Abstract
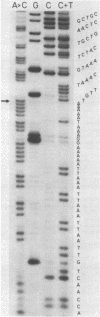
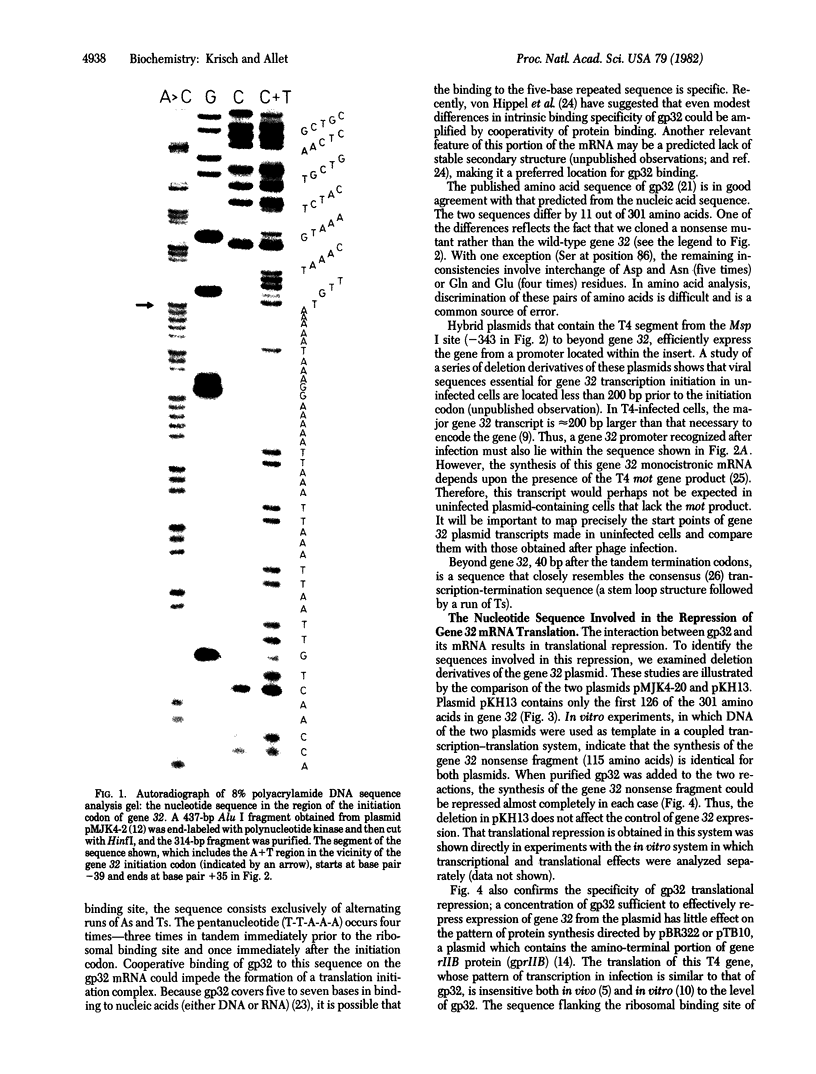
We have determined the nucleotide sequence of a cloned segment of the bacteriophage T4D chromosome, which contains the regulatory sequences and the structural gene (gene 32) for the single-stranded DNA binding protein (gp32). The amino acid sequence predicted by translation of the structural gene agrees well with that published for gp32 [Williams, K. R., Lo-Presti, M. B., Setoguchi, M. & Konigsberg, W. H. (1980) Proc. Natl. Acad. Sci. USA 77, 4614-4617]. To localize the nucleotide sequence involved in translational self-regulation of gene 32, we have constructed a series of plasmids in which gene 32 is fused to an amino-terminal deletion mutant of the beta-galactosidase gene of Escherichia coli. Expression of a beta-galactosidase fusion protein that contains only the first seven amino acids of gp32 is still repressed by gp32. The ribosomal binding site of gene 32 is flanked by a repetitive A+T-rich sequence. Preferential and cooperative binding of gp32 to this region of its mRNA could inhibit translation initiation and, thus, would account for the autoregulation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Frey L. T4 bacteriophage gene 32: a structural protein in the replication and recombination of DNA. Nature. 1970 Sep 26;227(5265):1313–1318. doi: 10.1038/2271313a0. [DOI] [PubMed] [Google Scholar]
- Baldy M. W. The UV sensitivity of some early-function temperature-sensitive mutants of phage T4. Virology. 1970 Feb;40(2):272–287. doi: 10.1016/0042-6822(70)90403-4. [DOI] [PubMed] [Google Scholar]
- Belin D., Epstein R. H. A temperature-sensitive rIIB mutation which affects the synthesis of bacteriophage T4 rIIB protein. Virology. 1977 May 15;78(2):537–553. doi: 10.1016/0042-6822(77)90129-5. [DOI] [PubMed] [Google Scholar]
- Belin D., Hedgpeth J., Selzer G. B., Epstein R. H. Temperature-sensitive mutation in the initiation codon of the rIIB gene of bacteriophage T4. Proc Natl Acad Sci U S A. 1979 Feb;76(2):700–704. doi: 10.1073/pnas.76.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H., Warren A. J., Fry K. E. Variations in genetic recombination due to amber mutations in T4D bacteriophage. J Virol. 1969 Feb;3(2):171–175. doi: 10.1128/jvi.3.2.171-175.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesteland R. F. Isolation and characterization of ribonuclease I mutants of Escherichia coli. J Mol Biol. 1966 Mar;16(1):67–84. doi: 10.1016/s0022-2836(66)80263-2. [DOI] [PubMed] [Google Scholar]
- Gold L., O'Farrell P. Z., Russel M. Regulation of gene 32 expression during bacteriophage T4 infection of Escherichia coli. J Biol Chem. 1976 Nov 25;251(22):7251–7262. [PubMed] [Google Scholar]
- Gray H. B., Jr, Ostrander D. A., Hodnett J. L., Legerski R. J., Robberson D. L. Extracellular nucleases of Pseudomonas BAL 31. I. Characterization of single strand-specific deoxyriboendonuclease and double-strand deoxyriboexonuclease activities. Nucleic Acids Res. 1975 Sep;2(9):1459–1492. doi: 10.1093/nar/2.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt J. F., Allet B., Weil R., Ahmad-Zadeh C. Synthesis of the tumour antigen and the major capsid protein of simian virus 40 in a cell-free system derived from Escherichia coli. J Mol Biol. 1976 Dec;108(2):361–379. doi: 10.1016/s0022-2836(76)80125-8. [DOI] [PubMed] [Google Scholar]
- Jensen D. E., Kelly R. C., von Hippel P. H. DNA "melting" proteins. II. Effects of bacteriophage T4 gene 32-protein binding on the conformation and stability of nucleic acid structures. J Biol Chem. 1976 Nov 25;251(22):7215–7228. [PubMed] [Google Scholar]
- Krisch H. M., Bolle A., Epstein R. H. Regulation of the synthesis of bacteriophage T4 gene 32 protein. J Mol Biol. 1974 Sep 5;88(1):89–104. doi: 10.1016/0022-2836(74)90296-4. [DOI] [PubMed] [Google Scholar]
- Krisch H. M., Selzer G. B. Construction and properties of a recombinant plasmid containing gene 32 of bacteriophage T4D. J Mol Biol. 1981 May 25;148(3):199–218. doi: 10.1016/0022-2836(81)90535-0. [DOI] [PubMed] [Google Scholar]
- Krisch H. M., Van Houwe G., Belin D., Gibbs W., Epstein R. H. Regulation of the expression of bacteriophage T4 genes 32 and 43. Virology. 1977 May 1;78(1):87–98. doi: 10.1016/0042-6822(77)90080-0. [DOI] [PubMed] [Google Scholar]
- Krisch H. M., Van Houwe G. Stimulation of the synthesis of bacteriophage T4 gene 32 protein by ultraviolet light irradiation. J Mol Biol. 1976 Nov;108(1):67–81. doi: 10.1016/s0022-2836(76)80095-2. [DOI] [PubMed] [Google Scholar]
- Lemaire G., Gold L., Yarus M. Autogenous translational repression of bacteriophage T4 gene 32 expression in vitro. J Mol Biol. 1978 Nov 25;126(1):73–90. doi: 10.1016/0022-2836(78)90280-2. [DOI] [PubMed] [Google Scholar]
- Mattson T., Van Houwe G., Epstein R. H. Isolation and characterization of conditional lethal mutations in the mot gene of bacteriophage T4. J Mol Biol. 1978 Dec 15;126(3):551–570. doi: 10.1016/0022-2836(78)90058-x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mosig G., Luder A., Garcia G., Dannenberg R., Bock S. In vivo interactions of genes and proteins in DNA replication and recombination of phage T4. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):501–515. doi: 10.1101/sqb.1979.043.01.056. [DOI] [PubMed] [Google Scholar]
- Polisky B., Greene P., Garfin D. E., McCarthy B. J., Goodman H. M., Boyer H. W. Specificity of substrate recognition by the EcoRI restriction endonuclease. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3310–3314. doi: 10.1073/pnas.72.9.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Russel M., Gold L., Morrissett H., O'Farrell P. Z. Translational, autogenous regulation of gene 32 expression during bacteriophage T4 infection. J Biol Chem. 1976 Nov 25;251(22):7263–7270. [PubMed] [Google Scholar]
- Selzer G., Bolle A., Krisch B., Epstein R. Construction and properties of recombinant plasmids containing the rII genes of bacteriophage T4. Mol Gen Genet. 1978 Feb 27;159(3):301–309. doi: 10.1007/BF00268267. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. R., LoPresti M. B., Setoguchi M., Konigsberg W. H. Amino acid sequence of the T4 DNA helix-destabilizing protein. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4614–4617. doi: 10.1073/pnas.77.8.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]