Abstract
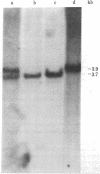
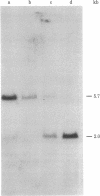
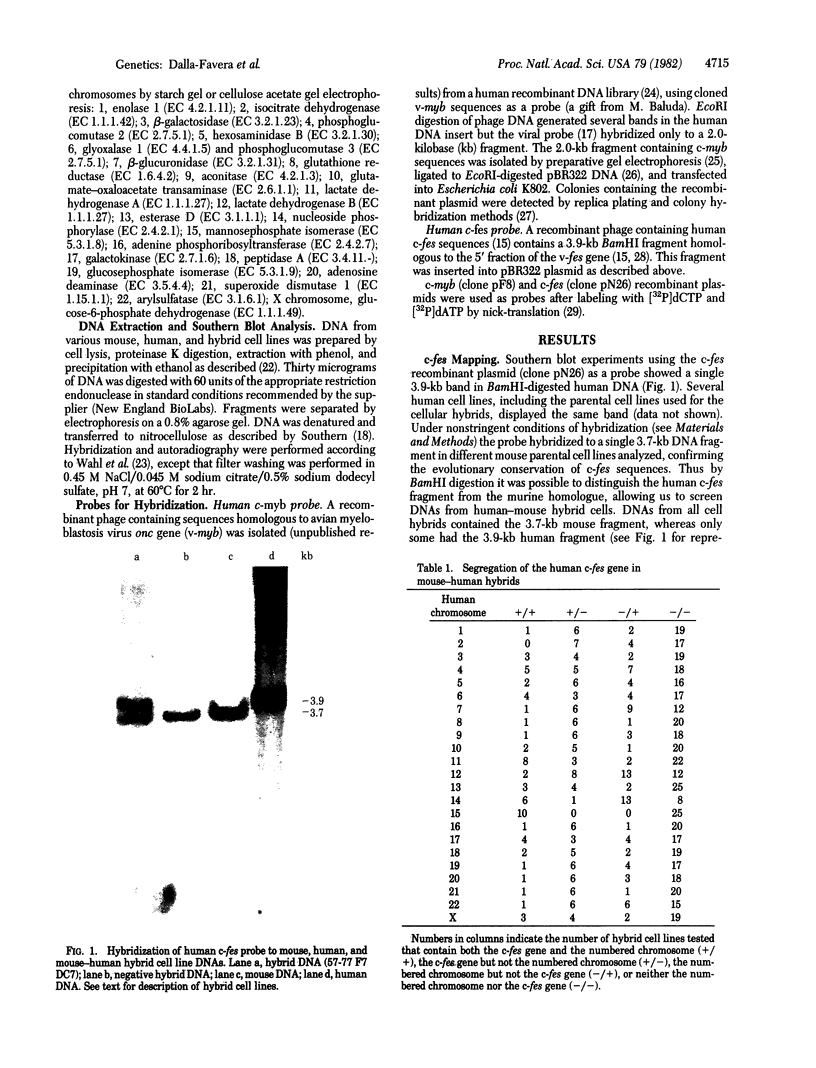
Retroviral transforming genes, v-onc genes, are derived from normal cellular sequences that are called cellular onc (c-onc) genes. DNA from mouse-human somatic cell hybrids that have selectively lost human chromosomes was used in Southern blots to map the chromosomal location of two human onc genes. Cloned human homologues of retroviral onc genes were used as probes. Because the human c-fes gene, which is homologous to feline sarcoma virus, segregates concordantly with human chromosome 15, and the human c-myb gene, which is homologous to avian myeloblastosis virus onc genes, segregates concordantly with human chromosome 6, we have assigned the c-fes and the c-myb genes to human chromosomes 15 and 6, respectively. Nonrandom chromosomal defects involving these human chromosomes have been observed in neoplasms. These studies should be valuable in determining whether specific rearrangements involving these chromosomes result in the abnormal expression of these onc genes in human malignancies.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M., Beemon K., Devare S. G. Origin and functional properties of the major gene product of the Snyder-Theilen strain of feline sarcoma virus. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5158–5162. doi: 10.1073/pnas.77.9.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheim A., Berger R., Lenoir G. Cytogenetic studies on African Burkitt's lymphoma cell lines: t(8;14), t(2;8) and t(8;22) translocations. Cancer Genet Cytogenet. 1981 Jun;3(4):307–315. doi: 10.1016/0165-4608(81)90039-x. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Cairns J. The origin of human cancers. Nature. 1981 Jan 29;289(5796):353–357. doi: 10.1038/289353a0. [DOI] [PubMed] [Google Scholar]
- Chern C. J., Kennett R., Engel E., Mellman W. J., Croce C. M. Assignment of the structural genes for the alpha subunit of hexosaminidase A, mannosephosphate isomerase, and pyruvate kinase to the region q22-qter of human chromosome 15. Somatic Cell Genet. 1977 Nov;3(6):553–560. doi: 10.1007/BF01539065. [DOI] [PubMed] [Google Scholar]
- Croce C. M., Aden C., Koprowski H. Tumorigenicity of mouse-human diploid hybrids in nude mice. Science. 1975 Dec 19;190(4220):1200–1202. doi: 10.1126/science.173020. [DOI] [PubMed] [Google Scholar]
- Croce C. M., Shander M., Martinis J., Cicurel L., D'Ancona G. G., Dolby T. W., Koprowski H. Chromosomal location of the genes for human immunoglobulin heavy chains. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3416–3419. doi: 10.1073/pnas.76.7.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H. Transforming genes of retroviruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):13–29. doi: 10.1101/sqb.1980.044.01.005. [DOI] [PubMed] [Google Scholar]
- Erikson J., Martinis J., Croce C. M. Assignment of the genes for human lambda immunoglobulin chains to chromosome 22. Nature. 1981 Nov 12;294(5837):173–175. doi: 10.1038/294173a0. [DOI] [PubMed] [Google Scholar]
- FOLEY G. E., LAZARUS H., FARBER S., UZMAN B. G., BOONE B. A., MCCARTHY R. E. CONTINUOUS CULTURE OF HUMAN LYMPHOBLASTS FROM PERIPHERAL BLOOD OF A CHILD WITH ACUTE LEUKEMIA. Cancer. 1965 Apr;18:522–529. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Fedele L. A., Even J., Garon C. F., Donner L., Sherr C. J. Recombinant bacteriophages containing the integrated transforming provirus of Gardner--Arnstein feline sarcoma virus. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4036–4040. doi: 10.1073/pnas.78.7.4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraisse J., Lenoir G., Vasselon C., Jaubert J., Brizard C. P. Variant translocation in Burkitt's lymphoma: 8;22 translocation in a French patient with an Epstein-Barr virus-associated tumor. Cancer Genet Cytogenet. 1981 Mar;3(2):149–153. doi: 10.1016/0165-4608(81)90070-4. [DOI] [PubMed] [Google Scholar]
- Gardner M. B., Rongey R. W., Arnstein P., Estes J. D., Sarma P., Huebner R. J., Rickard C. G. Experimental transmission of feline fibrosarcoma to cats and dogs. Nature. 1970 May 30;226(5248):807–809. doi: 10.1038/226807a0. [DOI] [PubMed] [Google Scholar]
- Golomb H. M., Rowley J., Vardiman J., Baron J., Locker G., Krasnow S. Partial deletion of long arm of chromosome 17: a specific abnormality in acute promyelocytic leukemia? Arch Intern Med. 1976 Jul;136(7):825–828. [PubMed] [Google Scholar]
- Graf T., Beug H. Avian leukemia viruses: interaction with their target cells in vivo and in vitro. Biochim Biophys Acta. 1978 Nov 17;516(3):269–299. doi: 10.1016/0304-419x(78)90011-2. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayata I., Oshimura M., Minowada J., Sandberg A. A. Chromosomal banding of cultured T and B lymphocytes. In Vitro. 1975 Nov-Dec;11(6):361–368. doi: 10.1007/BF02616372. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Klein G. The role of gene dosage and genetic transpositions in carcinogenesis. Nature. 1981 Nov 26;294(5839):313–318. doi: 10.1038/294313a0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Manolov G., Manolova Y. Marker band in one chromosome 14 from Burkitt lymphomas. Nature. 1972 May 5;237(5349):33–34. doi: 10.1038/237033a0. [DOI] [PubMed] [Google Scholar]
- McBride O. W., Hieter P. A., Hollis G. F., Swan D., Otey M. C., Leder P. Chromosomal location of human kappa and lambda immunoglobulin light chain constant region genes. J Exp Med. 1982 May 1;155(5):1480–1490. doi: 10.1084/jem.155.5.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough S. K., Larsen S., Brodey R. S., Stock N. D., Hardy W. D., Jr A transmissible feline fibrosarcoma of viral origin. Cancer Res. 1971 Jul;31(7):953–956. [PubMed] [Google Scholar]
- Minowada J., Onuma T., Moore G. E. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972 Sep;49(3):891–895. [PubMed] [Google Scholar]
- Neel B. G., Hayward W. S., Robinson H. L., Fang J., Astrin S. M. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell. 1981 Feb;23(2):323–334. doi: 10.1016/0092-8674(81)90128-8. [DOI] [PubMed] [Google Scholar]
- Nowell P. C., Finan J. B., Vonderheid E. C. Clonal characteristics of cutaneous T cell lymphomas: cytogenetic evidence from blood, lymph nodes, and skin. J Invest Dermatol. 1982 Jan;78(1):69–75. doi: 10.1111/1523-1747.ep12497947. [DOI] [PubMed] [Google Scholar]
- Oskarsson M., McClements W. L., Blair D. G., Maizel J. V., Vande Woude G. F. Properties of a normal mouse cell DNA sequence (sarc) homologous to the src sequence of Moloney sarcoma virus. Science. 1980 Mar 14;207(4436):1222–1224. doi: 10.1126/science.6243788. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Bishop J. M., Varmus H. E. Multiple arrangements of viral DNA and an activated host oncogene in bursal lymphomas. Nature. 1982 Jan 21;295(5846):209–214. doi: 10.1038/295209a0. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Courtneidge S. A., Crittenden L. B., Fadly A. M., Bishop J. M., Varmus H. E. Analysis of avian leukosis virus DNA and RNA in bursal tumours: viral gene expression is not required for maintenance of the tumor state. Cell. 1981 Feb;23(2):311–322. doi: 10.1016/0092-8674(81)90127-6. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rowley J. D., Golomb H. M., Vardiman J., Fukuhara S., Dougherty C., Potter D. Further evidence for a non-random chromosomal abnormality in acute promyelocytic leukemia. Int J Cancer. 1977 Dec 15;20(6):869–872. doi: 10.1002/ijc.2910200608. [DOI] [PubMed] [Google Scholar]
- Rowley J. D. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973 Jun 1;243(5405):290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- Scott A. F., Phillips J. A., 3rd, Migeon B. R. DNA restriction endonuclease analysis for localization of human beta- and delta-globin genes on chromosome 11. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4563–4565. doi: 10.1073/pnas.76.9.4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O. Recovery of DNA from gels. Methods Enzymol. 1980;65(1):371–380. doi: 10.1016/s0076-6879(80)65048-4. [DOI] [PubMed] [Google Scholar]
- Snyder S. P., Theilen G. H. Transmissible feline fibrosarcoma. Nature. 1969 Mar 15;221(5185):1074–1075. doi: 10.1038/2211074a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Trus M. D., Sodroski J. G., Haseltine W. A. Isolation and characterization of a human locus homologous to the transforming gene (v-fes) of feline sarcoma virus. J Biol Chem. 1982 Mar 25;257(6):2730–2733. [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake N., Hreshchyshyn M. M., Piver S. M., Matsui S., Sandberg A. A. Specific cytogenetic changes in ovarian cancer involving chromosomes 6 and 14. Cancer Res. 1980 Dec;40(12):4512–4518. [PubMed] [Google Scholar]
- Westin E. H., Gallo R. C., Arya S. K., Eva A., Souza L. M., Baluda M. A., Aaronson S. A., Wong-Staal F. Differential expression of the amv gene in human hematopoietic cells. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2194–2198. doi: 10.1073/pnas.79.7.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Staal F., Dalla-Favera R., Franchini G., Gelmann E. P., Gallo R. C. Three distinct genes in human DNA related to the transforming genes of mammalian sarcoma retroviruses. Science. 1981 Jul 10;213(4504):226–228. doi: 10.1126/science.6264598. [DOI] [PubMed] [Google Scholar]
- Wong-Staal F., Reitz M. S., Jr, Gallo R. C. Retrovirus sequences in a leukemic gibbon and its contact: evidence for partial provirus in the nonleukemic gibbon. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2032–2036. doi: 10.1073/pnas.76.4.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]