:F305–F309. doi: 10.1136/adc.2002.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000–2009. JAMA Advance online publication. 2012 doi: 10.1001/jama.2012.3951. http://jama.ama-assn.org. [DOI] [PubMed] [Google Scholar]
- Rasband WS. Bethesda, Maryland, USA: U. S. National Institutes of Health; 2011. [accessed August 30, 2011]. ImageJ. http://imagej.nih.gov/ij/, 1997–2011. [Google Scholar]
- Reeves P. The response of the average pupil to various intensities of light. J. Opt. Soc. Am. 1920;4:35–43. [Google Scholar]
- Roarty JD, Keltner JL. Normal pupil size and anisocoria in newborn infants. Arch. Ophthalmol. 1990;108:94–95. doi: 10.1001/archopht.1990.01070030100037. [DOI] [PubMed] [Google Scholar]
- Shea SL, Doussard-Roosevelt JA, Aslin RN. Pupillary measures of binocular luminance summation in infants and stereoblind adults. Invest. Ophthalmol. Vis. Sci. 1985;26:1064–1070. [PubMed] [Google Scholar]
- Tress KH, El-Sobky AA. Pupil responses to intravenous heroin (diamorphine) in dependent and non-dependent humans. Br. J. Clin. Pharmacol. 1979;7:213–217. doi: 10.1111/j.1365-2125.1979.tb00924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tress KH, El-Sobky AA, Aherne W, Piall E. Degree of tolerance and the relationship between plasma morphine concentration and pupil diameter following intravenous heroin in man. Br. J. Clin. Pharmacol. 1978;5:299–303. [Google Scholar]
- Twa MD, Bailey MD, Hayes J, Bullimore M. Estimation of pupil size by digital photography. J. Cataract Refract. Surg. 2004;30:381–389. doi: 10.1016/S0886-3350(03)00619-9. [DOI] [PubMed] [Google Scholar]
- Velez ML, Jansson LM, Schroeder J, Williams E. Prenatal methadone exposure and neonatal neurobehavioral functioning. Pediatr. Res. 2009;66:704–709. doi: 10.1203/PDR.0b013e3181bc035d. [DOI] [PMC free article] [PubMed] [Google Scholar]