Pure vertical nystagmus typically signifies brainstem or cerebellar dysfunction and can sometimes be modulated by head position. Here we report the first case of positional periodic alternating vertical nystagmus as the sole manifestation of a PCA-Tr–associated paraneoplastic encephalitis associated with Hodgkin lymphoma (HL).
Case reports.
A 25-year-old graduate student was evaluated for a 1-year history of positional vertigo. He slept upright because lying supine would produce 10-second-long attacks of vertigo recurring twice each minute as long as he maintained that position. He similarly avoided looking up or bending forward too long for the same reason. He had no other neurologic or constitutional symptoms. He took no medications and had no other medical history.
Neurologic examination including gait and tandem Romberg was normal. Ocular motor and vestibular examination revealed a small exophoria, normal extraocular range, trace gaze-evoked nystagmus in far rightward gaze, and normal saccades, pursuit, convergence, optokinetic responses, vestibulo-ocular reflex (VOR) suppression, and head impulse testing. Infrared video oculography while seated revealed no spontaneous nystagmus or any Valsalva-, headshaking-, or hyperventilation-induced nystagmus.
While gradually moving into the supine position he developed slow small-amplitude asymptomatic upbeat nystagmus. Once supine, a burst of rapid downbeat nystagmus (DBN) lasting 10 seconds coincided with his typical vertigo symptoms, followed by the return of slow upbeat nystagmus (video on the Neurology® Web site at www.neurology.org). This pattern of symptomatic downbeat alternating with asymptomatic upbeat nystagmus continued every 20–60 seconds while supine or with head-hanging. No DBN occurred supine with either ear down. Alternatively, prone head positioning produced intense DBN and vertigo lasting between 20 and 60 seconds.
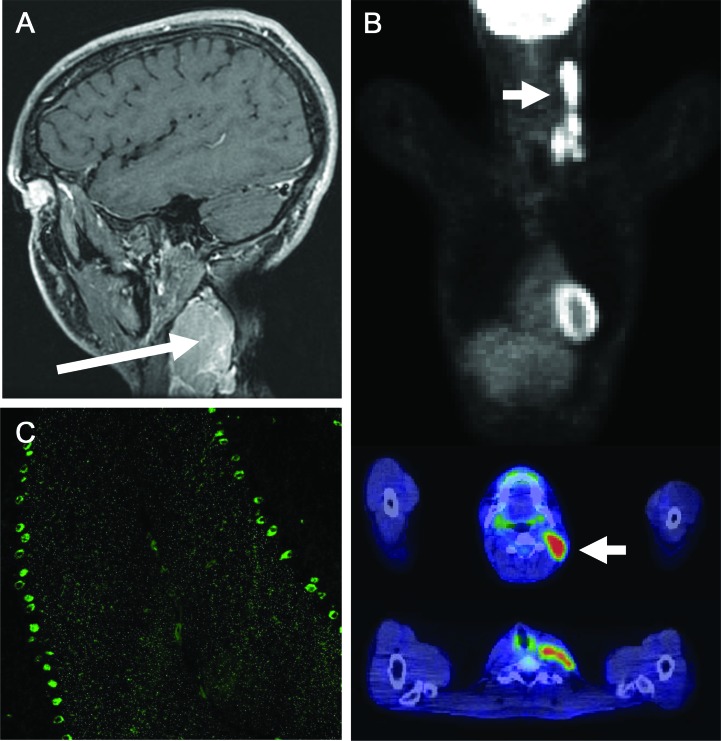
Initial evaluation revealed elevated tissue transglutaminase immunoglobulin A (IgA) (27.7 U/mL, normal <4.0), endomysial (1:80), and gliadin immunoglobulin G (50.1 U, >30 positive) and IgA (54.7 U, >30 positive) antibodies. Duodenal biopsy was consistent with celiac sprue. Vitamin B12 level was normal. Serum copper was 0.45 μg/mL (normal 0.75–1.45). The 25-hydroxy vitamin D level was 17 ng/mL (optimum > 24). Brain MRI and CSF examination were normal. Subsequent examination uncovered a 5-cm left cervical mass (figure, A). Biopsy revealed classic HL; large atypical cells expressed CD30, CD15, and PAX5. PET revealed limited disease (figure, B). A comprehensive paraneoplastic evaluation of patient's serum detected Purkinje cell antibody (PCA)-Tr (end point dilution 480, normal <120; figure, C) by indirect immunofluorescence.
Figure. Findings in paraneoplastic cerebellar degeneration related to Hodgkin lymphoma.
(A) Gadolinium-enhanced T1-weighted sagittal MRI demonstrates lymphadenopathy (long arrow) between the sternocleidomastoid and trapezius. (B) Staging 18F FDG PET with CT fusion imaging demonstrates hypermetabolic adenopathy limited to the left neck and supraclavicular region (short arrows). (C) Indirect immunofluorescence shows binding of patient immunoglobulin G to a frozen composite substrate of mouse cerebellum in a typical Purkinje cell antibody–Tr pattern (magnification ×200): staining of the soma of Purkinje neurons with distinctive punctate reactivity of the molecular layer (4-μm sections prefixed with 10% formalin).
The patient received 2 cycles of ABVD (Adriamycin, bleomycin, vinblastine, and dacarbazine) and 20 Gy radiation, with a subsequently normal PET scan and complete remission. The positional nystagmus did not change.
Discussion.
DBN is a common sign in cerebellar flocculus/paraflocculus dysfunction, typically associated with abnormalities of gaze-holding, pursuit, and VOR suppression. The influence of gravity on DBN is thought to be mediated by otolithic pathways and modulated by flocculonodular inputs such that cerebellar dysfunction may create or unmask asymmetry of vertical ocular motor signals in different pitch plane positions.1 Nodulus lesions classically produce horizontal periodic alternating nystagmus (PAN) from disinhibition of the vestibular velocity storage mechanism, though isolated nodulus infarctions have also been associated with apogeotropic horizontal positional nystagmus.2 A case of periodic DBN with a 3.5-minute cycle not modulated by head hanging was reported to resolve after correcting severe hypomagnesemia.3 We are not aware of a published case of positional periodic alternating vertical nystagmus.
Paraneoplastic cerebellar degeneration in HL is uncommon but well-recognized.4,5 We suggest that our patient's vertical positional nystagmus represents a novel neurologic association of PCA-Tr related to paraneoplastic floccular/parafloccular Purkinje cell dysfunction. Paraneoplastic positional downbeat nystagmus/vertigo with more widespread ocular motor signs has also been reported in small cell carcinoma.6 We cannot completely exclude a nonparaneoplastic autoimmune etiology associated with celiac disease, which has been linked to cerebellar dysfunction including positional downbeat nystagmus.7
It is unclear whether nodulus involvement could account for the periodic alternating nature of our patient's nystagmus (we did not test tilt suppression of postrotatory nystagmus), though the duration of each cycle's downbeat and upbeat nystagmus was shorter and less predictable than in typical cases of horizontal PAN, the intensity of the downbeat and upbeat differed considerably, and baclofen produced no improvement as it commonly does in acquired PAN.
This case illustrates how manifestations of paraneoplastic cerebellar degeneration (PCD) can remain restricted to positional nystagmus/vertigo for an extended time. Such a diagnosis should be considered once structural etiologies like Chiari malformation are excluded, particularly HL in a young nonsmoking man. PCA-Tr antibodies are highly associated with PCD and HL4 and solidify the relationship in this case. The periodic alternating nature of this vertical nystagmus is enigmatic but could reflect limited involvement of the cerebellar nodulus.
Supplementary Material
Footnotes
Author contributions: Dr. Eggers: drafting and revising the manuscript for content, study concept/design, analysis/interpretation of data. Dr. Pittock: revising the manuscript for content, study concept/design, analysis/interpretation of data. Dr. Shepard: revising the manuscript for content, analysis/interpretation of data. Dr. Habermann: revising the manuscript for content, study concept/design, analysis/interpretation of data. Dr. Neff: revising the manuscript for content. Ms. Klebig: revising the manuscript for content.
Dr. Eggers reports no disclosures. Dr. Pittock and Mayo Clinic have a financial interest in the technology entitled “Aquaporin-4 autoantibody as a cancer marker.” This technology has been licensed to a commercial entity but no royalties have been received. In addition, Dr. Pittock is an inventor of technology entitled “Aquaporin-4 binding autoantibodies in patients with neuromyelitis optic impair glutamate transport by down-regulating EAAT2.” Mayo Clinic has filed a non-provisional patent application for this technology. Dr. Pittock has received a research grant from Alexion Pharmaceuticals for an investigator-initiated study and receives research support from NIH RO1 NS065829-01 and the Guthy Jackson Charitable Foundation. Dr. Shepard, Dr. Habermann, Dr. Neff, and Ms. Klebig report no disclosures. Go to Neurology.org for full disclosures.
References
- 1. Marti S, Palla A, Straumann D. Gravity dependence of ocular drift in patients with cerebellar downbeat nystagmus. Ann Neurol 2002; 52: 712– 721 [DOI] [PubMed] [Google Scholar]
- 2. Moon IS, Kim JS, Choi KD, et al. Isolated nodular infarction. Stroke 2009; 40: 487– 491 [DOI] [PubMed] [Google Scholar]
- 3. Du Pasquier R, Vingerhoets F, Safran AB, Landis T. Periodic downbeat nystagmus. Neurology 1998; 51: 1478– 1480 [DOI] [PubMed] [Google Scholar]
- 4. Bernal F, Shams'ili S, Rojas I, et al. Anti-Tr antibodies as markers of paraneoplastic cerebellar degeneration and Hodgkin's disease. Neurology 2003; 60: 230– 234 [DOI] [PubMed] [Google Scholar]
- 5. Briani C, Vitaliani R, Grisold W, et al. Spectrum of paraneoplastic disease associated with lymphoma. Neurology 2011; 76: 705– 710 [DOI] [PubMed] [Google Scholar]
- 6. Ogawa E, Sakakibara R, Kawashima K, et al. VGCC antibody-positive paraneoplastic cerebellar degeneration presenting with positioning vertigo. Neurol Sci Epub 2011. [DOI] [PubMed] [Google Scholar]
- 7. Versino M, Franciotta D, Colnaghi S, et al. Cerebellar signs in celiac disease. Neurology 2009; 72: 2046– 2048 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.