Abstract
trans-3,3'-Bis[alpha-(trimethylammonio)methyl]azobenzene bromide (BisQ) is a potent agonist of the acetylcholine receptor (AcChoR) of Electrophorus electricus. BisQ is highly constrained, suggesting that its structure is complementary to the combining site of the AcChoR when the latter is in its activated state. Antibodies produced in rabbits to a conjugate of bovine serum albumin and a derivative of BisQ mimicked the binding characteristics of the AcChoR with respect to the order of binding of a variety of agonists and to the preferred recognition of decamethonium ion (an agonist) over hexamethonium ion (an antagonist). Immunization of three rabbits with purified anti-BisQ yielded antisera having binding characteristics of anti-AcChoR in that, by complement fixation and enzyme immunoassay, crossreactions with receptor preparations from rat, Torpedo, and eel could be demonstrated in sera of all three rabbits immunized. Two of the three rabbits showed signs of muscle weakness similar to that seen after immunization with the AcChoR. One of the rabbits was injected intramuscularly with neostigmine and showed temporary improvement. Another showed post-tetanic exhaustion of hind-limb muscles after stimulation of the sciatic nerve at 50 Hz. Antibodies reactive with the AcChoR, therefore, were elicited by immunization with an antibody to a potent ligand of the AcChoR without the necessity of isolating the receptor itself. A similar mechanism may play a part in the etiology of at least some autoimmune diseases in which antibodies to various other receptors are involved.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEISER S. M., TANENBAUM S. W. Binding site topology of enzymes and antibodies induced by the same determinants. Ann N Y Acad Sci. 1963 May 8;103:595–610. doi: 10.1111/j.1749-6632.1963.tb53720.x. [DOI] [PubMed] [Google Scholar]
- Bartels E., Wassermann N. H., Erlanger B. F. Photochromic activators of the acetylcholine receptor. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1820–1823. doi: 10.1073/pnas.68.8.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockes J. P., Hall Z. W. Acetylcholine receptors in normal and denervated rat diaphragm muscle. I. Purification and interaction with [125I]-alpha-bungarotoxin. Biochemistry. 1975 May 20;14(10):2092–2099. doi: 10.1021/bi00681a008. [DOI] [PubMed] [Google Scholar]
- Eichmann K. Expression and function of idiotypes of lymphocytes. Adv Immunol. 1978;26:195–254. doi: 10.1016/s0065-2776(08)60231-x. [DOI] [PubMed] [Google Scholar]
- Engvall E. Enzyme immunoassay ELISA and EMIT. Methods Enzymol. 1980;70(A):419–439. doi: 10.1016/s0076-6879(80)70067-8. [DOI] [PubMed] [Google Scholar]
- Erlanger B. F. Principles and methods for the preparation of drug protein conjugates for immunological studies. Pharmacol Rev. 1973 Jun;25(2):271–280. [PubMed] [Google Scholar]
- Erlanger B. F., Sack R. A. Opertional normality of alpha-chymotrypsin solutions by a sensitive potentiometric technique using a fluoride electrode. Anal Biochem. 1970 Feb;33(2):318–322. doi: 10.1016/0003-2697(70)90302-7. [DOI] [PubMed] [Google Scholar]
- FARR R. S. A quantitative immunochemical measure of the primary interaction between I BSA and antibody. J Infect Dis. 1958 Nov-Dec;103(3):239–262. doi: 10.1093/infdis/103.3.239. [DOI] [PubMed] [Google Scholar]
- Flier J. S., Kahn C. R., Roth J., Bar R. S. Antibodies that impair insulin receptor binding in an unusual diabetic syndrome with severe insulin resistance. Science. 1975 Oct 3;190(4209):63–65. doi: 10.1126/science.170678. [DOI] [PubMed] [Google Scholar]
- Heidmann T., Changeux J. P. Structural and functional properties of the acetylcholine receptor protein in its purified and membrane-bound states. Annu Rev Biochem. 1978;47:317–357. doi: 10.1146/annurev.bi.47.070178.001533. [DOI] [PubMed] [Google Scholar]
- Hough C. A., Edwardson J. A. Antibodies to thaumatin as a model of the sweet taste receptor. Nature. 1978 Jan 26;271(5643):381–383. doi: 10.1038/271381a0. [DOI] [PubMed] [Google Scholar]
- Karlin A. The acetylcholine receptor: progress report. Life Sci. 1974 Apr 16;14(8):1385–1415. doi: 10.1016/0024-3205(74)90150-7. [DOI] [PubMed] [Google Scholar]
- Marasco W. A., Becker E. L. Anti-idiotype as antibody against the formyl peptide chemotaxis receptor of the neutrophil. J Immunol. 1982 Feb;128(2):963–968. [PubMed] [Google Scholar]
- Meunier J. C., Changeux J. P. Comparison between the affinities for reversible cholinergic ligands of a purified and membrane bound state of the acetylcholine-receptor protein from Electrophorus electricus. FEBS Lett. 1973 May 15;32(1):143–148. doi: 10.1016/0014-5793(73)80758-6. [DOI] [PubMed] [Google Scholar]
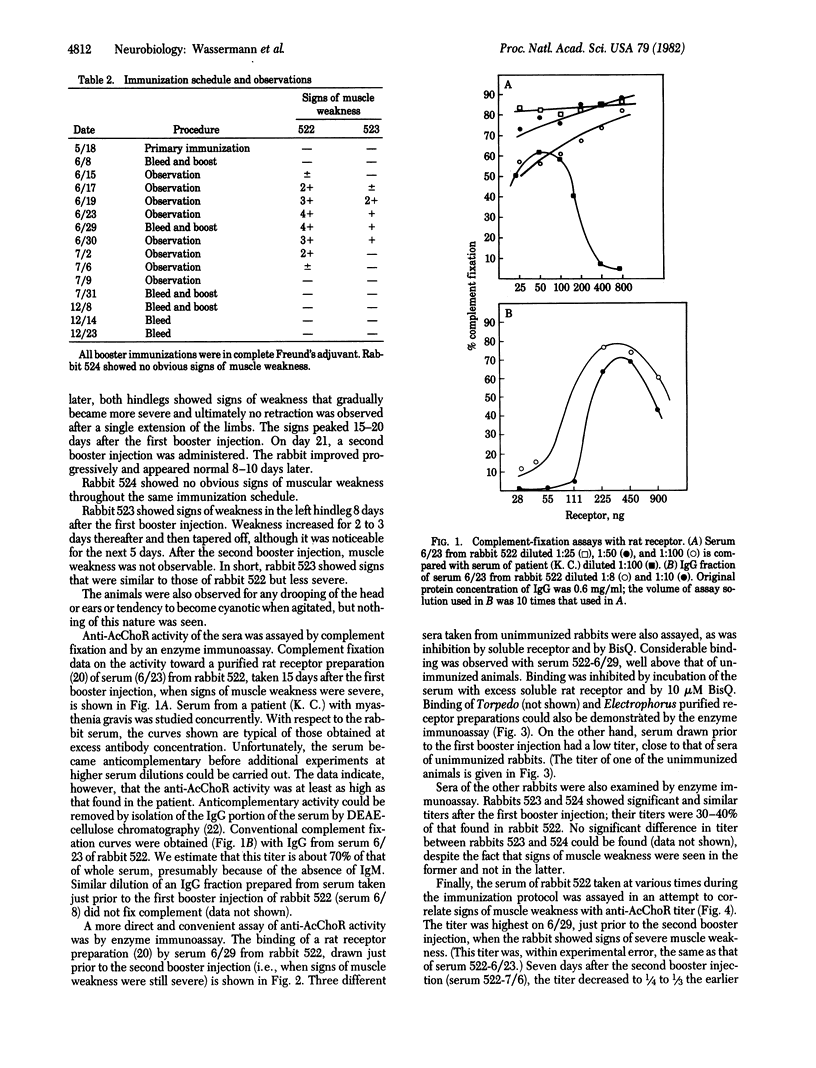
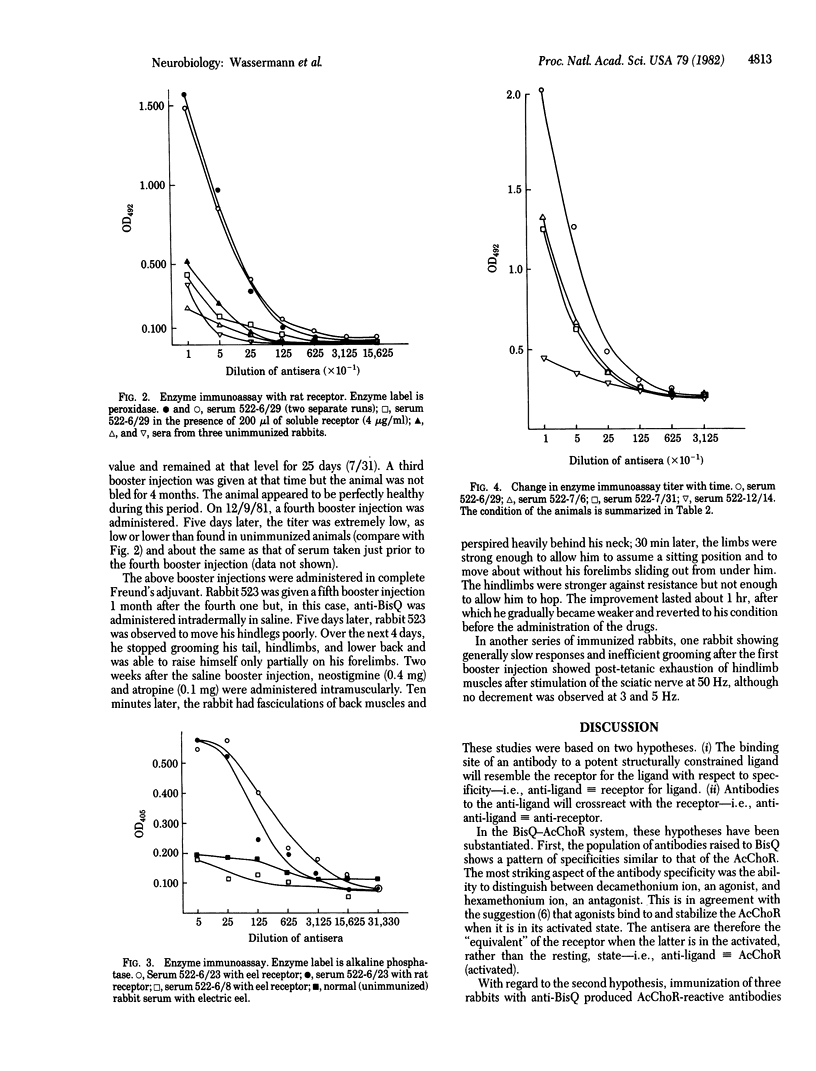
- Norcross N. L., Griffith I. J., Lettieri J. A. Measurement of acetylcholine receptor and anti-receptor antibodies by ELISA. Muscle Nerve. 1980 Jul-Aug;3(4):345–349. doi: 10.1002/mus.880030412. [DOI] [PubMed] [Google Scholar]
- Patrick J., Lindstrom J. Autoimmune response to acetylcholine receptor. Science. 1973 May 25;180(4088):871–872. doi: 10.1126/science.180.4088.871. [DOI] [PubMed] [Google Scholar]
- Penn A. S., Chang H. W., Lovelace R. E., Niemi W., Miranda A. Antibodies to acetylcholine receptors in rabbits: immunochemical and electrophysiological studies. Ann N Y Acad Sci. 1976;274:354–376. doi: 10.1111/j.1749-6632.1976.tb47697.x. [DOI] [PubMed] [Google Scholar]
- Schreiber A. B., Couraud P. O., Andre C., Vray B., Strosberg A. D. Anti-alprenolol anti-idiotypic antibodies bind to beta-adrenergic receptors and modulate catecholamine-sensitive adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7385–7389. doi: 10.1073/pnas.77.12.7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sege K., Peterson P. A. Anti-idiotypic antibodies against anti-vitamin A transporting protein react with prealbumin. Nature. 1978 Jan 12;271(5641):167–168. doi: 10.1038/271167a0. [DOI] [PubMed] [Google Scholar]
- Sege K., Peterson P. A. Use of anti-idiotypic antibodies as cell-surface receptor probes. Proc Natl Acad Sci U S A. 1978 May;75(5):2443–2447. doi: 10.1073/pnas.75.5.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. R., Hall R. Thyroid-stimulating immunoglobulins in Graves' disease. Lancet. 1974 Aug 24;2(7878):427–431. doi: 10.1016/s0140-6736(74)91815-7. [DOI] [PubMed] [Google Scholar]
- Spector S., Felix A., Semenuk G., Finberg J. P. Development of a specific radioimmunoassay for acetylcholine. J Neurochem. 1978 Apr;30(4):685–689. doi: 10.1111/j.1471-4159.1978.tb10772.x. [DOI] [PubMed] [Google Scholar]
- WASSERMAN E., LEVINE L. Quantitative micro-complement fixation and its use in the study of antigenic structure by specific antigen-antibody inhibition. J Immunol. 1961 Sep;87:290–295. [PubMed] [Google Scholar]
- Wassermann N. H., Bartels E., Erlanger B. F. Conformational properties of the acetylcholine receptor as revealed by studies with constrained depolarizing ligands. Proc Natl Acad Sci U S A. 1979 Jan;76(1):256–259. doi: 10.1073/pnas.76.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann N. H., Erlanger B. F. Agents related to a potent activator of the acetylcholine receptor of Electrophorus electricus. Chem Biol Interact. 1981 Sep;36(3):251–258. doi: 10.1016/0009-2797(81)90069-7. [DOI] [PubMed] [Google Scholar]


