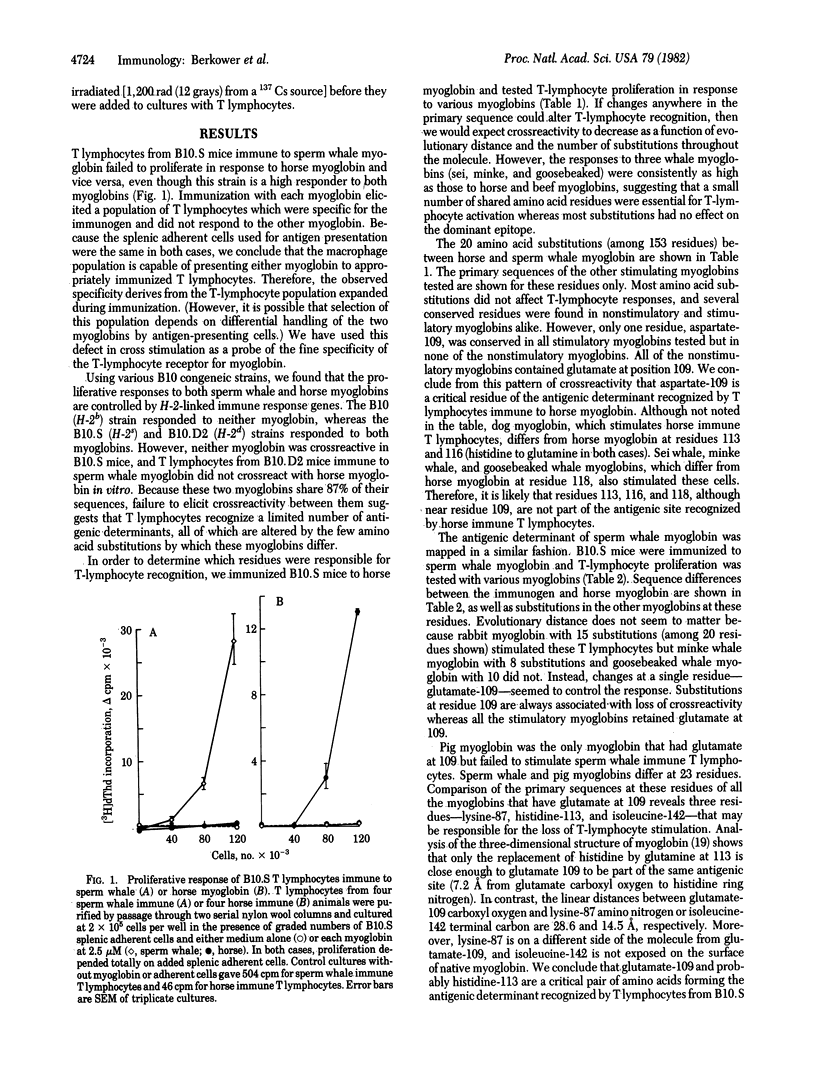
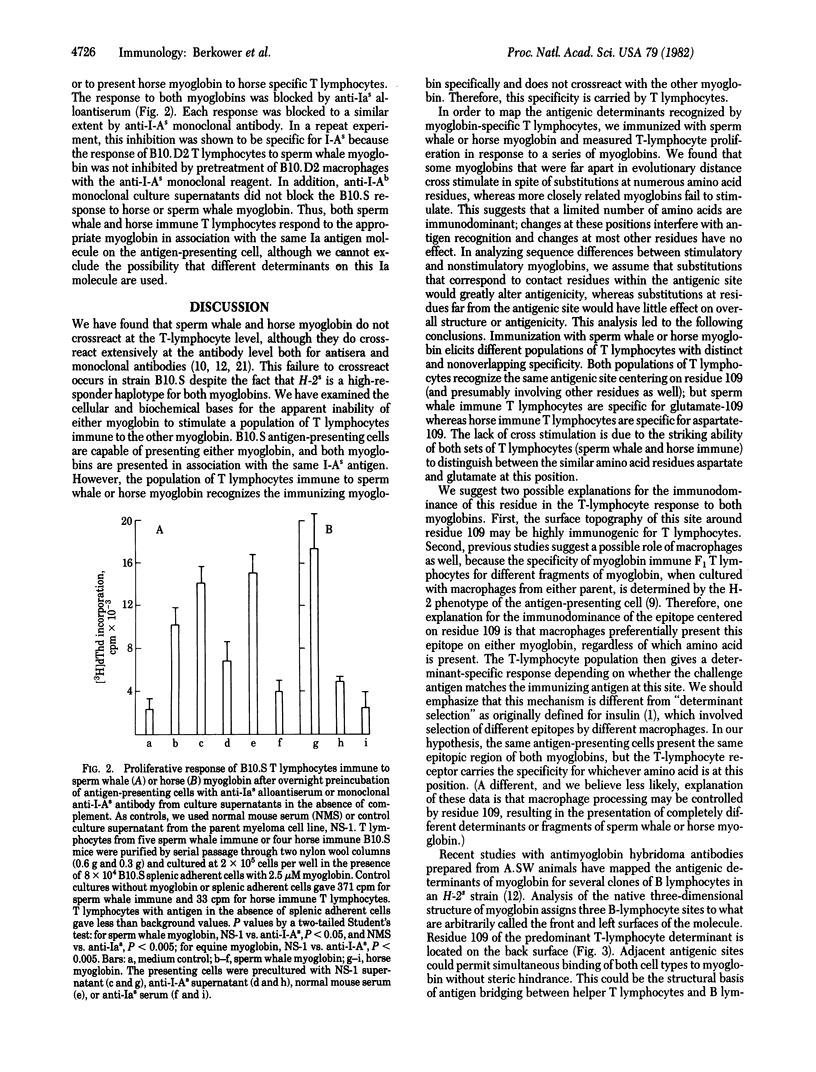
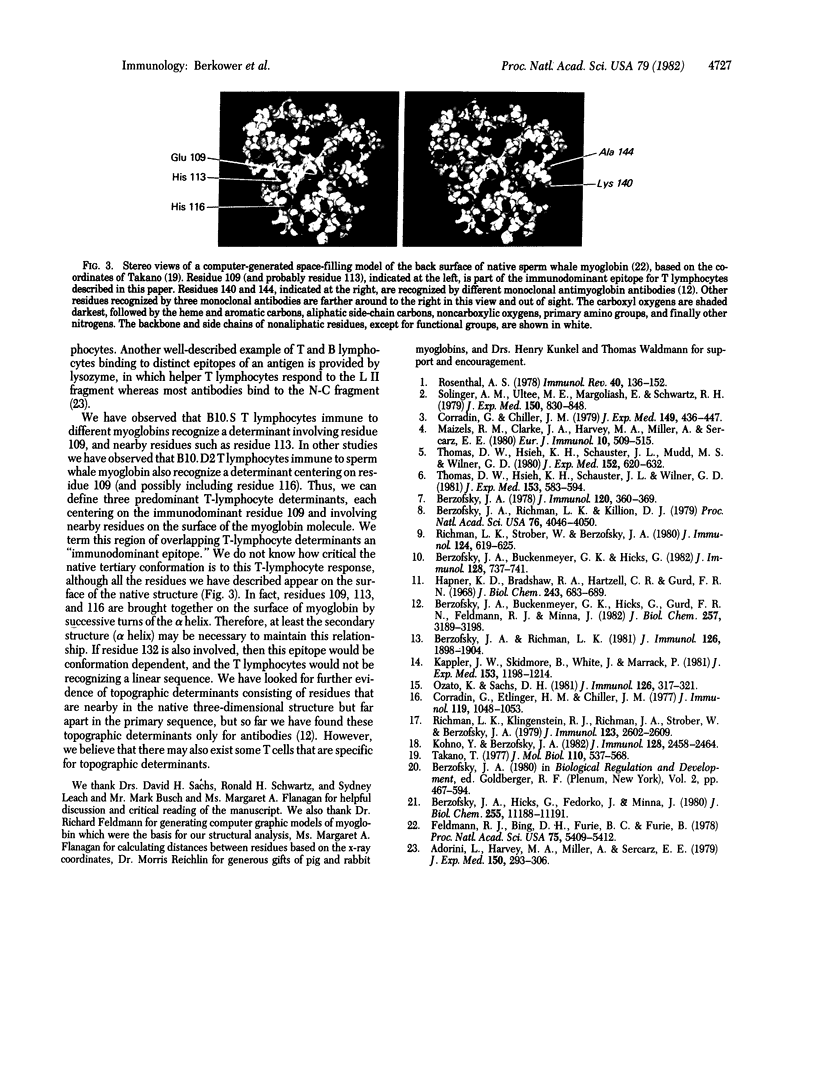
Abstract
We find that a single region on the surface of different species of myoglobin appears to be immunodominant for T lymphocytes, even though the residues in that region vary sufficiently that the T cells immune to one myoglobin do not crossreact with other myoglobins bearing substitutions at that site. Immunization of B10.S mice with sperm whale myoglobin elicits T-lymphocyte populations capable of recognizing sperm whale myoglobin but not horse myoglobin, whereas the converse is true when these mice are immunized with horse myoglobin. Using a series of myoglobin variants, we tested the effect of changes in primary sequence on the T-lymphocyte proliferative response. We were able to divide the myoglobin variants into two groups, depending on whether they cross stimulate sperm whale immune or horse immune T lymphocytes. The patterns of cross stimulation of both populations of myoglobin immune T lymphocytes were explained by amino acid substitutions at position 109. However, because sperm whale and horse myoglobin differ at this residue (glutamate vs. aspartate, respectively), T lymphocytes immune to each myoglobin do not crossreact with the other myoglobin. Additional data suggest that this immunodominant epitope also includes other residues nearby on the surface of the native molecule. Mixing experiments showed that the specificity was that of T lymphocytes and not antigen-presenting cells. Monoclonal anti-I-A blocking studies showed that both myoglobins are presented in association with the same Ia antigen. Possible explanations for the apparent immunodominance of this antigenic epitope, consisting of residue 109 and nearby residues on the surface of both myoglobins, include a peculiar immunogenicity of the surface topography of this site of a preferred orientation of the molecule imposed by antigen-presenting cells when T cells first encounter the antigen. The latter explanation is related to but distinct from "determinant selection." T-cell recognition of conformation is discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adorini L., Harvey M. A., Miller A., Sercarz E. E. Fine specificity of regulatory T cells. II. Suppressor and helper T cells are induced by different regions of hen egg-white lysozyme in a genetically nonresponder mouse strain. J Exp Med. 1979 Aug 1;150(2):293–306. doi: 10.1084/jem.150.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzofsky J. A., Buckenmeyer G. K., Hicks G. Genetic control of the immune response to myoglobins. VI. Distinct Ir genes for different myoglobins: complementing genes in I-A and H-2D for equine myoglobin. J Immunol. 1982 Feb;128(2):737–741. [PubMed] [Google Scholar]
- Berzofsky J. A., Buckenmeyer G. K., Hicks G., Gurd F. R., Feldmann R. J., Minna J. Topographic antigenic determinants recognized by monoclonal antibodies to sperm whale myoglobin. J Biol Chem. 1982 Mar 25;257(6):3189–3198. [PubMed] [Google Scholar]
- Berzofsky J. A. Genetic control of the immune response to mammalian myoglobins in mice I. More than one I-region gene in H-2 controls the antibody response. J Immunol. 1978 Feb;120(2):360–369. [PubMed] [Google Scholar]
- Berzofsky J. A., Hicks G., Fedorko J., Minna J. Properties of monoclonal antibodies specific for determinants of a protein antigen, myoglobin. J Biol Chem. 1980 Dec 10;255(23):11188–11191. [PubMed] [Google Scholar]
- Berzofsky J. A., Richman L. K. Genetic control of the immune response to myoglobins. IV. Inhibition of determinant-specific Ir gene-controlled antigen presentation and induction of suppression by pretreatment of presenting cells with anti-Ia antibodies. J Immunol. 1981 May;126(5):1898–1904. [PubMed] [Google Scholar]
- Berzofsky J. A., Richman L. K., Killion D. J. Distinct H-2-linked Ir genes control both antibody and T cell responses to different determinants on the same antigen, myoglobin. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4046–4050. doi: 10.1073/pnas.76.8.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradin G., Chiller J. M. Lymphocyte specificity to protein antigens. II. Fine specificity of T-cell activation with cytochrome c and derived peptides as antigenic probes. J Exp Med. 1979 Feb 1;149(2):436–447. doi: 10.1084/jem.149.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradin G., Etlinger H. M., Chiller J. M. Lymphocyte specificity to protein antigens. I. Characterization of the antigen-induced in vitro T cell-dependent proliferative response with lymph node cells from primed mice. J Immunol. 1977 Sep;119(3):1048–1053. [PubMed] [Google Scholar]
- Feldmann R. J., Bing D. H., Furie B. C., Furie B. Interactive computer surface graphics approach to study of the active site of bovine trypsin. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5409–5412. doi: 10.1073/pnas.75.11.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapner K. D., Bradshaw R. A., Hartzell C. R., Gurd F. R. Comparison of myoglobins from harbor seal, porpoise, and sperm whale. I. Preparation and characterization. J Biol Chem. 1968 Feb 25;243(4):683–689. [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno Y., Berzofsky J. A. Genetic control of the immune response to myoglobin. V. Antibody production in vitro is macrophage and T cell-dependent and is under control of two determinant-specific Ir genes. J Immunol. 1982 Jun;128(6):2458–2464. [PubMed] [Google Scholar]
- Maizels R. M., Clarke J. A., Harvey M. A., Miller A., Sercarz E. E. Epitope specificity of the T cell proliferative response to lysozyme: proliferative T cells react predominantly to different determinants from those recognized by B cells. Eur J Immunol. 1980 Jul;10(7):509–515. doi: 10.1002/eji.1830100705. [DOI] [PubMed] [Google Scholar]
- Ozato K., Sachs D. H. Monoclonal antibodies to mouse MHC antigens. III. Hybridoma antibodies reacting to antigens of the H-2b haplotype reveal genetic control of isotype expression. J Immunol. 1981 Jan;126(1):317–321. [PubMed] [Google Scholar]
- Richman L. K., Klingenstein R. J., Richman J. A., Strober W., Berzofsky J. A. The murine Kupffer cell. I. Characterization of the cell serving accessory function in antigen-specific T cell proliferation. J Immunol. 1979 Dec;123(6):2602–2609. [PubMed] [Google Scholar]
- Richman L. R., Strober W., Berzofsky J. A. Genetic control of the immune response to myoglobin. III. Determinant-specific, two Ir gene phenotype is regulated by the genotype of reconstituting Kupffer cells. J Immunol. 1980 Feb;124(2):619–625. [PubMed] [Google Scholar]
- Rosenthal A. S. Determinant selection and macrophage function in genetic control of the immune response. Immunol Rev. 1978;40:136–152. doi: 10.1111/j.1600-065x.1978.tb00404.x. [DOI] [PubMed] [Google Scholar]
- Solinger A. M., Ultee M. E., Margoliash E., Schwartz R. H. T-lymphocyte response to cytochrome c. I. Demonstration of a T-cell heteroclitic proliferative response and identification of a topographic antigenic determinant on pigeon cytochrome c whose immune recognition requires two complementing major histocompatibility complex-linked immune response genes. J Exp Med. 1979 Oct 1;150(4):830–848. doi: 10.1084/jem.150.4.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T. Structure of myoglobin refined at 2-0 A resolution. I. Crystallographic refinement of metmyoglobin from sperm whale. J Mol Biol. 1977 Mar 5;110(3):537–568. doi: 10.1016/s0022-2836(77)80111-3. [DOI] [PubMed] [Google Scholar]
- Thomas D. W., Hsieh K. H., Schauster J. L., Mudd M. S., Wilner G. D. Nature of T lymphocyte recognition of macrophage-associated antigens. V. Contribution of individual peptide residues of human fibrinopeptide B to T lymphocyte responses. J Exp Med. 1980 Sep 1;152(3):620–632. doi: 10.1084/jem.152.3.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. W., Hsieh K. H., Schauster J. L., Wilner G. D. Fine specificity of genetic regulation of guinea pig T lymphocyte responses to angiotensin II and related peptides. J Exp Med. 1981 Mar 1;153(3):583–594. doi: 10.1084/jem.153.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]