Abstract
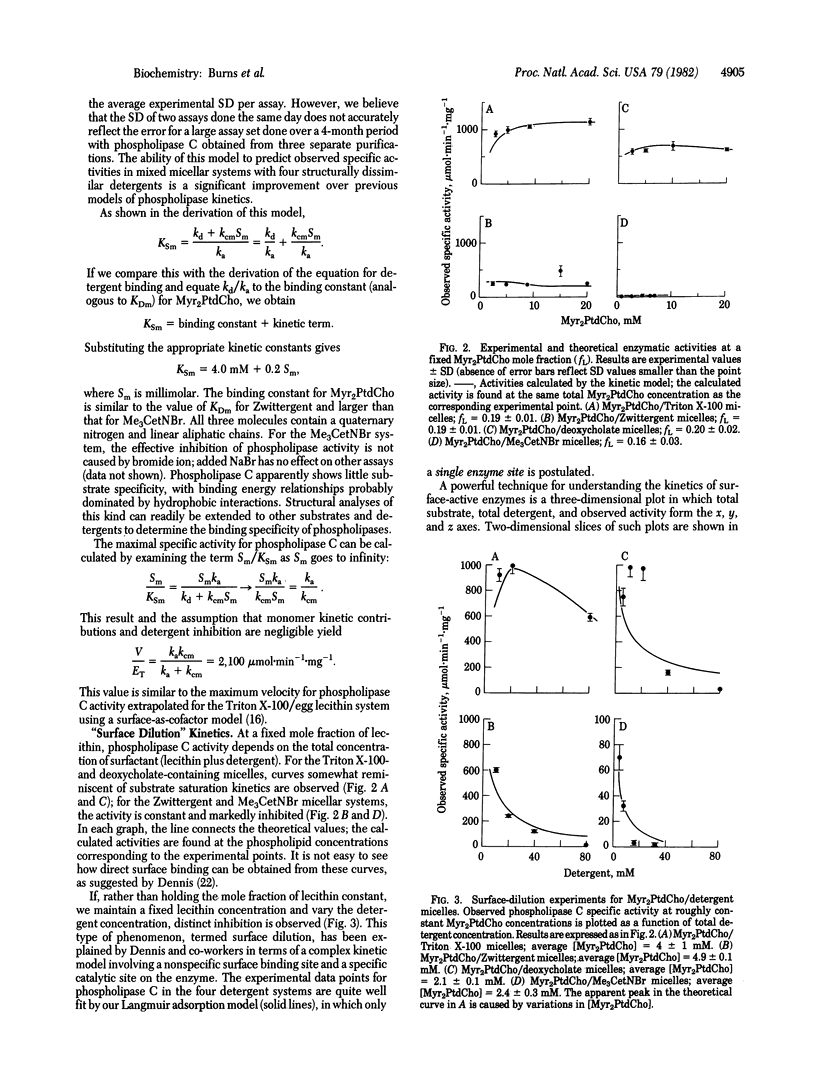
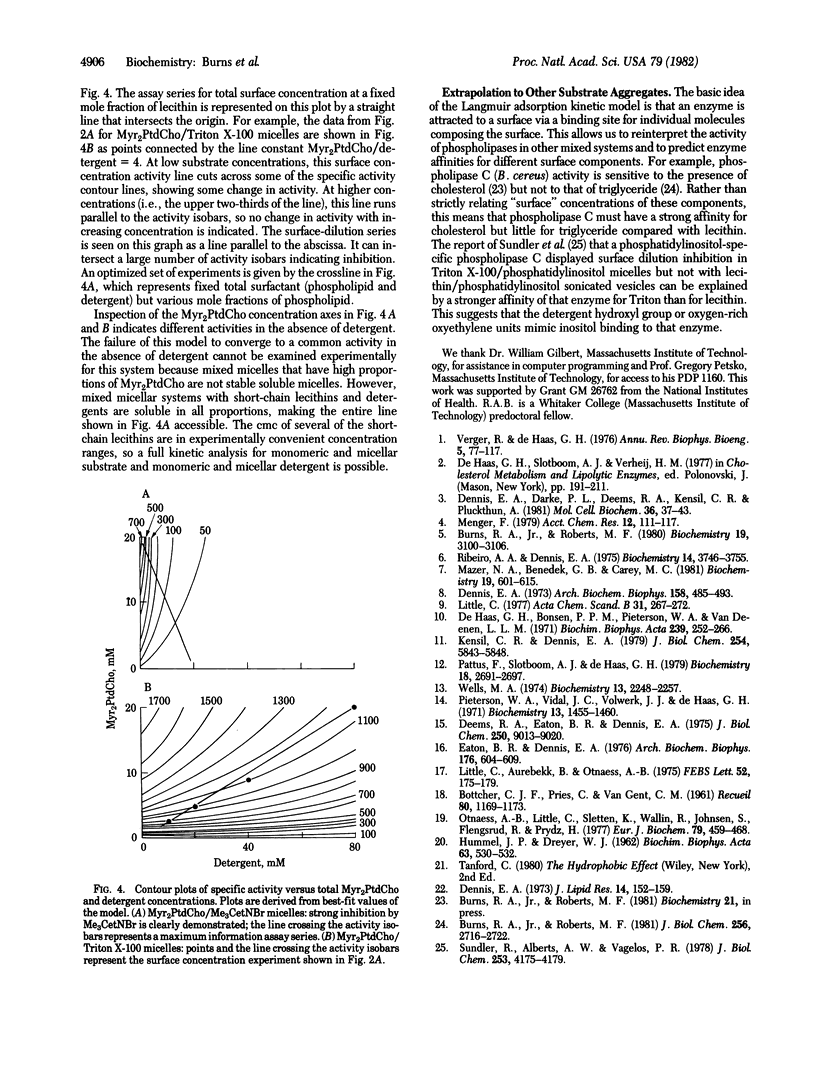
A simple kinetic model for the enzymatic activity of surface-active proteins against mixed micelles has been developed. This model uses the Langmuir adsorption isotherm, the classic equation for the binding of gas molecules to metal surfaces, to characterize enzyme adsorption to micelles. The number of available enzyme binding sites is equated with the number of substrate and inhibitor molecules attached to micelles; enzyme molecules are attracted to the micelle due to the affinity of the enzyme active site for the molecules in the micelle. Phospholipase C (Bacillus cereus) kinetics in a wide variety of dimyristoyl phosphatidylcholine/detergent micelles are readily explained by this model and the assumption of competitive binding of the detergent at the enzyme active site. Binding of phospholipase C to pure detergent micelles is demonstrated by gel filtration chromatography. The experimentally determined enzyme-detergent micelle binding constants are used directly in the rate equation. The Langmuir adsorption model predicts a variety of the characteristics observed for phospholipase kinetics, such as differential inhibition by various charged, uncharged, and zwitterionic detergents and surface-dilution inhibition. The essential idea of this model, that proteins can be attracted and bound to bilayers or micelles by possessing a binding site for the molecules composing the surface, may have wider application in the study of water-soluble (extrinsic) protein-membrane interactions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burns R. A., Jr, Roberts M. F. Carbon-13 nuclear magnetic resonance studies of short-chain lecithins. Motional and conformational characteristics of micellar and monomeric phospholipid. Biochemistry. 1980 Jun 24;19(13):3100–3106. doi: 10.1021/bi00554a041. [DOI] [PubMed] [Google Scholar]
- Burns R. A., Jr, Roberts M. F. Physical characterization and lipase susceptibility of short chain lecithin/triglyceride mixed micelles. Potential lipoprotein models. J Biol Chem. 1981 Mar 25;256(6):2716–2722. [PubMed] [Google Scholar]
- Deems R. A., Eaton B. R., Dennis E. A. Kinetic analysis of phospholipase A2 activity toward mixed micelles and its implications for the study of lipolytic enzymes. J Biol Chem. 1975 Dec 10;250(23):9013–9020. [PubMed] [Google Scholar]
- Dennis E. A., Darke P. L., Deems R. A., Kensil C. R., Plückthun A. Cobra venom phospholipase A2: a review of its action toward lipid/water interfaces. Mol Cell Biochem. 1981 Apr 13;36(1):37–45. doi: 10.1007/BF02354830. [DOI] [PubMed] [Google Scholar]
- Dennis E. A. Kinetic dependence of phospholipase A 2 activity on the detergent Triton X-100. J Lipid Res. 1973 Mar;14(2):152–159. [PubMed] [Google Scholar]
- Dennis E. A. Phospholipase A2 activity towards phosphatidylcholine in mixed micelles: surface dilution kinetics and the effect of thermotropic phase transitions. Arch Biochem Biophys. 1973 Oct;158(2):485–493. doi: 10.1016/0003-9861(73)90540-7. [DOI] [PubMed] [Google Scholar]
- Eaton B. R., Dennis E. A. Analysis of phospholipase C (Bacillus cereus) action toward mixed micelles of phospholipid and surfactant. Arch Biochem Biophys. 1976 Oct;176(2):604–609. doi: 10.1016/0003-9861(76)90204-6. [DOI] [PubMed] [Google Scholar]
- HUMMEL J. P., DREYER W. J. Measurement of protein-binding phenomena by gel filtration. Biochim Biophys Acta. 1962 Oct 8;63:530–532. doi: 10.1016/0006-3002(62)90124-5. [DOI] [PubMed] [Google Scholar]
- Kensil C. R., Dennis E. A. Action of cobra venom phospholipase A2 on the gel and liquid crystalline states of dimyristoyl and dipalmitoyl phosphatidylcholine vesicles. J Biol Chem. 1979 Jul 10;254(13):5843–5848. [PubMed] [Google Scholar]
- Little C., Aurebekk B., Otnaess A. B. Purification by affinity chromatography of phospholipase C from Bacillus cereus. FEBS Lett. 1975 Apr 1;52(2):175–179. doi: 10.1016/0014-5793(75)80800-3. [DOI] [PubMed] [Google Scholar]
- Little C. Phospholipase C from Bacillus cereus. Action on some artificial lecithins. Acta Chem Scand B. 1977;31(4):267–272. doi: 10.3891/acta.chem.scand.31b-0267. [DOI] [PubMed] [Google Scholar]
- Mazer N. A., Benedek G. B., Carey M. C. Quasielastic light-scattering studies of aqueous biliary lipid systems. Mixed micelle formation in bile salt-lecithin solutions. Biochemistry. 1980 Feb 19;19(4):601–615. doi: 10.1021/bi00545a001. [DOI] [PubMed] [Google Scholar]
- Otnaess A. B., Little C., Sletten K., Wallin R., Johnsen S., Flengsrud R., Prydz H. Some characteristics of phospholipase C from Bacillus cereus. Eur J Biochem. 1977 Oct 3;79(2):459–468. doi: 10.1111/j.1432-1033.1977.tb11828.x. [DOI] [PubMed] [Google Scholar]
- Pattus F., Slotboom A. J., de Haas G. H. Regulation of phospholipase A2 activity by the lipid-water interface: a monolayer approach. Biochemistry. 1979 Jun 26;18(13):2691–2697. doi: 10.1021/bi00580a001. [DOI] [PubMed] [Google Scholar]
- Pieterson W. A., Vidal J. C., Volwerk J. J., de Haas G. H. Zymogen-catalyzed hydrolysis of monomeric substrates and the presence of a recognition site for lipid-water interfaces in phospholipase A2. Biochemistry. 1974 Mar 26;13(7):1455–1460. doi: 10.1021/bi00704a021. [DOI] [PubMed] [Google Scholar]
- Ribeiro A. A., Dennis E. A. Proton magnetic resonance relaxation studies on the structure of mixed micelles of Triton X-100 and dimyristoylphosphatidylcholine. Biochemistry. 1975 Aug 26;14(17):3746–3755. doi: 10.1021/bi00688a005. [DOI] [PubMed] [Google Scholar]
- Sundler R., Alberts A. W., Vagelos P. R. Enzymatic properties of phosphatidylinositol inositolphosphohydrolase from Bacillus cereus. Substrate dilution in detergent-phospholipid micelles and bilayer vesicles. J Biol Chem. 1978 Jun 25;253(12):4175–4179. [PubMed] [Google Scholar]
- Verger R. Interfacial enzyme kinetics of lipolysis. Annu Rev Biophys Bioeng. 1976;5:77–117. doi: 10.1146/annurev.bb.05.060176.000453. [DOI] [PubMed] [Google Scholar]
- Wells M. A. The mechanism of interfacial activation of phospholipase A2. Biochemistry. 1974 May 21;13(11):2248–2257. doi: 10.1021/bi00708a002. [DOI] [PubMed] [Google Scholar]
- de Haas G. H., Bonsen P. P., Pieterson W. A., van Deenen L. L. Studies on phospholipase A and its zymogen from porcine pancreas. 3. Action of the enzyme on short-chain lecithins. Biochim Biophys Acta. 1971 Jul 13;239(2):252–266. doi: 10.1016/0005-2760(71)90171-8. [DOI] [PubMed] [Google Scholar]


