Abstract
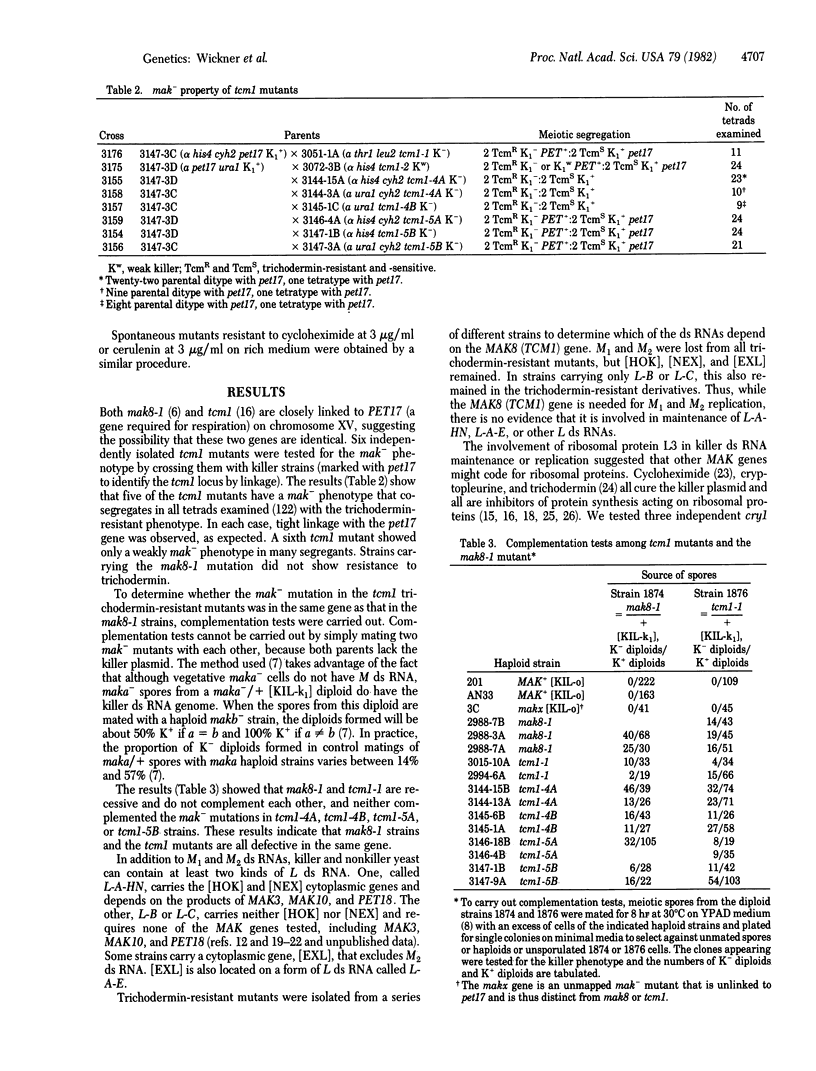
Ability to secrete the K1 (or K2) toxin protein and immunity to that toxin [the K1 (or K2) killer trait] are determined by a double-stranded (ds) RNA, called M1 (or M2), whose replication and maintenance depend on at least one of the larger (L) ds RNAs and 29 chromosomal genes, called MAK genes (maintenance of killer). The location of the MAK8 gene near TCM1 (trichodermin resistance) on the yeast map suggested the possible identity of these two genes. Of six independently isolated tcm1 mutants, five were clearly mak-, and the sixth was weakly mak-. In each case, the mak- phenotype and the trichodermin-resistant phenotypes cosegregated in meiosis and showed the expected tight linkage to pet17. The mak- mutations in the trichodermin-resistant strains did not complement mak8-1, indicating that MAK8 and TCM1 are the same gene. The mak8-1 mutation does not make strains resistant to trichodermin, and one tcm1 mutation is only slightly mak-. Whereas tcm1 mutants lose M1 or M2 ds RNA, they do not lose L ds RNA. Because TCM1 codes for ribosomal protein L3 [Fried, H. M. & Warner, J. R. (1981) Proc. Natl. Acad. Sci, USA 78, 238--242], we conclude that ribosomal protein L3 is involved in the replication and maintenance of M ds RNA. Mutations in cyh2 or cry1, producing resistance to cycloheximide and crytopleurine due to mutant ribosomal proteins, do not produce a mak- phenotype. In analogy with bacterial ribosome assembly mutants, yeast low-temperature-sensitive (lts) mutants may have defective ribosomes. We thus examined mutants for an effect on the killer system. An lts5 mutant, unable to grow at 5 degrees C, also has a mak- phenotype (at 30 degrees C) that cosegregates in meiosis with the lts- phenotype. Mutations in seven other lts genes do not result in the mak- phenotype.
Full text
PDF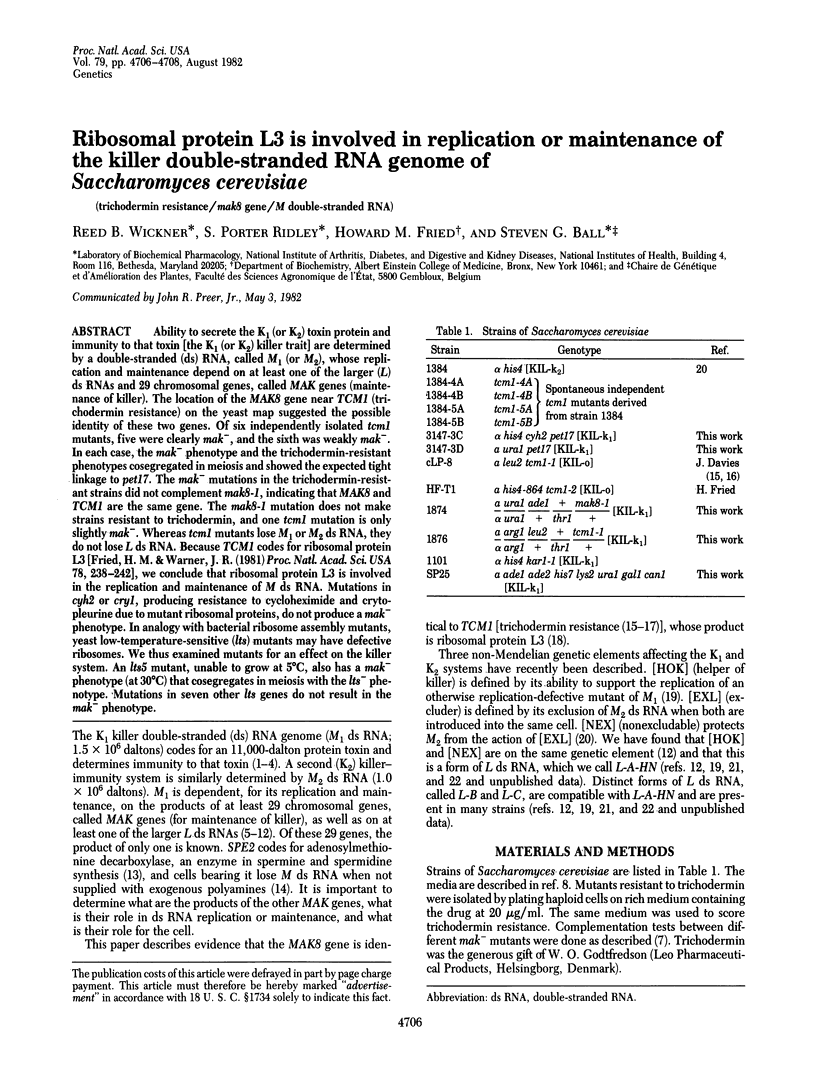

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevan E. A., Herring A. J., Mitchell D. J. Preliminary characterization of two species of dsRNA in yeast and their relationship to the "killer" character. Nature. 1973 Sep 14;245(5420):81–86. doi: 10.1038/245081b0. [DOI] [PubMed] [Google Scholar]
- Blumenthal T., Carmichael G. G. RNA replication: function and structure of Qbeta-replicase. Annu Rev Biochem. 1979;48:525–548. doi: 10.1146/annurev.bi.48.070179.002521. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Hopper J. E., Rogers D. T., Tipper D. J. Translational analysis of the killer-associated virus-like particle dsRNA genome of S. cerevisiae: M dsRNA encodes toxin. Cell. 1980 Feb;19(2):403–414. doi: 10.1016/0092-8674(80)90514-0. [DOI] [PubMed] [Google Scholar]
- Carrasco L., Barbacid M., Vazquez D. The trichodermin group of antibiotics, inhibitors of peptide bond formation by eukaryotic ribosomes. Biochim Biophys Acta. 1973 Jun 23;312(2):368–376. doi: 10.1016/0005-2787(73)90381-x. [DOI] [PubMed] [Google Scholar]
- Cohn M. S., Tabor C. W., Tabor H. Isolation and characterization of Saccharomyces cerevisiae mutants deficient in S-adenosylmethionine decarboxylase, spermidine, and spermine. J Bacteriol. 1978 Apr;134(1):208–213. doi: 10.1128/jb.134.1.208-213.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn M. S., Tabor C. W., Tabor H., Wickner R. B. Spermidine or spermine requirement for killer double-stranded RNA plasmid replication in yeast. J Biol Chem. 1978 Aug 10;253(15):5225–5227. [PubMed] [Google Scholar]
- Fink G. R., Styles C. A. Curing of a killer factor in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2846–2849. doi: 10.1073/pnas.69.10.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried H. M., Warner J. R. Cloning of yeast gene for trichodermin resistance and ribosomal protein L3. Proc Natl Acad Sci U S A. 1981 Jan;78(1):238–242. doi: 10.1073/pnas.78.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant P. G., Schindler D., Davies J. E. Mapping of trichodermin resistance in Saccharomyces cerevisiae: a genetic locus for a component of the 60S ribsomal subunit. Genetics. 1976 Aug;83(4):667–673. doi: 10.1093/genetics/83.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry-Kopecko P., Wickner R. B. Isolation and characterization of temperature-sensitive mak mutants of Saccharomyces cerevisiae. J Bacteriol. 1980 Dec;144(3):1113–1118. doi: 10.1128/jb.144.3.1113-1118.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie C., Nashimoto H., Nomura M. Structure and function of E. coli ribosomes. 8. Cold-sensitive mutants defective in ribosome assembly. Proc Natl Acad Sci U S A. 1969 Jun;63(2):384–391. doi: 10.1073/pnas.63.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper J. E., Bostian K. A., Rowe L. B., Tipper D. J. Translation of the L-species dsRNA genome of the killer-associated virus-like particles of Saccharomyces cerevisiae. J Biol Chem. 1977 Dec 25;252(24):9010–9017. [PubMed] [Google Scholar]
- Meade J. H., Riley M. I., Manney T. R. Expression of cryptopleurine resistance in Saccharomyces cerevisiae. J Bacteriol. 1977 Mar;129(3):1428–1434. doi: 10.1128/jb.129.3.1428-1434.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palfree R. G., Bussey H. Yeast killer toxin: purification and characterisation of the protein toxin from Saccharomyces cerevisiae. Eur J Biochem. 1979 Feb 1;93(3):487–493. doi: 10.1111/j.1432-1033.1979.tb12847.x. [DOI] [PubMed] [Google Scholar]
- Rouslin W. Effects of cerulenin upon the syntheses of lipid and protein and upon the formation of respiratory enzymes in adapting, lipid-limited Saccharomyces cerevisiae. J Bacteriol. 1979 Aug;139(2):502–506. doi: 10.1128/jb.139.2.502-506.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler D., Grant P., Davies J. Trichodermin resistance--mutation affecting eukaryotic ribosomes. Nature. 1974 Apr 5;248(448):535–536. doi: 10.1038/248535a0. [DOI] [PubMed] [Google Scholar]
- Singh A., Manney T. R. Genetic analysis of mutations affecting growth of Saccharomyces cerevisiae at low temperature. Genetics. 1974 Aug;77(4):651–659. doi: 10.1093/genetics/77.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skogerson L., McLaughlin C., Wakatama E. Modification of ribosomes in cryptopleurine-resistant mutants of yeast. J Bacteriol. 1973 Nov;116(2):818–822. doi: 10.1128/jb.116.2.818-822.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers J. M., Bevan E. A. The inheritance of the killer character in yeast. Genet Res. 1969 Feb;13(1):71–83. doi: 10.1017/s0016672300002743. [DOI] [PubMed] [Google Scholar]
- Sommer S. S., Wickner R. B. Co-curing of plasmids affecting killer double-stranded RNAs of Saccharomyces cerevisiae: [HOK], [NEX], and the abundance of L are related and further evidence that M1 requires L. J Bacteriol. 1982 May;150(2):545–551. doi: 10.1128/jb.150.2.545-551.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai P. C., Kessler D. P., Ingraham J. Cold-sensitive mutations in Salmonella typhimurium which affect ribosome synthesis. J Bacteriol. 1969 Mar;97(3):1298–1304. doi: 10.1128/jb.97.3.1298-1304.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodkin M., Katterman F., Fink G. R. Yeast killer mutants with altered double-stranded ribonucleic acid. J Bacteriol. 1974 Feb;117(2):681–686. doi: 10.1128/jb.117.2.681-686.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B., Leibowitz M. J. Chromosomal genes essential for replication of a double-stranded RNA plasmid of Saccharomyces cerevisiae: the killer character of yeast. J Mol Biol. 1976 Aug 15;105(3):427–443. doi: 10.1016/0022-2836(76)90102-9. [DOI] [PubMed] [Google Scholar]
- Wickner R. B., Leibowitz M. J. Mak mutants of yeast: mapping and characterization. J Bacteriol. 1979 Oct;140(1):154–160. doi: 10.1128/jb.140.1.154-160.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Mapping chromosomal genes of Saccharomyces cerevisiae using an improved genetic mapping method. Genetics. 1979 Jul;92(3):803–821. doi: 10.1093/genetics/92.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Plasmids controlled exclusion of the K2 killer double-stranded RNA plasmid of yeast. Cell. 1980 Aug;21(1):217–226. doi: 10.1016/0092-8674(80)90129-4. [DOI] [PubMed] [Google Scholar]
- Wickner R. B. Twenty-six chromosomal genes needed to maintain the killer double-stranded RNA plasmid of Saccharomyces cerevisiae. Genetics. 1978 Mar;88(3):419–425. doi: 10.1093/genetics/88.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata H., Dombou M., Sato T., Mizushima S., Uchida H. Deletion mapping and heterogenote analysis of a mutation responsible for osmosis-sensitive growth, spectinomycin resistance, and alteration of cytoplasmic membrane in Escherichia coli. J Bacteriol. 1980 Aug;143(2):661–667. doi: 10.1128/jb.143.2.661-667.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata H., Uchida H. Spectinomycin resistance mutations affecting the stability of sex-factors in Escherichia coli. J Mol Biol. 1972 Jun 28;67(3):533–535. doi: 10.1016/0022-2836(72)90472-x. [DOI] [PubMed] [Google Scholar]


