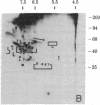
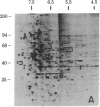
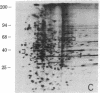
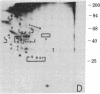
Abstract
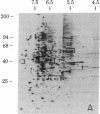
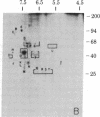
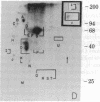
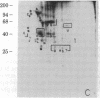
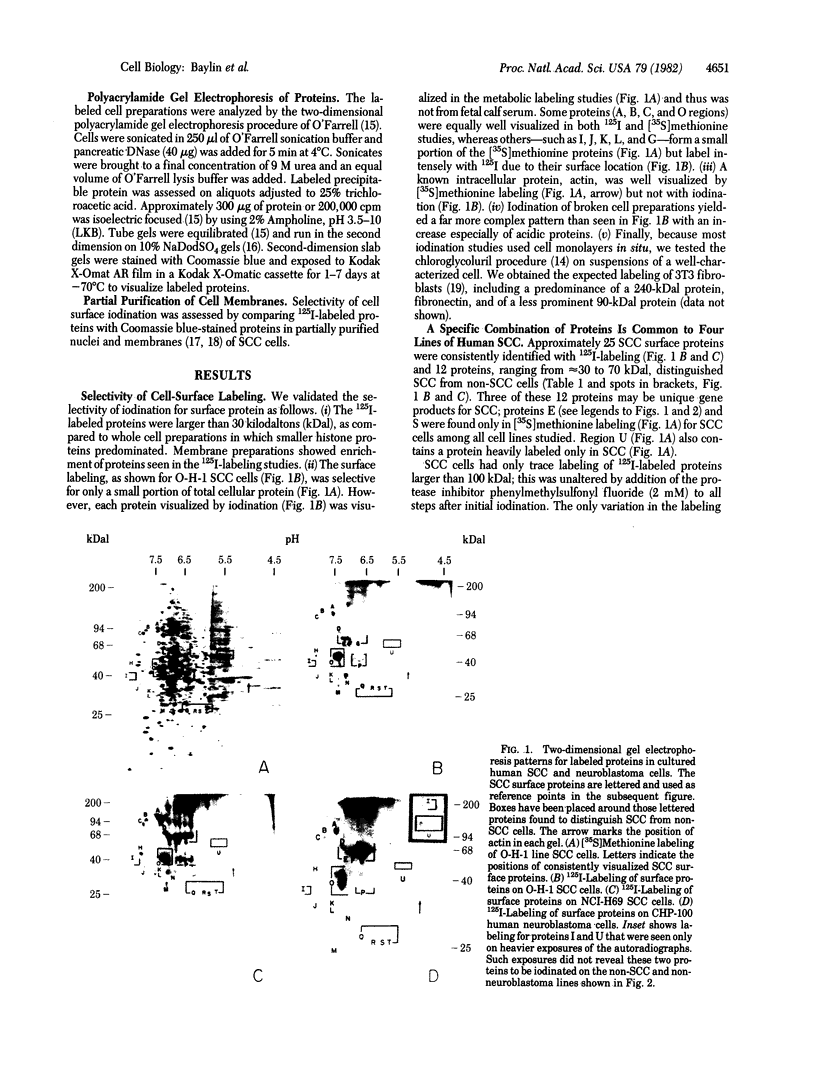
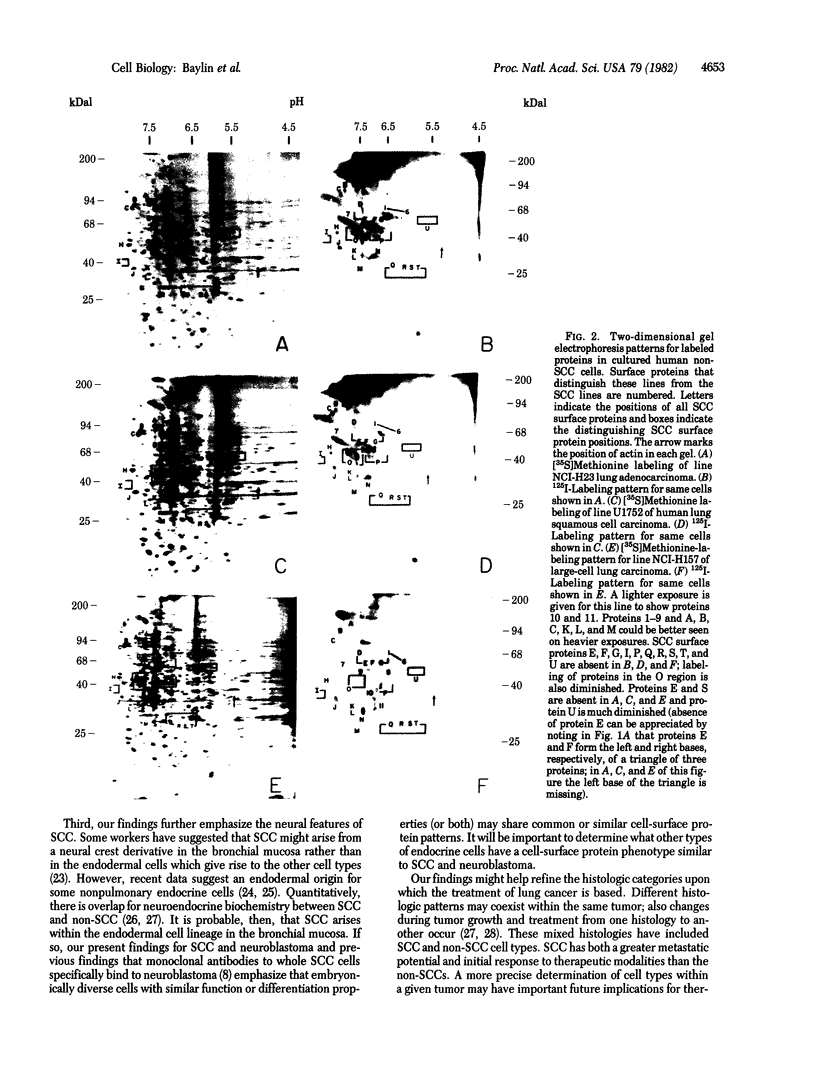
We have used radioiodination (125I) and two-dimensional polyacrylamide gel electrophoresis to determine that small- (oat) cell lung carcinoma (SCC)--a tumor with neuroendocrine features--possesses a surface protein pattern distinct from the other types of lung cancer cells (squamous, adeno-, and large-cell undifferentiated carcinoma). Twelve distinguishing proteins, 40 to 70 kilodaltons (kDal), characterized four separate lines of SCC; three of these, designated E (60 kDal; pI = 7.3), S (30 kDal; pI = 6.0), and U (57 kDal; pI = 5.6), may be unique SCC gene products and were identified only in [35S]methionine labeling of SCC and not in non-SCC or human fibroblasts. Two lines of adeno-, one of squamous, and one of undifferentiated large-cell lung carcinoma exhibited similar surface protein patterns to one another. Nine distinguishing proteins (40 to 100 kDal) and at least five large proteins (greater than 100 kDal) were unique to these lines. The surface protein phenotypes for SCC and non-SCC were distinct from those for human lymphoblastoid cells and fibroblasts. However, the neuroendocrine features of SCC were further substantiated because 6 of the 12 distinguishing SCC surface proteins, including E and U, were identified on human neuroblastoma cells. The proteins identified should (i) help define differentiation steps for normal and neoplastic bronchial epithelial cells, (ii) prove useful in better classifying lung cancers, and (iii) be instrumental in tracing formation of neuroendocrine cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeloff M. D., Eggleston J. C., Mendelsohn G., Ettinger D. S., Baylin S. B. Changes in morphologic and biochemical characteristics of small cell carcinoma of the lung. A clinicopathologic study. Am J Med. 1979 May;66(5):757–764. doi: 10.1016/0002-9343(79)91113-6. [DOI] [PubMed] [Google Scholar]
- Andersson L. C., Gahmberg C. G., Nilsson K., Wigzell H. Surface glycoprotein patterns of normal and malignant human lymphoid cells. I. T cells T blasts and leukemic T cell lines. Int J Cancer. 1977 Nov 15;20(5):702–707. doi: 10.1002/ijc.2910200509. [DOI] [PubMed] [Google Scholar]
- Andrew A. Further evidence that enterochromaffin cells are not derived from the neural crest. J Embryol Exp Morphol. 1974 Jun;31(3):589–598. [PubMed] [Google Scholar]
- Baylin S. B., Abeloff M. D., Goodwin G., Carney D. N., Gazdar A. F. Activities of L-dopa decarboxylase and diamine oxidase (histaminase) in human lung cancers and decarboxylase as a marker for small (oat) cell cancer in cell culture. Cancer Res. 1980 Jun;40(6):1990–1994. [PubMed] [Google Scholar]
- Berger C. L., Goodwin G., Mendelsohn G., Eggleston J. C., Abeloff M. D., Aisner S., Baylin S. B. Endocrine-related biochemistry in the spectrum of human lung carcinoma. J Clin Endocrinol Metab. 1981 Aug;53(2):422–429. doi: 10.1210/jcem-53-2-422. [DOI] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Lymphocytes as models for the study of mammalian cellular differentiation. Immunol Rev. 1977 Jan;33:105–124. doi: 10.1111/j.1600-065x.1977.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Cheng H., Leblond C. P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974 Dec;141(4):537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- Cuttitta F., Rosen S., Gazdar A. F., Minna J. D. Monoclonal antibodies that demonstrate specificity for several types of human lung cancer. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4591–4595. doi: 10.1073/pnas.78.7.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Gartler S. M. Apparent Hela cell contamination of human heteroploid cell lines. Nature. 1968 Feb 24;217(5130):750–751. doi: 10.1038/217750a0. [DOI] [PubMed] [Google Scholar]
- Gazdar A. F., Carney D. N., Russell E. K., Sims H. L., Baylin S. B., Bunn P. A., Jr, Guccion J. G., Minna J. D. Establishment of continuous, clonable cultures of small-cell carcinoma of lung which have amine precursor uptake and decarboxylation cell properties. Cancer Res. 1980 Oct;40(10):3502–3507. [PubMed] [Google Scholar]
- Keil-Dlouha V., Paulin D., Bagilet L. K., Keil B. A comparison of surface proteins in embryonal carcinoma cells and their differentiated derivatives. Biochim Biophys Acta. 1980 Mar 27;597(1):15–28. doi: 10.1016/0005-2736(80)90146-7. [DOI] [PubMed] [Google Scholar]
- Klein E., Klein G., Nadkarni J. S., Nadkarni J. J., Wigzell H., Clifford P. Surface IgM-kappa specificity on a Burkitt lymphoma cell in vivo and in derived culture lines. Cancer Res. 1968 Jul;28(7):1300–1310. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luk G. D., Goodwin G., Marton L. J., Baylin S. B. Polyamines are necessary for the survival of human small-cell lung carcinoma in culture. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2355–2358. doi: 10.1073/pnas.78.4.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- Nilsson K., Andersson L. C., Gahmberg C. G., Wigzell H. Surface glycoprotein patterns of normal and malignant human lymphoid cells. II. B cells, B blasts and Epstein-Barr virus (EBV)-positive and -negative B lymphoid cell lines. Int J Cancer. 1977 Nov 15;20(5):708–716. doi: 10.1002/ijc.2910200510. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pearse A. G. The cytochemistry and ultrastructure of polypeptide hormone-producing cells of the APUD series and the embryologic, physiologic and pathologic implications of the concept. J Histochem Cytochem. 1969 May;17(5):303–313. doi: 10.1177/17.5.303. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Yamada K. M. Isolation and immunological characterization of a glucose-regulated fibroblast cell surface glycoprotein and its nonglycosylated precursor. Cell. 1978 Jan;13(1):139–140. doi: 10.1016/0092-8674(78)90145-9. [DOI] [PubMed] [Google Scholar]
- Schlesinger H. R., Gerson J. M., Moorhead P. S., Maguire H., Hummeler K. Establishment and characterization of human neuroblastoma cell lines. Cancer Res. 1976 Sep;36(9 PT1):3094–3100. [PubMed] [Google Scholar]
- Sherwin S. A., Minna J. D., Gazdar A. F., Todaro G. J. Expression of epidermal and nerve growth factor receptors and soft agar growth factor production by human lung cancer cells. Cancer Res. 1981 Sep;41(9 Pt 1):3538–3542. [PubMed] [Google Scholar]
- Springer T. A., Mann D. L., DeFranco A. L., Strominger J. L. Detergent solubilization, purification, and separation of specificities of HLA antigens from a cultured human lymphoblastoid line, RPMI 4265. J Biol Chem. 1977 Jul 10;252(13):4682–4693. [PubMed] [Google Scholar]
- Stone K. R., Smith R. E., Joklik W. K. Changes in membrane polypeptides that occur when chick embryo fibroblasts and NRK cells are transformed with avian sarcoma viruses. Virology. 1974 Mar;58(1):86–100. doi: 10.1016/0042-6822(74)90143-3. [DOI] [PubMed] [Google Scholar]
- Welsh K. I., Dorval G., Nilsson K., Clements G. B., Wigzell H. Quantitation of beta2-microglobulin and HLA on the surface of human cells. II. In vitro cell lines and their hybrids. Scand J Immunol. 1977;6(4):265–271. doi: 10.1111/j.1365-3083.1977.tb00393.x. [DOI] [PubMed] [Google Scholar]
- Wylie D. E., Damsky C. H., Buck C. A. Studies on the function of cell surface glycoproteins. I. Use of antisera to surface membranes in the identification of membrane components relevant to cell-substrate adhesion. J Cell Biol. 1979 Feb;80(2):385–402. doi: 10.1083/jcb.80.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Olden K. Fibronectins--adhesive glycoproteins of cell surface and blood. Nature. 1978 Sep 21;275(5677):179–184. doi: 10.1038/275179a0. [DOI] [PubMed] [Google Scholar]