Abstract
The development of new immunosuppressive drugs for kidney transplantation resulted both in better short-term outcomes and in decreased metabolic, cardiovascular, and nephrotoxicity risk. Belatacept belongs to a new class of immunosuppressive drugs that selectively inhibits T-cell activation by preventing CD28 activation and by binding its ligands B7-1 and B7-2. The result is an inactivation of costimulatory pathways. A comparative analysis of the BENEFIT and BENEFIT-EXT datasets showed belatacept regimens resulted in better cardiovascular and metabolic risk profiles than did cyclosporin A (CsA) regimens: belatacept likewise outperformed CsA in terms of lower blood pressure and serum lipids and less new onset diabetes after transplantation. About 20% of belatacept-treated patients developed adverse effects which included anemia, pyrexia, neutropenia, diarrhea, urinary tract infection, headache, and peripheral edema. At present, belatacept does not seem to predispose patients to a higher rate of infection than CsA maintenance immunosuppression. The risk of posttransplant lymphoproliferative diseases was higher in Epstein–Barr virus (EBV)-seronegative patients than in EBV-seropositive patients, but the risk may be reduced by use of a less intensive regimen and avoidance of EBV-negative patients and of patients whose pretransplant EBV serology is unknown. Belatacept provides a new option for immunosuppressive therapy in kidney transplantation, but needs further evaluation in terms of the late effects that may derive from prolonged blockage of the costimulatory system and the induction of tolerance status.
Keywords: costimulation, organ transplantation, belatacept
Costimulatory pathways
T-cells are important mediators of the immune response, and their activation is closely regulated to prevent autoreactivity. It is now recognized that the process of T-cell activation involves multiple signals and distinctly regulated pathways.
To become fully activated, naïve T-cells require two signals. The first (signal 1) is antigen-specific and derives from the interaction of the T-cell receptor (TCR) with the major histocompatibilty complex and antigenic peptide complex that are both expressed on antigen-presenting cells (APCs). The second signal (signal 2), called the costimulatory signal, is antigen-nonspecific and is provided by a number of specialized cell surface receptors. This signal is required for the survival, clonal expansion, and differentiation of activated T-cells.1 Signal 2 is activated when B7-1 (CD80) and B7-2 (CD86), located on the surface of dentritic cells, bind CD28 on T-cells or on its homolog, cytotoxic T lymphocyte-associated antigen 4 (CTLA-4).
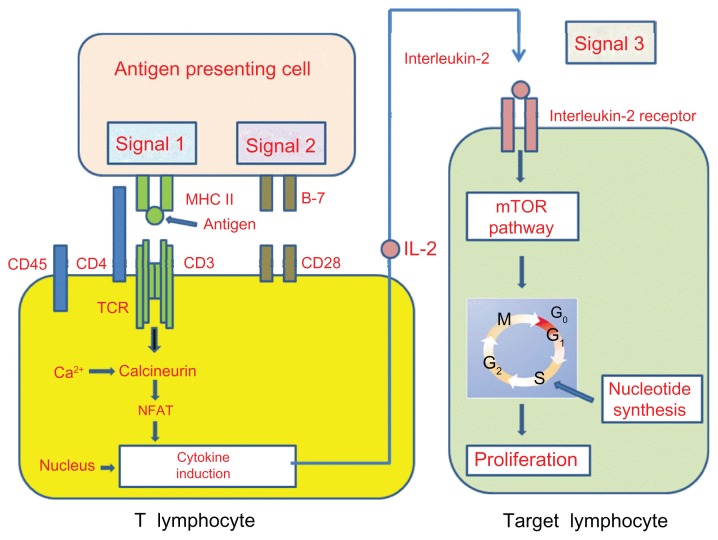
Subsequent to these two signals, various pathways are activated, including the calcium-calcineurin process, the ras/mitogen-activated protein kinase pathway, and the nuclear factor-kB. The activation of these transduction pathways leads to the linkage of IL-2 to its receptor (signal 3), whereupon the mammalian target of rapamycin is activated (Figure 1). These events collectively induce T-cell clonal proliferation and the generation of effector CD4+ T-cells (T-helpers) as well as a clonal expansion of activated CD8+ (cytotoxic T-cells). If the given pathway is blocked, T-cells do not receive a costimulation signal – they become anergic and undergo apoptosis.2,3
Figure 1.
T-cell response to alloantigens.
Notes: The T-cell response to alloantigens requires two different but synergistic signals. Signal 1 is antigen-specific and is delivered via TCR, following recognition of alloantigens coexpressed with an MHC molecule on APCs. Signal 2 is not antigen-specific and is characterized by a link with costimulatory receptor ligand. Signal 3: following these two signals, different pathways are activated. The activation of these transduction pathways leads to the link of IL-2 to its receptor, with activation of mTOR.
Abbreviations: TCR, T-cell receptor; MHC, major histocompatibilty complex; APCs, antigen-presenting cells; mTOR, mammalian target of rapamycin; IL, interleukin; NFAT, nuclear factor of activated T cells.
The costimulatory molecules may be divided into two categories: (1) positive costimulatory pathway (CD28/B7), which promotes T-cell activation; and (2) negative costimulatory pathway (CTLA4/B7), which antagonizes TCR signals and suppresses T-cell activation.4
The CD28 pathway
The best characterized T-cell costimulatory pathway involves the CD28 receptor, which binds to costimulatory molecules named B7-1 (CD80) and B7-2 (CD86).5,6 CD80 expression on resting cells is low, but it increases after prolonged T-cell stimulation, and it thus plays a role in perpetuating immune response. CD86 is expressed constitutively, is rapidly upregulated on APCs upon signal 1, and may be important in the mediation of initial T-cell activation.7
The CD28 molecule, which is constitutively expressed on all naïve CD4 and CD8 T-cells, is the most important activating costimulation receptor of T-cells, in concert with TCR. After interaction with its ligands, CD28 promotes T-cell differentiation into TH1 cells, enhances both the production of antibodies by B-cells and the proliferation of previously activated T-cells and causes the production of cytokine, including IL-2 and IFN-y.6 Moreover, the CD28/B-7 signals induce the development of a class of T-cells termed as regulatory T-cells (Tregs), which inhibit immune response and mantain self-tolerance.8
The CTLA-4 pathway
After the identification of the CD28 molecule and its role in T-cell activation, it was found that CTLA-4 (CD152) and CD28 bind the same ligands (B7-1 and B7-2), but CTLA-4 binds with far higher affinity: 2500-fold avidity for B7-1 and 500-fold for B7-2.9 Unlike CD28, CTLA-4 is exclusively expressed by activated T-cells.10 The engagement of CTLA-4 releases a negative costimulatory signal (known as the coin-hibitory signal), which inhibits TCR and CD28 mediated signal transduction in a B7-dependent way by inhibiting IL-2 production and blocking cell-cycle progression. Thus, CTLA-4 leads to the suppression of T cell activation, which in turn induces T-cell anergy.11
The CD40 pathway
The CD40 pathway has received attention because of its importance both in T-cell costimulation and in transplantation. CD40 is a member of the tumor necrosis factor receptor (TNFR) superfamily and is expressed, at low levels, on the surface of APCs, including B-cells, endothelial cells, keratinocytes, and fibroblasts.10 CD154 (CD40L) is the ligand of CD40, belongs to the TNFR superfamily, and is expressed on activated T-cells, subsets of NK cells, eosinophils, and platelets.12 The CD40/CD154 pathway was initially identified as crucial for B-cell activation and differentiation, but it was subsequently reported to be involved in T-cell activation by upregulating the B7 family ligands CD80 and CD86 in APCs.12 The interaction of CD40 with B-cells increases immunoglobulin (Ig) production and induces a switch of the Ig class, confirming its important role for humoral immunity.13 Upon cell activation, increased levels of CD40 lead to increased CD40/CD154 interaction, which in turn triggers vigorous exposition of antigen-specific signals. For graft survival, in experimental transplant models, the CD40/CD154 costimulation blockade prevents acute rejection, but not chronic rejection.14 Antihuman CD154 antibodies have been generated for therapeutic use, but trials are suspended because of thromboembolic side effects, which are related to the expression of CD154 on platelets.15
To avoid any such interference with platelets, anti-CD40 monoclonal antibodies (mAbs) have been generated for the purpose of blocking CD40/CD154 pathways. These mAbs are currently undergoing experimental and clinical investigation.
Costimulatory pathway in transplantation
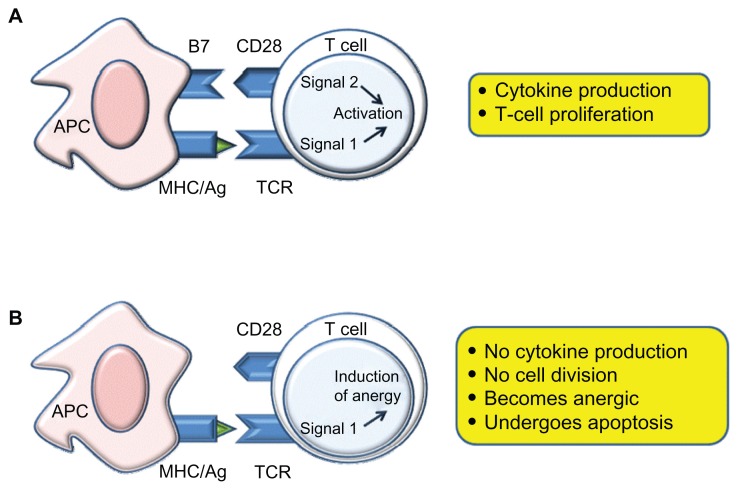
In the past, many groups demonstrated that a T-cell costimulatory signals blockade may both improve long-term graft survival and induce transplantation tolerance in mice and nonhuman primates.16 The efficacy of costimulation signal inactivation depends on the nonactivation of antigen-specific T-cells after TCR involvement (signal 1) and on the absence of costimulation (signal 2) (Figure 2).17
Figure 2.
Costimulatory molecules and biological pathways implicated in the targeting of B7 vs CD28.
Abbreviations: APC, antigen-presenting cell; MHC, major histocompatibilty complex; TCR, T-cell receptor.
Preclinical studies on outbred juvenile rhesus monkeys found that graft survival was prolonged by CTLA-4Ig-induced inhibition of antibody response. Subsequent follow-up demonstrated that CTLA-4Ig is unable to retain a hyporesponsive condition.18
While CD28 blockade is not as efficient in preventing allograft rejections in nonhuman primate models, the blockade of CD40-CD154 has shown promise for the induction of transplantation tolerance.18
CD152-Ig blocks CD28 adhesion on recently activated T-cells and thus prevents the in vitro activation of alloreactive T-cells in mixed leukocyte cultures.19 However, a CD28 blockade is not as efficient in preventing allograft rejection in nonhuman primate models.
Finally, the combined blockade of CD28 signaling with CD152-Ig and of CD40 signaling with anti-CD154 or anti-CD40 mAbs also induces a robust tolerance to alloantigens. Said tolerance extends to skin grafts and inhibits the development of chronic vascular rejection in primarily vascularized cardiac allografts.20
Belatacept, a second generation of CTLA-4Ig fusion protein, has made available a drug with increased affinity for CD80 and CD86. Larsen et al variously tested belatacept as a monotherapy and in combination with basiliximab, steroids, or mycophenolate mofetil (MMF). Used as a monotherapy, belatacept was superior to CTLA-4Ig monotherapy on kidney-graft survival. Thus, in the induction phase, the combination of belatacept with basiliximab, as well as with MMF and steroids, was not only safer but more effective.21
These promising results led to the commencement of testing in humans.
Belatacept (LEA29Y)
Belatacept belongs to a new class of immunosuppressive drugs, is a selective T-cell blocker, and includes two replaced aminoacids in the abatacept (L104E and A29Y). This drug markedly increases its activity in vivo by virtue of its great binding ability to CD80 and CD86.22
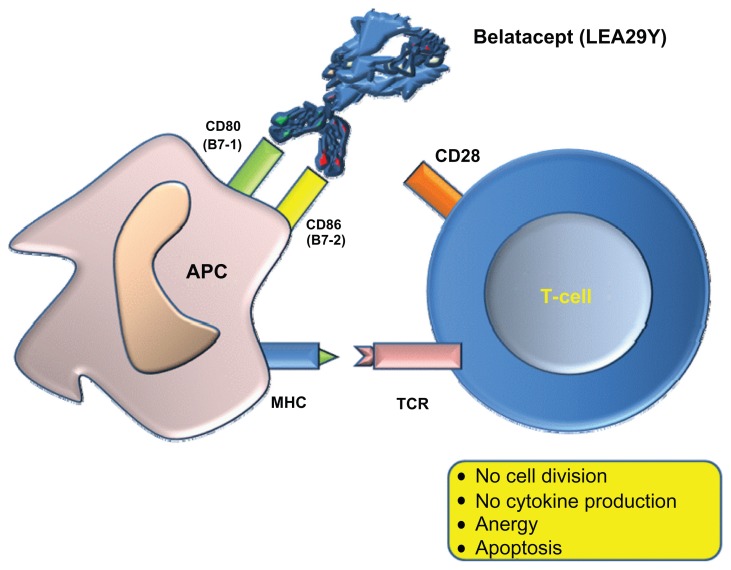
Belatacept is a human fusion protein that combines a modified extracellular portion of CTLA-4 with the Fc domain of human IgG1. It prevents the stimulation of CD28 antagonizing CD80 and CD86 on APCs, and thus blocks the three signals of the transduction pathway. This latter antagonist effect results in an inability to produce effector cell cytokines, such as IL-2. This interaction inhibits the complete activation of T-cells and promotes anergy and apoptosis (Figure 3).6 Moreover, belatacept does not act by depleting T-cells, is well-tolerated, and protects patients against the adverse renal, cardiovascular, and metabolic effects encountered with calcineurin inhibitors (CNIs).
Figure 3.
Belatacept binds to CD80 (B7-1) and CD86 (B7-2) and blocks costimulation.
Abbreviations: APC, antigen-presenting cell; MHC, major histocompatibilty complex; TCR, T-cell receptor.
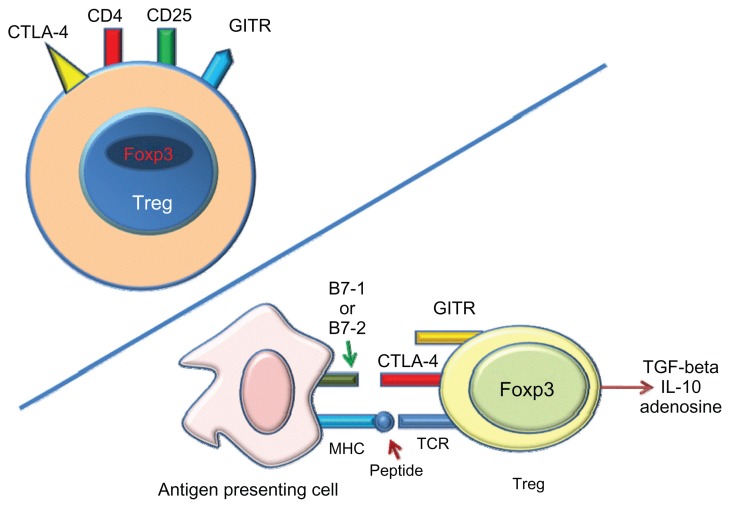
Tregs, a CD4+ CD25+ FOXP3+ subset of T-cells, are known to be able to suppress pathological immune response in autoimmune diseases, transplantation, and graft-versushost disease (Figure 4).23 Bluestone et al investigated the effect of costimulatory blockade on Tregs in kidney transplanted patients receiving belatacept treatment with basiliximab induction. The results show that, unlike CNIs, belatacept has no short- or long-term effects on the number or function of circulating Tregs.24 Great interest was aroused by the finding of a number of Tregs in graft biopsies during acute rejection that exceeded the number found in patients treated with CNIs. This finding suggests that belatacept may both promote Tregs infiltration in renal allografts and facilitate recovery from rejection.24
Figure 4.
Tregs expression.
Notes: Tregs express both the CD4 T-cell receptor and the CD25 IL-2 receptor. Thus, Tregs are CD4+ CD25+. Expression of the Foxp3 is the defining property that determines Tregs development and function. Tregs suppress activation, proliferation, and cytokine production of CD4+ and CD8+ T-cells, and may suppress activity of B-and dendritic cells. Moreover, Tregs can produce and release soluble messengers that have a suppressive function, such as TGF-beta, IL10, and adenosine.
Abbreviations: Tregs, regulatory T-cells; GITR, glucocorticoid-induced TNF receptor; Foxp3, nuclear transcription factor Forkhead box P3; APC, antigen-presenting cell; MHC, major histocompatibilty complex; TCR, T-cell receptor.
The pharmacokinetic parameters of belatacept are not influenced by age, sex, ethnicity, or comorbid conditions. Moreover, hemodialysis, diabetes, and hepatic/renal dysfunction do not affect the pharmacokinetics of belatacept, so that dosage adjustments are not required. The expected half-life of belatacept is about 11 days.25 In renal transplanted nonhuman primates, belatacept demonstrated better effectiveness in preventing acute rejection than abatacept.21
Phase II of a multicenter randomized study in renal transplantation compared data from an intensive regimen of belatacept with those from a less intensive regimen of belatacept or of cyclosporin A (CsA).26 The primary objective was to demonstrate the noninferiority of belatacept over CsA in the incidence of acute rejection at 6 months. Secondary endpoints were the incidence of acute rejection (biopsy-confirmed or presumed) at 6 and 12 months and glomerular filtration rate (GFR) at 1, 6, and 12 months. The incidence of acute rejection was similar at 6 months throughout the three groups recruited for the study. No episodes of acute rejection were reported after month 6 in any group. Moreover, the GFR at 12 months was significantly higher in patients receiving the more intensive (MI) and less intensive (LI) belatacept regimens than in those receiving CsA (P = 0.01 for the comparison between MI belatacept and CsA, P = 0.04 between LI belatacept and CsA). Finally, chronic allograft nephropathy was lower in belatacept-treated patients.26
This study was extended to 5 years and demonstrated stable renal function and a high level of safety. Interestingly, the CD86 receptor showed significant saturation in both 4 week and 8 week dosing regimens, thus suggesting that CD86 receptor binding by belatacept persists over many years of administration.27 These results correlate with data from Latek et al,28 which found that free CD86 receptor levels in belatacept treated patients were (1) significantly lower than they were prior to treatment and (2) lower than those of volunteers and of patients treated with CsA.
On the basis of these results, Phase III trials were carried out to verify the benefits of the costimulation blockade on kidneys from standard-criteria donors (BENEFIT [Belatacept Evaluation of Nephroprotection and Efficacy as First-line Immunosuppressive Trial]) and on kidneys from expanded criteria donors (ECDs) (BENEFIT-EXT [Belatacept Evaluation of Nephroprotection and Efficacy as First-line Immunosuppression – EXTended criteria donors]).
In the BENEFIT study, at 12 months, both belatacept regimens showed similar patient/graft survival to that found for CsA and GFR was significantly lower in the MI and LI belatacept group than in the CsA group (55% MI, 54% LI, and 78% CsA). Unexpectedly, the incidence of acute rejection was higher in belatacept patients than in the CsA group (22% MI, 17% LI, and 7% CsA). Amost 100% of rejection occurred within the first 6 months and were histologically more severe than in CsA-treated patients.29
Given the complexity of the CD28/B7 pathway, multiple factors are likely involved in the onset of acute rejection in belatacept-treated patients: (1) possible memory-cell resistance to costimulation blockade and the intervention of other costimulatory pathways in T-cell activation;3,30 (2) higher B7 occupation by belatacept interfering with inhibitory signals through CTLA-4;31 and (3) possible inhibition of Tregs through the abrogation of CD28 signals and the inhibition of CTLA-4 function.32
The use of the ECDs procedure is perceived as increasingly acceptable and attractive because it enables transplantation to be performed in patients who otherwise would not qualify to receive a donated kidney.
The BENEFIT-EXT trial is a recently published study that aims to underline the superiority of belatacept’s effectiveness compared with that of CsA in patients receiving ECD kidneys.33
The co-primary endpoints at 12 months were composite patient/graft survival and a renal impairment endpoint. Patient/graft survival with MI and LI belatacept was similar to CsA (86% MI, 89% LI, and 85% CsA) at 12 months. Secondary end points included GFR, cardiovascular profile, and metabolic profile, all of which showed better trends in belatecept than in CsA treated patients.
The incidence of acute rejection was similar across groups (18% MI, 18% LI, and 14% CsA). The authors conclude that belatacept provides better renal function and similar immunosuppressive efficacy in comparison with CsA, that it improves cardiovascular risk profile, and that tolerance is high. There were no clinically meaningful differences in efficacy or safety between the MI and LI regimen.
In an analysis of the respective BENEFIT and BENEFIT-EXT datasets, Vanrenterghem et al compared belatacept-based regimens with CsA at month 12. Both the given studies show that belatacept-based regimens had a better cardiovascular and metabolic risk profile, along with lower blood pressure and serum lipids levels and less new onset diabetes after transplant than the CsA-based regimen.34
Analogously comparing performance at 24 months, Larsen et al reported similarity in terms of graft function, but greater renal benefits for belatacept-treated patients than for CsA-treated patients. There were few new acute rejection episodes in either study at 12 and 24 months.35
Finally, in a 36-month study by Florman et al, belatacept demonstrated better renal function and comparable patient/graft survival when compared with CsA, regardless of standard criteria donors or ECD donor type throughout the 3-year period. As of month 24, new cases of acute rejection were infrequent.36
In conclusion, both the BENEFIT and BENEFIT-EXT trials showed that belatacept offers a more positive cardiovascular and metabolic profile, with lower incidence of new-onset diabetes after transplant than do currently used immunosuppressants, and that it might also prevent CNI-associated nephrotoxicity.
Safety of belatacept
About 20% of belatacept-treated patients developed adverse effects, which included anemia, pyrexia, neutropenia, diarrhea, urinary tract infection, headache, and peripheral edema. There were no reports of hypersensitivity or anaphylaxis related to the infusion of belatacept in any patient.37,38
A total of 263 (27.7%) of the 949 patients receiving belatacept had a diagnosis of urinary tract infection, which ranged from mild to severe. Upper respiratory infections were reported in the BENEFIT and BENEFIT-EXT trials and were found in 8.5% of the patients receiving the MI or LI regimen. Moreover, pneumonia occurred in 2.5% of patients.29,33
At 12 months, the respective infection rates for belatacept, as variously combined with MMF, sirolimus, or tacrolimus immunosuppression, were 21%, 15%, and 17% of patients.39
At the time of writing, it does not appear that belatacept predisposes patients to a higher rate of infection than does CsA maintenance immunosuppression.38
Progressive multifocal leukoencephalopathy (PML) occurred in one kidney transplant patient and in a liver transplant patient. Both patients received an MI belatacept regimen, and both died. No cases of PML were reported for the belatacept LI regimen or for control cases.40
The occurrence of posttransplant lymphoproliferative diseases (PTLD) may be related to various factors such as age, immunosuppressive burden, T-cell depleting therapy, Epstein-Barr infection (EBV), and others.41 Belatacept has been associated with an increased risk of PTLD, both in Phase II and Phase III trials, and a greater frequency (1.7%) than the CsA group (0.2%). PTLD occurred in both MI and LI belatacept regimens.37
The risk of PTLD was higher for Epstein–Barr virus (EBV)-seronegative patients than for EBV-seropositive patients. EBV-seropositive patients are defined as evidencing acquired immunity, as shown by the presence of IgG antibodies to viral capsid antigen and EBV nuclear antigen. All cases of PTLD reported over the 36-month study period in belataceptor CsA-treated patients presented within 18 months of transplantation. Overall, the rate of PTLD in 949 patients, treated with any of the belatacept regimens, was ninefold higher in those who were EBV-seronegative or EBV-serostatus unknown (8/139), than in those who were EBV-seropositive (5/810 patients). Belatacept is exclusively recommended for use in patients who are EBV-seropositive.42,43
In conclusion, PTLD risk may be reduced by the use of the LI regimen and by avoidance of patients who are EBV-seronegative or who have an unknown pretransplant EBV serology.
PTLD involving the central nervous system was reported more often in the belatacept groups than in the CsA groups, but it was higher in the MI regimen than in the LI regimen (MI: 6, LI: 3, CsA: 0). Among the nine belatacept central nervous system PTLD cases, the higher incidence was observed in EBV-seronegative patients (5%) as compared with EBV-seropositive patients (0.5%).42,43
Conclusion
With its FDA approval, belatacept is the first biology-based therapeutic agent, one that is based on the blockage of the costimulatory pathway and that, as such, creates a new therapeutic class. The results of the registration trials have allowed the therapy’s use as an immunosuppressant in EBV-seropositive renal transplant patients. Despite the clear benefits it offers in terms of metabolic, cardiovascular, and nephrotoxicity risks, belatacept needs further evaluation, specifically in terms of the late effects that might derive from a prolonged blockage of the costimulatory system and the induction of tolerance status.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bretscher PA. A two-step, two-signal model for the primary activation of precursor helper T cells. Proc Natl Acad Sci U S A. 1999;96(1):185–190. doi: 10.1073/pnas.96.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351(26):2715–2729. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 3.Li XC, Rothstein DM, Sayegh MH. Costimulatory pathways in transplantation: challenges and new developments. Immunol Rev. 2009;229(1):271–293. doi: 10.1111/j.1600-065X.2009.00781.x. [DOI] [PubMed] [Google Scholar]
- 4.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 5.Bromley SK, Iaboni A, Davis SJ, et al. The immunological synapse and CD28-CD80 interactions. Nat Immunol. 2001;2(12):1159–1166. doi: 10.1038/ni737. [DOI] [PubMed] [Google Scholar]
- 6.Alegre ML, Frauwirth KA, Thompson CB. T-cell regulation by CD28 and CTLA-4. Nat Rev Immunol. 2001;1(3):220–228. doi: 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- 7.McAdam AJ, Schweitzer AN, Sharpe AH. The role of B7 costimulation in activation and differentiation of CD4+ and CD8+ T cells. Immunol Rev. 1998;165:231–247. doi: 10.1111/j.1600-065x.1998.tb01242.x. [DOI] [PubMed] [Google Scholar]
- 8.Sharpe AH, Abbas AK. T-cell costimulation – biology, therapeutic potential, and challenges. N Engl J Med. 2006;355(10):973–975. doi: 10.1056/NEJMp068087. [DOI] [PubMed] [Google Scholar]
- 9.Greene JL, Leytze GM, Emswiler J, et al. Covalent dimerization of CD28/CTLA-4 and oligomerization of CD80/CD86 regulate T cell costimulatory interactions. J Biol Chem. 1996;271(43):26762–26771. doi: 10.1074/jbc.271.43.26762. [DOI] [PubMed] [Google Scholar]
- 10.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–252. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 11.Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1(5):405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 12.van Kooten C, Banchereau J. Functions of CD40 on B cells, dendritic cells and other cells. Curr Opin Immunol. 1997;9(3):330–337. doi: 10.1016/s0952-7915(97)80078-7. [DOI] [PubMed] [Google Scholar]
- 13.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu K, Schönbeck U, Mach F, Libby P, Mitchell RN. Host CD40 ligand deficiency induces long-term allograft survival and donor-specific tolerance in mouse cardiac transplantation but does not prevent graft arteriosclerosis. J Immunol. 2000;165(6):3506–3518. doi: 10.4049/jimmunol.165.6.3506. [DOI] [PubMed] [Google Scholar]
- 15.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000;6(2):114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 16.Ford ML, Larsen CP. Translating costimulation blockade to the clinic: lessons learned from three pathways. Immunol Rev. 2009;229(1):294–306. doi: 10.1111/j.1600-065X.2009.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 18.Kirk AD, Harlan DM, Armstrong NN, et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci U S A. 1997;94(16):8789–8794. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 20.Larsen CP, Elwood ET, Alexander DZ, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381(6581):434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 21.Larsen CP, Pearson TC, Adams AB, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5(3):443–453. doi: 10.1111/j.1600-6143.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 22.Emamaullee J, Toso C, Merani S, Shapiro AM. Costimulatory blockade with belatacept in clinical and experimental transplantation – a review. Expert Opin Biol Ther. 2009;9(6):789–796. doi: 10.1517/14712590902942284. [DOI] [PubMed] [Google Scholar]
- 23.Sakaguchi S, Sakaguchi N, Shimizu J, et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 24.Bluestone JA, Liu W, Yabu JM, et al. The effect of costimulatory and interleukin 2 receptor blockade on regulatory T cells in renal transplantation. Am J Transplant. 2008;8(10):2086–2096. doi: 10.1111/j.1600-6143.2008.02377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Z, Shen J, Hong Y, Kaul S, Pfister M, Roy A. Time-varying belatacept exposure and its relationship to efficacy/safety responses in kidney-transplant recipients. Clin Pharmacol Ther. 2012;92(2):251–257. doi: 10.1038/clpt.2012.84. [DOI] [PubMed] [Google Scholar]
- 26.Vincenti F, Larsen C, Durrbach A, et al. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005;353(8):770–781. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- 27.Vincenti F, Blancho G, Durrbach A, et al. Five-year safety and efficacy of belatacept in renal transplantation. J Am Soc Nephrol. 2010;21(9):1587–1596. doi: 10.1681/ASN.2009111109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Latek R, Fleener C, Lamian V, et al. Assessment of belatacept-mediated costimulation blockade through evaluation of CD80/86-receptor saturation. Transplantation. 2009;87(6):926–933. doi: 10.1097/TP.0b013e31819b5a58. [DOI] [PubMed] [Google Scholar]
- 29.Vincenti F, Charpentier B, Vanrenterghem Y, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study) Am J Transplant. 2010;10(3):535–546. doi: 10.1111/j.1600-6143.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 30.Adams AB, Pearson TC, Larsen CP. Heterologous immunity: an over-looked barrier to tolerance. Immunol Rev. 2003;196:147–160. doi: 10.1046/j.1600-065x.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- 31.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27(1):111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pilat N, Sayegh MH, Wekerle T. Costimulatory pathways in transplantation. Semin Immunol. 2011;23(4):293–303. doi: 10.1016/j.smim.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durrbach A, Prestana JM, Pearson T, et al. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study) Am J Transplant. 2010;10(3):547–557. doi: 10.1111/j.1600-6143.2010.03016.x. [DOI] [PubMed] [Google Scholar]
- 34.Vanrenterghem Y, Bresnahan B, Campistol J, et al. Belatacept-based regimens are associated with improved cardiovascular and metabolic risk factors compared with cyclosporine in kidney transplant recipients (BENEFIT and BENEFIT-EXT studies) Transplantation. 2011;91(9):976–983. doi: 10.1097/TP.0b013e31820c10eb. [DOI] [PubMed] [Google Scholar]
- 35.Larsen CP, Grinyó J, Medina-Pestana J, et al. Belatacept-based regimens versus a cyclosporine A-based regimen in kidney transplant recipients: 2-year results from the BENEFIT and BENEFIT-EXT studies. Transplantation. 2010;90(12):1528–1535. doi: 10.1097/TP.0b013e3181ff87cd. [DOI] [PubMed] [Google Scholar]
- 36.Florman S, Becker T, Bresnahan B, et al. Three-year outcomes by donor type in phase III studies of belatacept vs cyclosporine in kidney transplantation (BENEFIT and BENEFIT-EXT) Am J Transplant. 2011;11(Suppl S2):28–559. [Abstract 229] [Google Scholar]
- 37.Su VC, Harrison J, Rogers C, Ensom MH. Belatacept: a new biologic and its role in kidney transplantation. Ann Pharmacother. 2012;46(1):57–67. doi: 10.1345/aph.1Q537. [DOI] [PubMed] [Google Scholar]
- 38.Martin ST, Tichy EM, Gabardi S. Belatacept: a novel biologic for maintenance immunosuppression after renal transplantation. Pharmacotherapy. 2011;31(4):394–407. doi: 10.1592/phco.31.4.394. [DOI] [PubMed] [Google Scholar]
- 39.Ferguson R, Grinyó J, Vincenti F, et al. Immunosuppression with belatacept-based, corticosteroid-avoiding regimens in de novo kidney transplant recipients. Am J Transplant. 2011;11(1):66–76. doi: 10.1111/j.1600-6143.2010.03338.x. [DOI] [PubMed] [Google Scholar]
- 40.Archdeacon P, Dixon C, Belen O, Albrecht R, Meyer J. Summary of the US FDA approval of belatacept. Am J Transplant. 2012;12(3):554–562. doi: 10.1111/j.1600-6143.2011.03976.x. [DOI] [PubMed] [Google Scholar]
- 41.Ippoliti G, Rinaldi M, Pellegrini C, Viganò M. Incidence of cancer after immunosuppressive treatment for heart transplantation. Crit Rev Oncol Hematol. 2005;56(1):101–113. doi: 10.1016/j.critrevonc.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 42.NULOJIX® (belatacept) [package insert] Princeton, NJ: Bristol-Myers Squibb; 2011. [Accessed August 23, 2012]. Available from: http://packageinserts.bms.com/pi/pi_nulojix.pdf. [Google Scholar]
- 43.Grinyó J, Charpentier B, Pestana JM, et al. An integrated safety profile analysis of belatacept in kidney transplant recipients. Transplantation. 2010;90(12):1521–1527. doi: 10.1097/TP.0b013e3182007b95. [DOI] [PubMed] [Google Scholar]