Abstract
Activation of spermine/spermidine-N1-acetyltransferase (SSAT) leads to DNA damage and growth arrest in mammalian cells, and its ablation reduces the severity of ischemic and endotoxic injuries. Here we have examined the role of SSAT in the pathogenesis of toxic liver injury caused by carbon tetrachloride (CCl4). The expression and activity of SSAT increase in the liver subsequent to CCl4 administration. Furthermore, the early liver injury after CCl4 treatment was significantly attenuated in hepatocyte-specific SSAT knockout mice (Hep-SSAT-Cko) compared with wild-type (WT) mice as determined by the reduced serum alanine aminotransferase levels, decreased hepatic lipid peroxidation, and less severe liver damage. Cytochrome P450 2e1 levels remained comparable in both genotypes, suggesting that SSAT deficiency does not affect the metabolism of CCl4. Hepatocyte-specific deficiency of SSAT also modulated the induction of cytokines involved in inflammation and repair as well as leukocyte infiltration. In addition, Noxa and activated caspase 3 levels were elevated in the livers of WT compared with Hep-SSAT-Cko mice. Interestingly, the onset of cell proliferation was significantly more robust in the WT compared with Hep-SSAT Cko mice. The inhibition of polyamine oxidases protected the animals against CCl4-induced liver injury. Our studies suggest that while the abrogation of polyamine back conversion or inhibition of polyamine oxidation attenuate the early injury, they may delay the onset of hepatic regeneration.
Keywords: hepatotoxicity, carbon tetrachloride, polyamine
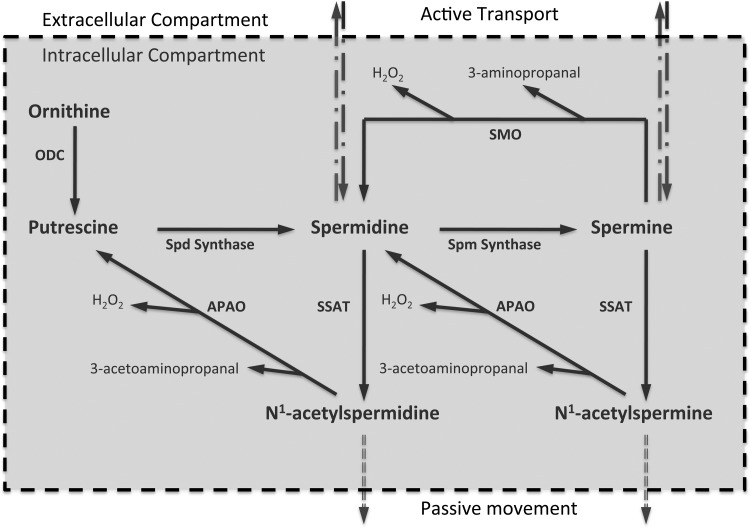
polyamines interact with nucleic acids and proteins and through these interactions play important roles in gene transcription, signal transduction, and cell proliferation (6, 12, 14, 15, 20). The cellular levels of polyamines are tightly regulated through import, export, synthesis and catabolism (Fig. 1). The initial step in polyamine synthesis is the decarboxylation of ornithine by ornithine decarboxylase (ODC) to form putrescine (Put). Sequential addition of aminopropyl residues to Put and spermidine (Spd) lead to the formation of Spd and spermine (Spm). Polyamines are degraded through their back conversion by Spm/Spd-N1-acetyltransferase (SSAT) and N1-acetylpolyamine oxidase (APAO) or by oxidation of Spm by Spm oxidase (SMO). Oxidation of acetylated Spm and Spd by APAO and Spm by SMO generates cytotoxic molecules such as H2O2 and aldehydes (i.e., 3-aminopropanal and 3-acetoaminopropanal; Fig. 1).
Fig. 1.
Regulation of cellular polyamine homeostasis. The cellular levels of polyamines are tightly regulated through import, export, synthesis, and catabolism. The initial step in polyamine synthesis is the decarboxylation of ornithine by ODC to form Put. Sequential addition of aminopropyl residues to Put and Spd leads to the formation of Spd and Spm. Polyamines are degraded through back conversion (SSAT and APAO cascade) and oxidation of Spm by SMO. Oxidation of acetylated polyamines by APAO and Spm by SMO generate cytotoxic molecules such as 3-aminopropanal, 3-acetoaminopropanal, and H2O2. SSAT, spermine/spermidine-N1-acetyltransferase; ODC, ornithine decarboxylase; Put, putrescine; Spd, spermidine; Spm, spermine; APAO, N1-acetylpolyamine oxidase; SMO, Spm oxidase.
The expression and activity of SSAT increases in organs (e.g., liver, kidney, and brain) subjected to ischemia-reperfusion and septic and traumatic injuries (4, 35, 37, 39). The ablation of the SSAT gene reduces the severity of renal and hepatic ischemia-reperfusion and endotoxin-induced renal injuries (35, 37). Transgenic animals that express high levels of SSAT develop several pathologies, including skin lesions and pancreatitis (1, 24, 25). In vitro, expression of SSAT causes oxidative stress, DNA damage, cell cycle arrest, and apoptosis (7, 11). These results suggest that elevated SSAT levels contribute to the onset of cell damage and tissue injury.
The mouse model of carbon tetrachloride (CCl4)-induced hepatic injury has been used to investigate the factors involved in the mediation of toxic liver damage (16, 23). Although SSAT expression increases in CCl4-induced liver injury its role and the contribution of enhanced polyamine catabolism to the pathophysiology of hepatotoxic injuries in general, and CCl4-induced liver injury in particular, are not understood (21). Recent studies indicate that α-methylspermidine, a stable polyamine mimetic, has an ameliorative effect on CCl4-induced liver toxicity, suggesting that polyamines and their catabolism are important mediators of this injury (13). We hypothesize that increased SSAT expression and activity in the hepatocytes of animals treated with CCl4 contributes to liver injury. To test this hypothesis we generated a hepatocyte-specific SSAT knockout mouse and determined the impact of SSAT ablation on the severity of CCl4-induced liver damage.
MATERIALS AND METHODS
Reagents.
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise indicated. Oligonucleotides were purchased from Invitrogen (Carlsbad, CA). Monoclonal anti-proliferating cell nuclear antigen (anti-PCNA; Calbiochem La Jolla, CA), rabbit anti-cytochrome P450 2e1 (CYP2e1; ProSci Poway, CA), rabbit anti-actin (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-mouse Noxa (Santa Cruz Biotechnology), rabbit anti-pro and cleaved caspase 3 (H-277; Santa Cruz Biotechnology), rabbit anti-cleaved caspase 3 (Sigma-Aldrich), and rat monoclonal anti-neutrophil (AbD Serotech, Oxford, UK) antibodies were used in these studies. All secondary antibodies were purchased from Molecular Probes (Eugene, OR).
Generation and genotyping of hepatocyte-specific SSAT-deficient mice.
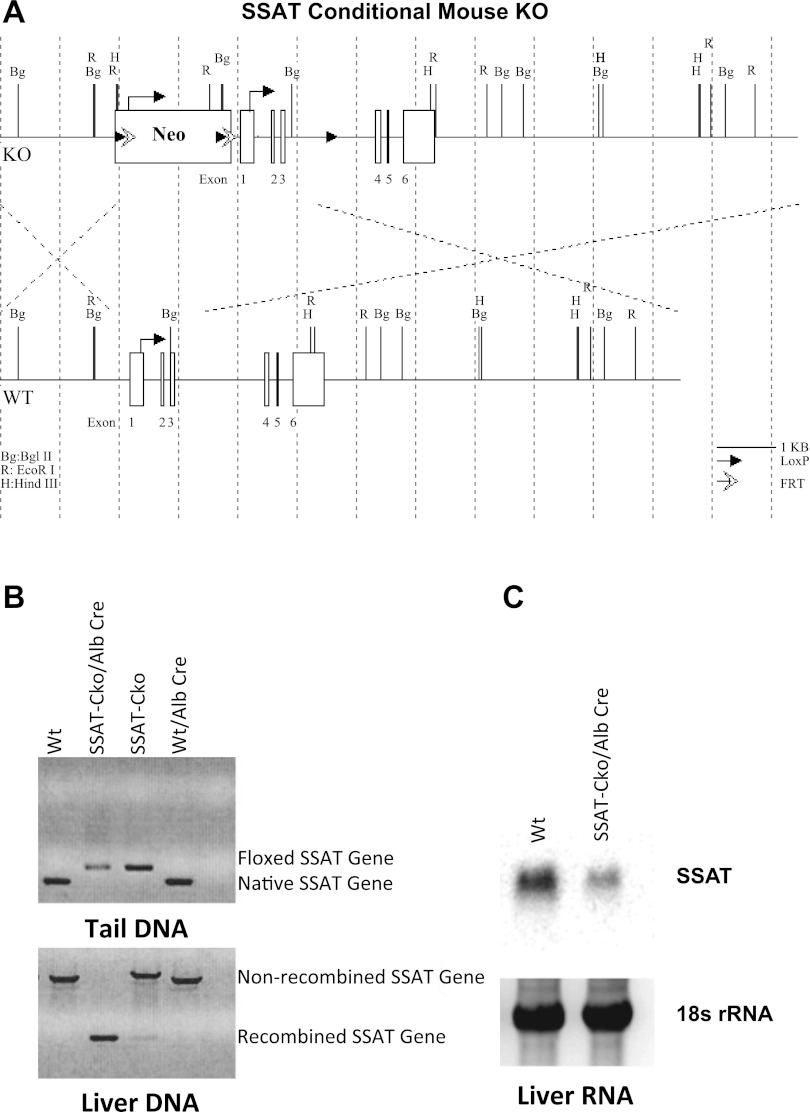
Hepatocyte-specific SSAT-deficient animals were generated in our laboratory. The arrangement of the LoxP sites in the knockout construct was such that after the deletion of the neomycin resistance gene and the middle LoxP site, the remaining two LoxP sites flanked the 5′ untranslated region, first and second exons and introns, third exon, and parts of the third intron of the SSAT gene (the structure of LoxP-SSAT construct is shown in Fig. 2A). After elimination of the neomycin resistance and FLP recombinase genes, the resulting SSAT-CkoNeo−/FLP− mice were back-crossed to C57BL6 mice for 15 generations to obtain SSAT-CkoNeo−/FLP− mice on C57BL6 genetic background. Hepatocyte-specific SSAT-deficient (Hep-SSAT-Cko) mice were generated by breeding the C57BL6-SSAT-CkoNeo−/Flp− with C57BL6-albumin-promoter-driven Cre-recombinase transgenic mice (Jackson Laboratories, Bar Harbor, ME). The Cre-recombinase-mediated disruption of SSAT gene was confirmed by examining the genomic DNA. Briefly, isolated genomic DNA was amplified (94°C for 3 min-94°C 30 s, 50°C 30 s, 72°C 90 s for 35 cycles-72°C for 10 min) using 5′F-SSAT (5′-GCTTCCTGAGTTTGCTTTTCTGC-3′) and 3′R-SSAT (5′-CTCGTGACACCCATGGCCAAACC-3′) primers. The amplified DNA was examined for the presence of ≃1450 bp wild-type (WT) and ≃240 bp (SSAT-Cko) products (Fig. 2B). The effect of SSAT gene ablation on the expression of its mRNA in the liver was examined by Northern blot analysis (Figs. 2B and see also 7, A–B) and analysis of SSAT activity (see Fig. 7C).
Fig. 2.
Targeting strategy and characterization of hepatocyte-specific SSAT knockout mice. A: structure of LoxP-SSAT construct and the gene ablation strategy are depicted (top and bottom). B: disruption of SSAT gene was confirmed by examining the genomic DNA from the tail (top) and liver (bottom) of Alb-SSAT-Cko (Hep-SSAT-Cko) and their Cre-deficient (SSAT-CkoNeo−/Flp−) or wild-type (Wt, WT) littermates. C: examination of SSAT mRNA expression in the livers of hepatocyte-specific SSAT knockout mice (Hep-SSAT-Cko), WT, and SSAT-Cko Neo−/Flp− mice.
Fig. 7.
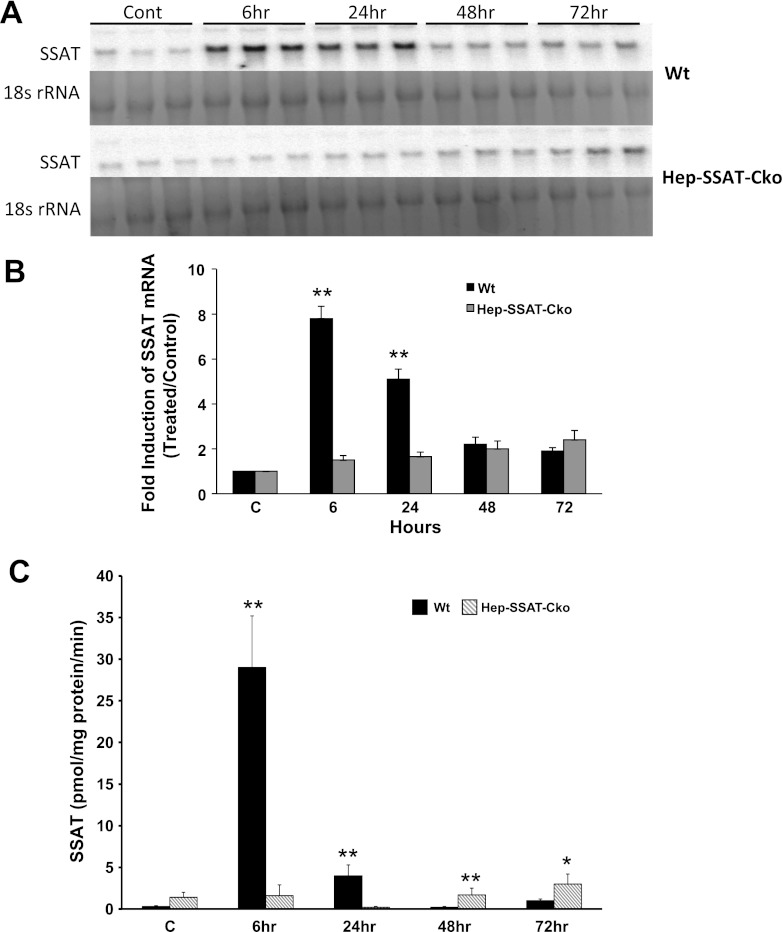
Comparison of the time course of expression and activity of SSAT in the livers of control and CCl4-treated WT and Hep-SSAT-Cko mice. A: liver RNA from control and CCl4-treated WT and Hep-SSAT-Cko mice (n = 3/group) was size fractionated and subjected to Northern blot analysis to compare the expression levels of SSAT transcript. B: intensity of the SSAT mRNA bands were normalized against their corresponding 18s rRNA bands. These results were used to compare the induction of SSAT transcript in the livers of CCl4- or vehicle-treated WT and Hep-SSAT-Cko animals. The induction values are presented as fold increase over control. C: enzymatic activity of SSAT was measured and compared in the livers of time-matched control and CCl4-treated WT and Hep-SSAT-Cko mice (n = 3/group). *P < 0.05 is considered significant; **P < 0.01.
Induction of CCl4-mediated hepatic injury.
Male (28–32 g) WT and Hep-SSAT-Cko mice were given a single intraperitoneal injection of CCl4 (10 μl/g of 1:30 dilution of CCl4 in sunflower seed oil) or vehicle (10 μl/g of sunflower seed oil). At timed intervals (6, 24, 48 and 72 h) animals from each treatment group were killed (n = 6/group). Blood was collected and processed to obtain serum. Livers were harvested and fixed in paraformaldehyde or frozen in liquid nitrogen. Paraformaldehyde fixed livers sections were used for hematoxylin and eosin or immunohistochemical staining. Studies assessing the effect of inhibition of polyamine oxidases on the severity of CCl4-induced hepatic injury were performed as outlined above, except animals (n = 5/group) were treated with vehicle (saline) or polyamine oxidase inhibitor MDL72527 (100 mg/kg ip), 30 min prior to administration of sunflower seed oil (control) or CCl4 followed by once daily treatment with MDL72527 (100 mg/kg ip) for the duration of the study. Animal studies were performed according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” following a protocol approved by the University of Cincinnati Animal Care and Use Committee. All animal work complied with the National Institutes of Health guidelines.
Northern blot analysis.
RNA was extracted and subjected to Northern blot analysis as previously described (32, 36). Equal loading of RNA samples was confirmed by assessment of the 28 s or 18 s rRNA band intensities. For quantitative analysis of mRNA expression signal intensity of each band was normalized against the intensity of its corresponding rRNA band. The results were then expressed as fold increase over the control samples.
Preparation of liver extracts and Western blot analysis.
Liver samples (50 mg) were pulverized, washed with ice-cold PBS, and spun down at 700 g for 5 min to remove non-liver cell material. Subsequently, 200 μl of extraction buffer (45 mM HEPES, 0.4 M KCl, 1 mM EDTA, 0.1 mM dithiothreitol, 10% glycerol, pH 7.8) was added to the liver samples. Resulting suspensions were mixed vigorously, snap frozen in liquid nitrogen, and immediately thawed. Then 30 μl of 1% Triton X-100 in buffer A was added to a 100 μl aliquot of each sample. Samples were mixed vigorously and incubated for 5 min on ice. After centrifugation at 14,000 g for 5 min at 4°C to remove cell debris, the supernatants were collected, and their protein contents were determined by BCA assay (Thermo Scientific, Rockford, IL). Then 30 μg of each extract was size fractionated by gel electrophoresis, transferred to nitrocellulose membrane, and subjected to Western blot analysis as previously described (39).
Assessment of liver function.
Serum alanine aminotransferase (ALT) levels of vehicle and CCl4-treated animals were measured using a commercially available kit (Cayman Chemicals, Ann Arbor, MI).
Measurement of tissue polyamine levels, and ODC and SSAT activities.
The liver polyamine pools, as well as ODC and SSAT activities were analyzed as described previously (17, 18).
Lipid peroxidation assay.
Lipid peroxidation was assessed in the serum and liver extracts using a commercially available kit (Enzo Life Sciences, Plymouth Meeting, PA).
Immunofluorescent microscopic analysis of liver sections.
Liver sections (5 μm) were processed for immunofluorescent detection of neutrophils, PCNA, Noxa, and cleaved caspase 3 as described previously (10, 35, 38). Quantitation of neutrophil infiltration was performed by determining the number of infiltrating neutrophils in 10 independent fields from three different liver samples from each treatment group. The results were expressed as means ± SE and subjected to statistical analysis.
Data analysis.
Values are expressed as means ± SE. The significance of differences between mean values of multiple samples was examined using ANOVA. A P value of < 0.05 was considered statistically significant.
RESULTS
The expression of SSAT, SMO, and ODC mRNA is elevated in the livers of CCl4-treated mice.
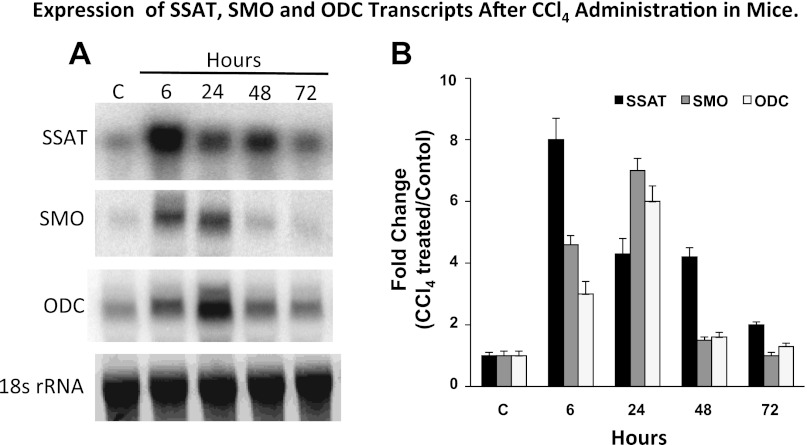
The hepatic expression of SSAT, SMO, and ODC transcripts is induced as early as 6 h post-CCl4 administration (Fig. 3, A and B). While SSAT levels are at their peak in the aforementioned time point, SMO and ODC transcript levels reach their highest levels at 24 h after CCl4 administration (Fig. 3, A and B). Expression levels of all transcripts remained elevated throughout the 72-h duration of these studies (Fig. 3, A and B).
Fig. 3.
Effect of carbon tetrachloride (CCl4) treatment on the hepatic expression of SSAT, SMO, and ODC mRNAs. Liver RNA samples (15 μg/well of total RNA) from control and CCl4-treated animals were size fractionated and subjected to Northern blot analysis to assess the expression of SSAT, SMO, and ODC transcripts. A: Northern blot analysis results indicate that all three transcripts are upregulated in the livers of CCl4-treated animals. B: densitometric analysis of the Northern blot analysis results were normalized against the intensity of their corresponding 18s rRNA band. These results were used to compare the induction of each transcript in the livers of CCl4- to that of vehicle-treated animals. The induction values are presented as fold increase over control. The blots are representative of 3 independent studies.
Hepatocyte-specific deficiency of SSAT reduces the severity of CCl4-induced hepatic injury.
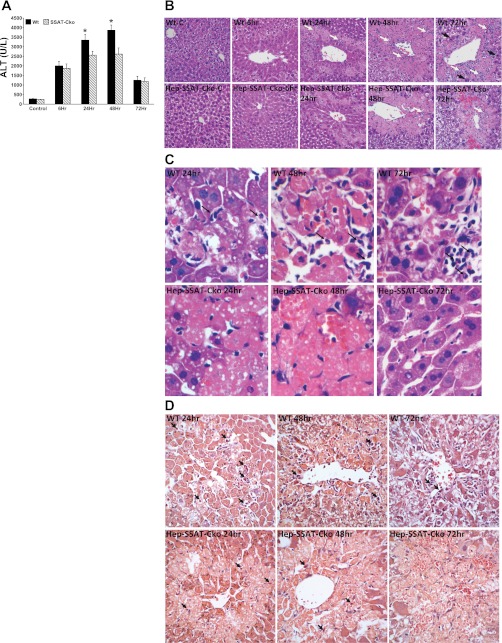
Increased expression of SSAT, and the attendant enhancement of polyamine catabolism contribute to cell damage and organ dysfunction in a variety of injuries (4, 35, 37, 39). To determine the role of SSAT in CCl4-induced liver injury, WT and Hep-SSAT-Cko mice were given a single intraperitoneal injection of CCl4. Serum ALT levels in all CCl4-treated animals were significantly higher than those of control animals (Fig. 4A). Furthermore, serum ALT levels were significantly (P < 0.05) lower in Hep-SSAT-Cko compared with WT mice at 24 and 48 h post-CCl4 administration (Fig. 4A). Comparison of the liver histopathology of WT to Hep-SSAT-Cko animals revealed that WT animals had more profuse and diffuse hepatocyte swelling compared with Hep-SSAT-Cko mice as early as 6 h post-CCl4 administration (Fig. 4B, WT vs. Hep-SSAT-Cko 6 h). Hepatic damage was limited to pericentral (zone 3) areas in both WT and Hep-SSAT-Cko animals. Hepatocyte necrosis and infiltration of acute inflammatory cells were apparent in zone 3 at 24 h [black arrows in hematoxylin and eosin (Fig. 4C) and chloroacetate esterase (Fig. 4D) stained slides]. The necrosis and inflammatory cell infiltration were more severe in the WT compared with Hep-SSAT-Cko mice (Fig. 4, B–D, WT vs. Hep-SSAT-Cko 24 and 48 h). Comparison of the livers of CCl4-treated WT to Hep-SSAT-Cko mice at 48 and 72 h revealed that the necrosis in the WT animals had begun to resolve, with a higher number of chronic inflammatory cells (large white arrows) in zone 3 and increased number of mitotic cells (large black arrows) in the areas adjacent to necrotic regions, while the Hep-SSAT-Cko animals had an ongoing necrotic and a nascent regenerative/repair response (Fig. 4, B and C, WT vs. Hep-SSAT-Cko 72 h). Increased mitotic activity in the liver sections was further confirmed by immunostaining for PCNA expression. The number of PCNA positive cells are significantly increased in the livers of CCl4-treated WT compared with similarly treated Hep-SSAT-Cko mice at 48 and 72 h time points (Fig. 5).
Fig. 4.
Assessing the effect of hepatocyte-specific SSAT deficiency on the severity of CCl4-induced liver injury. WT and Hep-SSAT-Cko were given intraperitoneal injections of CCl4 or vehicle. Animals (n = 6/treatment group/genotype) were killed at timed intervals after treatment. A: serum alanine aminotransferase (ALT) levels of control and CCl4-treated WT and Hep-SSAT-Cko mice (n = 6/group) were compared following the protocol outlined in materials and methods. *P < 0.05 WT compared with SSAT-Cko. B: liver histology (magnification, ×400) of control and injured animals from both genotypes were compared. Infiltrating neutrophils (small white arrows), chronic inflammatory cells (large white arrows) and mitotic bodies (large black arrows) are marked. C: liver histology of CCl4-treated WT and Hep-SSAT-Cko mice was examined at ×600 magnification (black arrows mark the infiltrating neutrophils). D: livers of CCl4-treated WT and Hep-SSAT-Cko mice were subjected to chloroacetate estrase staining to identify the infiltrating neutrophils (black arrows).
Fig. 5.
Comparison of the number of proliferating cells in the livers of WT and Hep-SSAT-Cko mice after CCl4-induced liver injury. Expression of proliferating cell nuclear antigen (PCNA), a marker of proliferating cells, was compared in the livers of WT and Hep-SSAT-Cko animals after CCl4-induced liver injury.
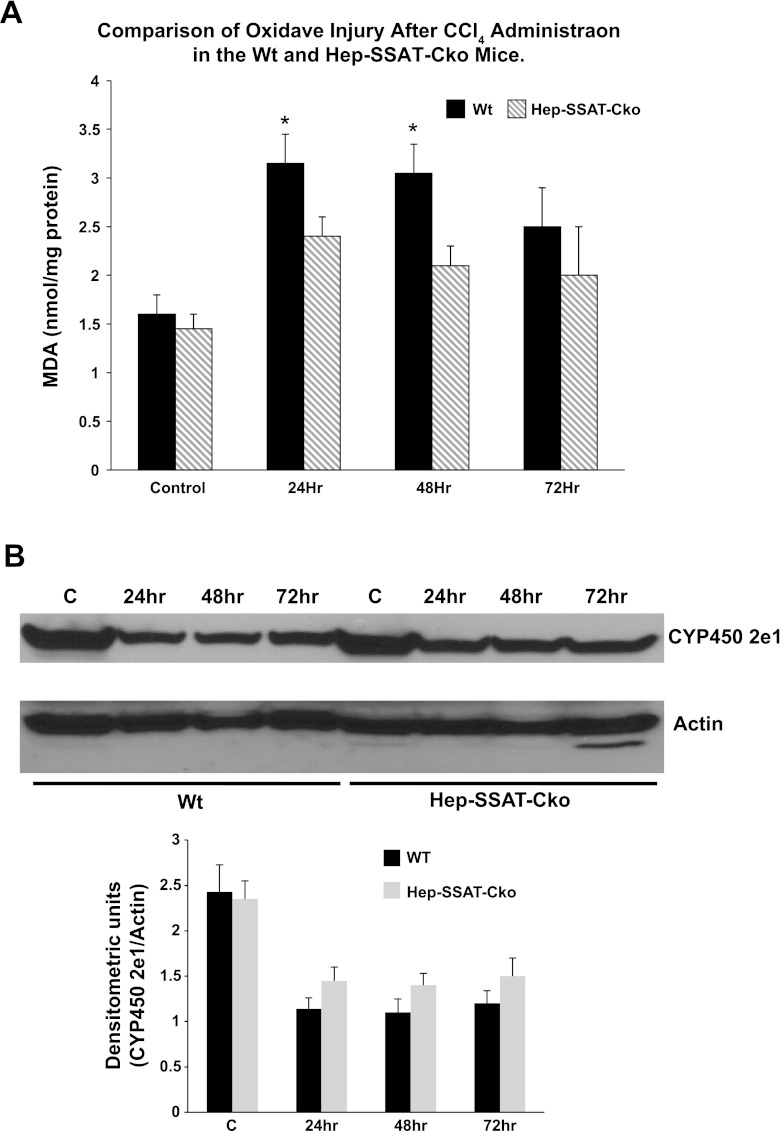
The injurious effect of CCl4 is the result of the metabolism of trichloromethyl radical by CYP2e1 to trichloromethyl peroxy radical, a highly toxic molecule that can lead to oxidative injury (33). We compared the extent of lipid peroxidation and CYP2e1 levels in CCl4-treated WT and Hep-SSAT-Cko animals. Comparison of lipid peroxidation levels in the serum and liver extracts of WT and Hep-SSAT-Cko mice revealed that serum (data not shown) and liver malondialdehyde levels in both genotypes were significantly higher in CCl4-treated animals than controls at 24 and 48 h posttreatment. However, the levels of malondialdehyde were significantly lower in the liver extracts of Hep-SSAT-Cko compared with WT mice at 24 and 48 h post-CCl4 administration (Fig. 6A), suggesting that the extent of oxidative injury was reduced in the former. Comparison of CYP2e1 levels revealed that the hepatic levels of this protein were nearly identical in untreated animals of both genotypes. CYP2e1 levels decrease in both WT and Hep-SSAT-Cko mice after CCl4 treatment and the reduction in CYP2e1 levels are of similar magnitudes in the two genotypes (Fig. 6B).
Fig. 6.
Comparison of the severity of oxidative injury in WT and Hep-SSAT-Cko mice. A: onset of oxidative damage in the liver of WT and Hep-SSAT-Cko animals (n = 5/group/genotype) after CCl4-induced hepatotoxic injury was compared by determining the extent of lipid peroxidation as measured by the malondialdehyde (MDA) levels in the liver extracts. *Levels were significantly higher in WT vs. Hep-SSAT-Cko at 24 h and 48 h. B: adaptive downregulation of liver CYP2e1 was compared in WT and Hep-SSAT-Cko mice. CYP2e1 and actin signals from Western blot analyses of liver extracts from control and CCl4-treated WT and Hep-SSAT-Cko mice were subjected to densitometric analysis to compare the effect of CCl4-induced liver injury on the expression of CYP2e1. The blots are representative and the densitometry studies are the average ± SD of 3 independent samples.
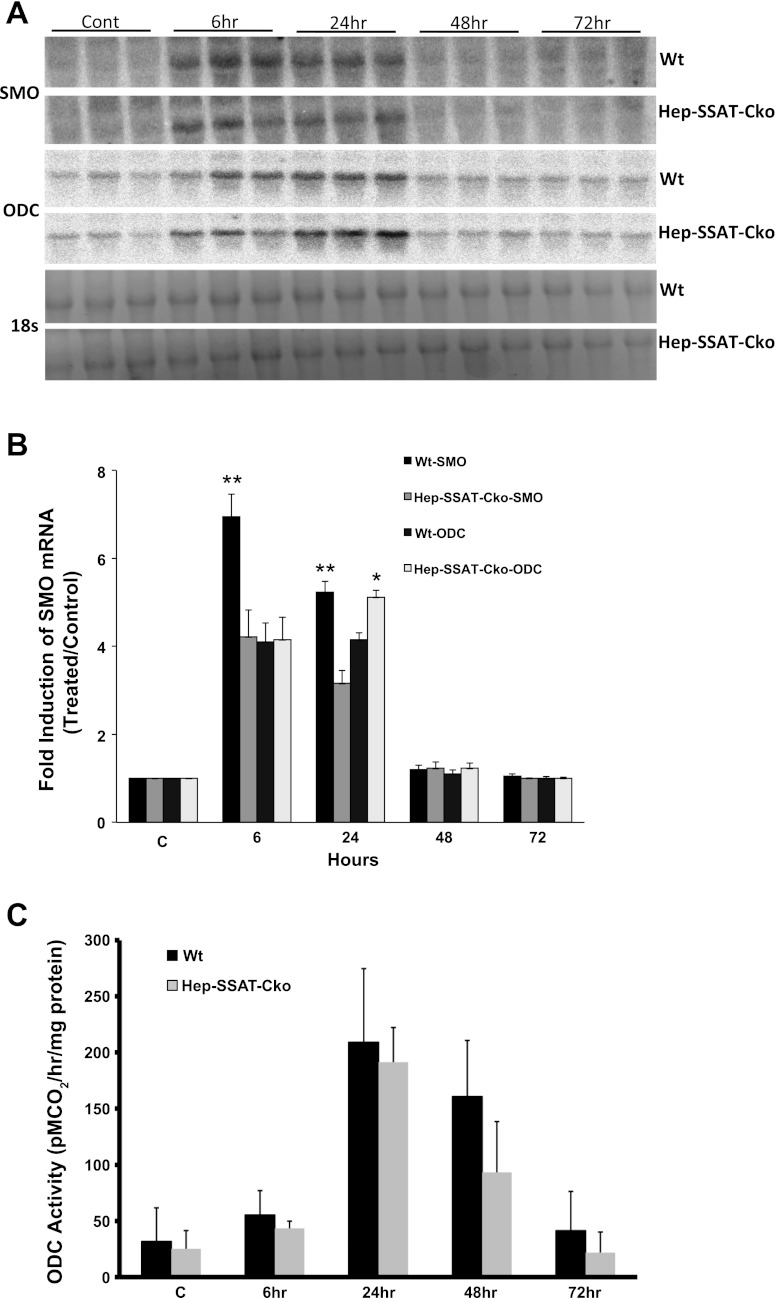
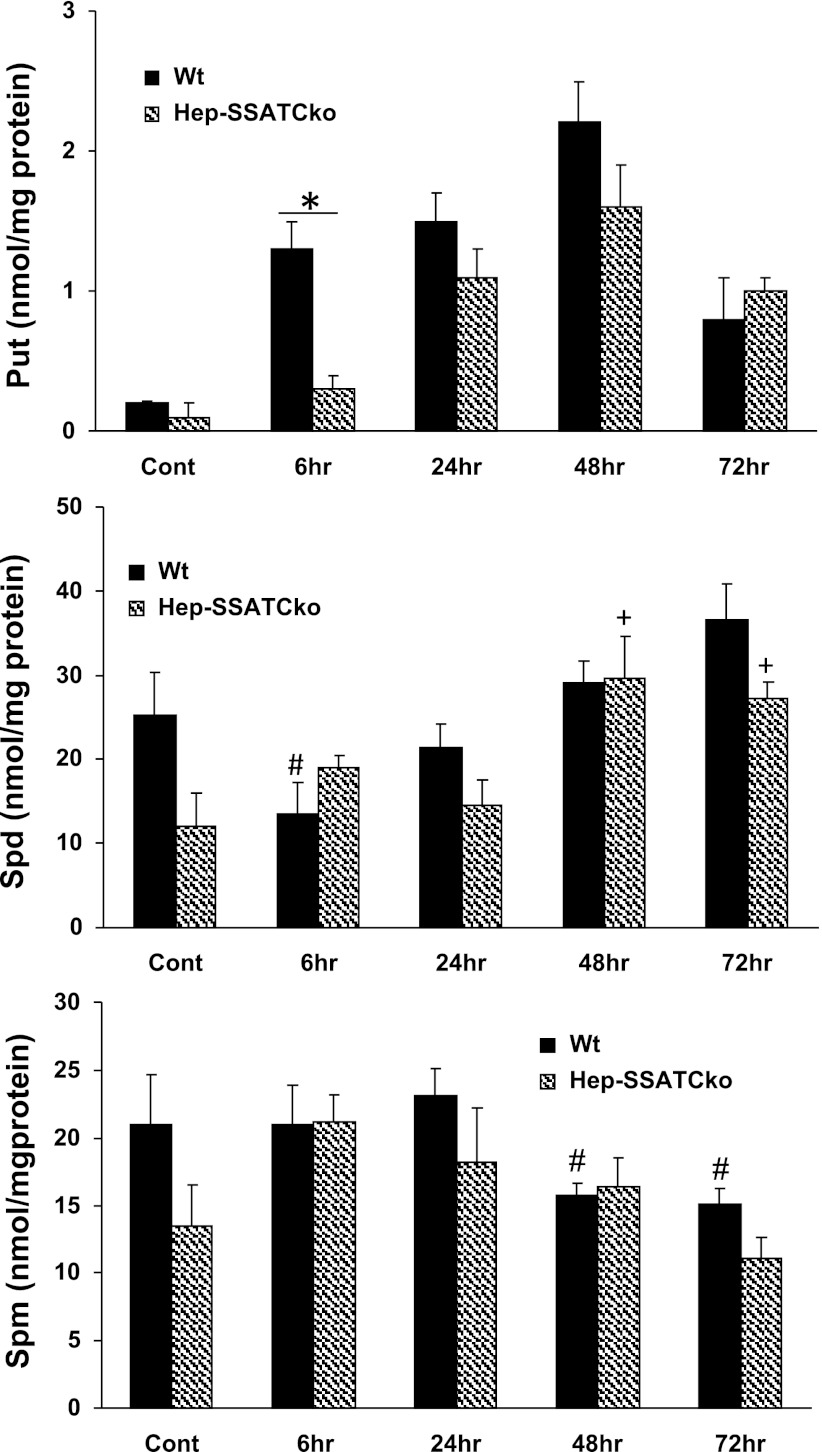
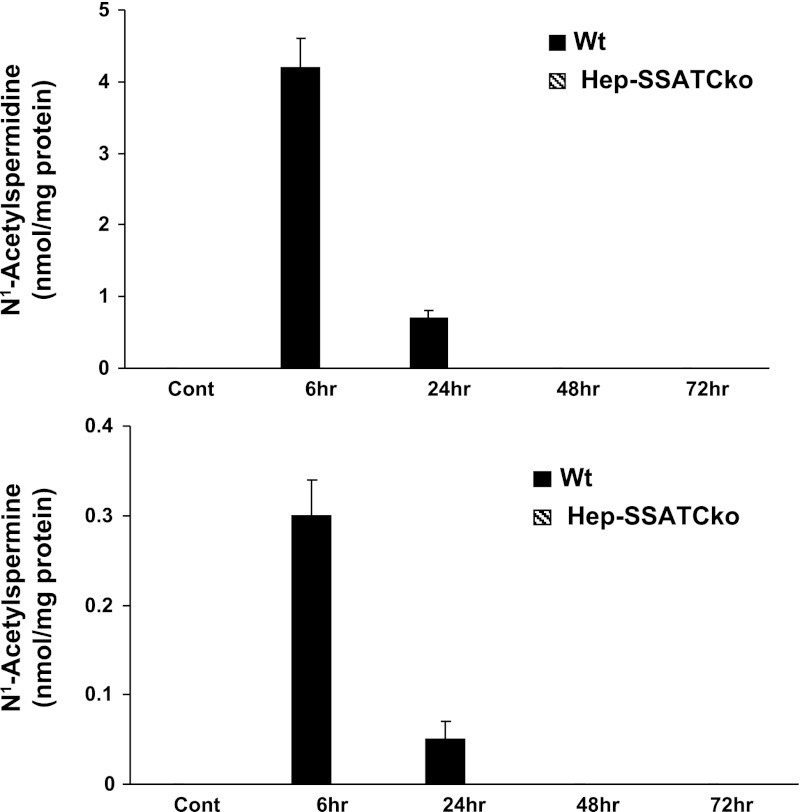
Next, to confirm that the SSAT gene is deactivated and polyamine metabolism is altered, we compared the expression and activity of SSAT and ODC as well as expression of SMO in WT and Hep-SSAT-Cko mice treated with CCl4. As expected, our results indicate that SSAT expression (Fig. 7, A and B) and activity (Fig. 7C) was elevated in CCl4-treated WT but not in similarly treated Hep-SSAT-Cko animals. The mRNA levels of and enzymatic activity of ODC were elevated in the livers of CCl4-treated animals of both genotypes (Fig. 8, A–C). The induction of ODC mRNA and its activity levels were similar in WT and Hep-SSAT-Cko animals (Fig. 8, A–C). On the other hand, while the expression of SMO transcript was elevated in both genotypes, based on densitometric measurement of the Northern blot analyses results, SMO mRNA levels were significantly (P < 0.05) higher in the WT compared with Hep-SSAT-Cko animals at 6 and 24 h after CCl4 treatment (Fig. 8B). Comparison of liver polyamine levels of the CCl4-treated animals indicates that compared with vehicle-treated controls the hepatic levels of Spd decreased significantly by 6 h after CCl4 treatment in WT but remained essentially the same in Hep-SSAT-Cko mice, while the Spm levels remained essentially unaltered in the early time points and were only marginally lower in CCl4-treated WT animals at 48 and 72 h post-CCl4 administration (Fig. 9). The hepatic Put levels were significantly higher as early as 6 h after CCl4-treatment in WT mice and remained above normal levels through the duration of the study (Fig. 9). On the other hand, Put levels remained low during the early time point (6 h) of injury but increased significantly in the latter time points of the study in Hep-SSAT-Cko mice (Fig. 9). Comparison of hepatic acetylated polyamine levels revealed the complete absence of these in Hep-SSAT-Cko mice (Fig. 10).
Fig. 8.
Comparison of the time course of expression of SMO and ODC in the livers of vehicle and CCl4-treated WT and Hep-SSAT-Cko mice. A: liver RNA from control and CCl4-treated WT and Hep-SSAT-Cko mice (n = 3/group) were subjected to Northern blot analysis to compare the expression levels of SMO and ODC transcripts. B: intensity of SMO and ODC mRNA bands for each sample was determined by densitometry and normalized against the corresponding 18s rRNA band. The induction values are presented as fold increase over control. C: ODC activity was measured in the livers of vehicle and CCl4-treated WT and Hep-SSAT-Cko animals. *P < 0.05 is considered significant; **P < 0.01.
Fig. 9.
Comparison of the polyamine content in the livers of control and CCl4-treated WT and Hep-SSAT-Cko mice. The content of Put, Spd, and Spm (top, middle, and bottom, respectively) in the livers of control and CCl4-treated WT and Hep-SSAT-Cko mice were measured by HPLC (results are means ± SE of n = 3/treatment group/genotype). Hepatic content of each polyamine was compared in CCl4-treated and control samples as well as time-matched samples from both genotypes. Polyamine levels are expressed as pmol/mg of protein. P > 0.05 is considered significant. *A significant difference between WT and Hep-SSAT-Cko. #Significant differences between vehicle-treated controls and CCl4-treated WT animals. +Significant differences between vehicle-treated controls and CCl4-treated Hep-SSAT-Cko mice.
Fig. 10.
Comparison of the acetylated polyamine content in the livers of vehicle and CCl4-treated WT and Hep-SSAT-Cko mice. The content of N1-acetylspermidine and N1-acetylspermine (top and bottom, respectively) in the livers of control (Cont) and CCl4-treated WT and Hep-SSAT-Cko mice were measured by HPLC (results are means ± SE of n = 3/treatment group/genotype). Hepatic levels of acetylated polyamines were compared in CCl4-treated and control samples as well as time-matched samples from both genotypes. Polyamine levels are expressed as pmol/mg of protein.
Hepatocyte-specific ablation of SSAT modulates the inflammatory response.
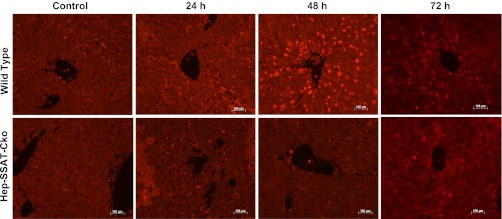
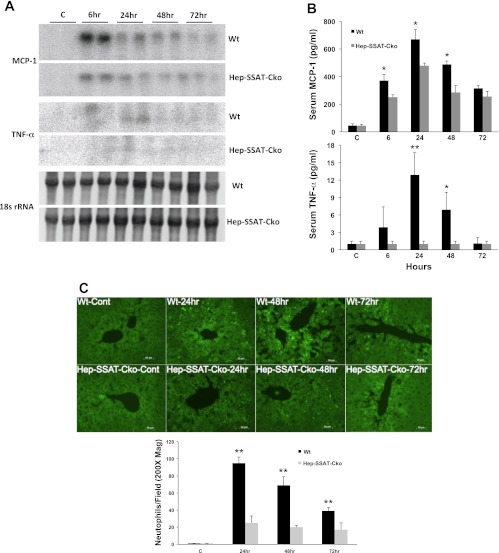
We next examined the effect of hepatocyte-specific deficiency of SSAT on the severity of the inflammatory response in CCl4 injury. The hepatic levels of monocyte chemotactic protein-1 (MCP-1) and to a lesser extent TNF-α, but not IL-6 (data not shown) transcripts were elevated in the livers of WT compared with Hep-SSAT-Cko CCl4-treated animals (Fig. 11A). Examination of the serum levels of MCP-1 (368 ± 8.1 vs. 248 ± 16.2, 671 ± 18 vs. 470 ± 11, and 495 ± 10.2 vs. 278 ± 30 pg/ml in WT vs. Hep-SSAT-Cko mice at 6, 24, and 48 h after CCl4 treatment) and TNF-α (14 ± 3.2 vs. below detectable limits at 24 after CCl4 treatment) revealed that the circulating levels of both cytokines are also significantly (for P values refer to Fig. 11B, legend) higher in the CCl4-treated WT compared with similarly treated Hep-SSAT-Cko mice (Fig. 11B). Our results indicate that the levels of MCP-1 are significantly increased as early as 6 h post-CCl4 administration, while significant increase in TNF-α levels was first observed at 24 h post-CCl4 treatment (Fig. 11, A and B). The number of infiltrating neutrophils was determined in the WT and Hep-SSAT-Cko mice by chlorocetate esterase (Fig. 4C) and immunofluorescent staining (Fig. 11C). The comparison of the infiltrating cells in the livers of WT and Hep-SSAT-Cko mice subjected to CCl4-induced injury revealed that compared with Hep-SSAT-Cko mice, the WT mice had significantly higher (P < 0.01) number of infiltrating neutrophils at 24, 48, and 72 h post-CCl4 administration (Figs. 4D and 11C).
Fig. 11.
Comparison of the inflammatory response of WT and Hep-SSAT-Cko animals subjected to CCl4-induced liver injury. A: liver RNA form control and CCl4-treated WT and Hep-SSAT-Cko mice (n = 2/group) was subjected to Northern blot analysis to compare the monocyte chemotactic protein-1 (MCP-1) and TNF-α levels. Data is representative of 2 independent studies. B: serum levels of MCP-1 and TNF-α were compared in control and CCl4-treated WT and Hep-SSAT-Cko mice (n = 3/group). *P < 0.05 is considered significant; **P < 0.01. C: infiltration of neutrophils in livers of vehicle and CCl4-treated mice was assessed by immunofluorescent microscopy (magnification, ×200). The number of neutrophils was determined in 10 independent fields (magnification, ×200), and the results were expressed as means ± SD. **P < 0.01.
Hepatocyte-specific ablation of SSAT modulates the onset of apoptosis in CCl4-induced liver injury.
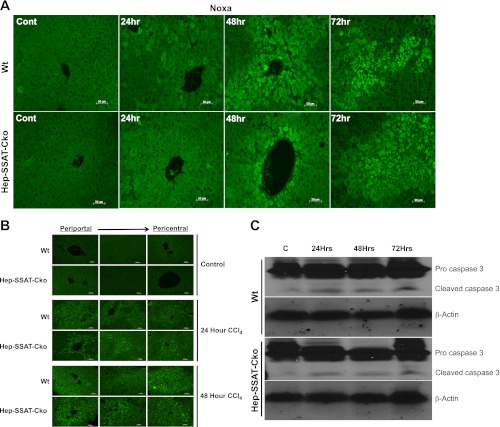
We next examined the effect of SSAT deficiency on the onset of apoptosis by examining the expression of proapoptotic BH3 only protein Noxa, a transcripts that is induced in SSAT expressing cultured cells (data not shown) and the hepatic levels of cleaved caspase 3. Immunohistochemical studies indicate that the expression of Noxa is higher at 24 and 48 h post-CCl4 administration in the WT compared with Hep-SSAT-Cko mice (Fig. 12A). To examine the effect of SSAT deficiency and associated reduction in Noxa expression on the onset of apoptosis, we compared the levels of activated caspase 3 in WT and Hep-SSAT-Cko animals (Fig. 12B). At 24 h post-CCl4 treatment few caspase 3 positive cells were detected in the pericentral area, while there were substantial number of capase 3 positive cells and cell clusters in the intermediate and periportal zones in both genotypes (Fig. 12B). Comparison of the two genotypes revealed the WT animals to have higher numbers of cleaved caspase 3 staining cells (Fig. 12B, 24 h WT vs. Hep-SSAT-Cko). At 48 h posttreatment, the numbers of cells staining with anti-cleaved caspase 3 antibody increased substantially in both genotypes in zones 1 through 3, with WT animals still having substantially increased numbers of cleaved caspase 3 staining cells (Fig. 12B, 24 h WT vs. Hep-SSAT-Cko). These results were further confirmed by Western blot analyses comparing the content of uncleaved and cleaved caspase 3 in the hepatic extracts of WT and Hep-SSAT-Cko mice treated with vehicle or CCl4. Our results indicate that the increase in cleaved caspase 3 levels are more substantial in the WT compared with Hep-SSAT-Cko mice at 24, 48, and 72 h post-CCl4 treatment (Fig. 12C).
Fig. 12.
Comparison of Noxa and activated caspase 3 levels in the livers of vehicle and CCl4-treated WT and Hep-SSAT-Cko animals. A: expression of Noxa was assessed by immunofluorescent staining of liver sections from control and CCl4-treated WT and Hep-SSAT-Cko mice (magnification, ×200). B: activated/cleaved caspase 3 levels in the livers of control and CCl4-treated WT and Hep-SSAT-Cko mice were examined by immunofluorescent microscopy (magnification, ×200). C: levels of pro and cleaved caspase 3 were examined in the liver extracts of vehicle and CCl4-treated WT and Hep-SSAT-Cko mice. Equal loading was confirmed by determining the actin levels in the size-fractionated extracts. The blots are representative of 3 independent samples.
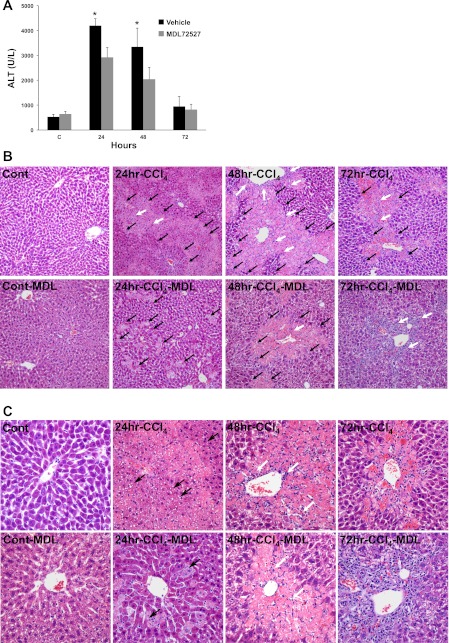
Inhibition of polyamine oxidases (APAO and SMO) reduces the severity of CCl4-induced hepatic injury.
Enhanced polyamine catabolism, in addition to disruption of polyamine homeostasis, leads to production of H2O2 and aldehyde molecules through the activity of polyamine oxidases (APAO and SMO) and can cause oxidative injury. Constitutively expressed peroxisomal enzyme APAO is responsible for oxidation of acetylated polyamines, while SMO, which is induced in coincident with increased expression of SSAT (32), specifically oxidizes Spm. Since hepatic polyamine levels were minimally disturbed, we examined the role of polyamine oxidases, APAO and SMO, in the mediation of hepatic injury. This was accomplished by examining the effect of their inhibition, by MDL72527, on the severity of CCl4-induced hepatic damage. Our results indicate that the serum ALT levels are significantly (P < 0.05) lower in MDL72527-treated animals relative to saline-treated animals subjected to CCl4-induced liver injury (Fig. 13A). Histological examination of the livers revealed that treatment with MDL72527 reduces the severity of hepatic injury as evidenced by reductions in the size of necrotic areas, diminished bridging necrosis between zones 2 and 3 (parenchyma and portal triad) at 24 and 48 h, and lower numbers of inflammatory cell infiltrates in the livers of CCl4/MDL72527-treated Hep-SSAT-Cko compared with CCl4-treated mice (Fig. 13, B and C).
Fig. 13.
Examining the role of polyamine oxidases downstream of SSAT in CCl4-induced liver injury. A: serum ALT levels of control and CCl4-treated mice injected with MDL72527 or vehicle (n = 6/group) were compared following the protocol outlined in the materials and methods. *P < 0.05 is considered significant. Results are means ± SE of 3 independent samples. B: liver histology (magnification, ×200) of control and injured animals from vehicle and MDL72527-treated groups were compared. White arrows, infiltrating cells; black arrows, areas of necrosis. C: liver histology (magnification, ×400) of control and injured animals from vehicle and MDL72527-treated animals were compared. White arrows, infiltrating cells; black arrows, steatotic vesicles.
DISCUSSION
Metabolic activity of the liver is important in detoxification of alcohol and toxic, therapeutic, and carcinogenic agents. Injuries caused by these agents lead to acute hepatitis, cholestasis, and cirrhosis. The mouse model of CCl4-induced hepatic injury has been extensively used to investigate the factors involved in the mediation of hepatotoxicity (16, 23). Previous studies indicate that the expression of SSAT is elevated and treatment with a catabolically stable polyamine mimetic, α-methylspermidine, reduces the severity of CCl4-induced liver injury (13, 21). Studies described here examine the role of hepatocyte-specific expression of SSAT, the rate-limiting enzyme in polyamine back conversion, in the mediation of tissue damage in CCl4-induced liver injury. Examination of liver function and histology at timed intervals (6 to 72 h) after CCl4 administration revealed the presence of elevated serum ALT levels and increased hepatic damage as early as 24 h after CCl4 treatment (data not shown), confirming the onset of acute liver injury. Our results also demonstrated that the expression of SSAT, SMO, and ODC increased in the livers of mice given CCl4 as early as 6 h posttreatment (Fig. 3). The expression levels of these transcripts remained elevated through the duration of the study.
Previous studies comparing the extent of organ injury and dysfunction in WT and SSAT-ko mice indicate that SSAT deficiency reduces the severity of a variety of injuries (35, 37). On the basis of the aforementioned observations, we set out to test the hypothesis that enhanced expression of SSAT by hepatocytes contributes to their damage and liver dysfunction in CCl4-induced injury. This was accomplished by examining the effect of hepatocyte-specific ablation of the SSAT gene on the severity of CCl4-induced liver injury.
Enhanced SSAT mRNA expression and activity in response to CCl4 treatment was observed in WT but not in Hep-SSAT-Cko mice (Fig. 7). The latter results, in addition to the absence of hepatic acetylated polyamines in Hep-SSAT-Cko mice (Fig. 10) and the examination of isolated DNA and SSAT mRNA levels from the livers of WT and Hep-SSAT-Cko animals (Fig. 2), confirm that the SSAT gene is inactive and polyamine back conversion is short circuited in the livers of the Hep-SSAT-Cko animals.
Determination of Spd and Spm levels in the livers of control and injured WT and Hep-SSAT-Cko mice revealed that the levels of Spm remained fairly stable in both WT and SSAT-deficient mice (Fig. 9). On the other hand, Spd levels showed a significant initial (6 h) decrease in the WT but not in Hep-SSAT-Cko mice, which was followed by a build up of this molecule in the late time points of the study in both genotypes. The increase in Put levels was delayed in Hep-SSAT-Cko compared with WT mice (Fig. 9). Furthermore, acetylated polyamine levels were not detected in the livers of CCl4-treated Hep-SSAT-Cko mice (Fig. 10), indicating that the activity of SSAT was absent in the hepatocytes of these animals. The similarities between hepatic Spm and Spd contents in WT and Hep-SSAT-Cko animals in matching time points suggest that reductions in the hepatic levels of these polyamines per se may not play a direct role in the differences observed in the severity of CCl4-induced liver injury in WT and Hep-SSAT-Cko mice. Niiranen et al. (22) have also demonstrated that alterations in the hepatic content of Spm and Spm are similar in CCl4-treated WT and SSAT-ko mice. The latter findings in addition to our results suggest that factors other than the depletion of polyamines, such as enhanced polyamine oxidation and generation of toxic molecules (e.g., H2O2 and aminopropanal) may contribute to the induction of hepatic injury.
Comparison of serum ALT levels, hepatic histology, and the extent of lipid peroxidation in CCl4-treated WT and Hep-SSAT-Cko mice revealed that hepatocyte-specific deficiency of SSAT reduces the severity of CCl4-induced liver injury (Fig. 4). Our results also indicate that the hepatic CYP2e1 levels were nearly identical in the control animals of both genotypes and were not differentially affected in the WT and Hep-SSAT-Cko mice (Fig. 4E), suggesting that adaptive reduction in the metabolism of CCl4 to trichloromethyl radical through downregulation of CYP2e1 does not play a significant role in the modulation of hepatic injury in Hep-SSAT-Cko mice. This observation is supported by previous report that maximal bioactivation and covalent adduct formation by CCl4 occurs with in the first 2 h of its application, which is prior to peak induction of polyamine back conversion or onset of polyamine flux in this model of hepatic injury (28). The latter results suggest that alterations in polyamine catabolism play a role in the mediation of tissue damage subsequent to the primary insult caused by the reactive metabolites of CCl4 bioactivation. We therefore examined the potential role of SSAT in the mediation of tissue damage secondary to the initial insult and independent of CCl4 metabolism. Compared with WT mice, Hep-SSAT-Cko mice had reduced hepatic mRNA and serum levels of MCP-1 and TNF-α, and decreased number of infiltrating leukocytes and diminished oxidative injury (Figs. 4, 6, and 11). The onset of apoptosis in hepatocytes, as determined by the immunofluorescent microscopic assessment of Noxa and activated caspase 3 levels, as well as levels of uncleaved and cleaved caspase 3, revealed that compared with WT mice, the Hep-SSAT-Cko mice had a muted apoptotic response (Fig. 12). The primary mode of cell death in the pericentral zone during the acute phase of CCl4 injury is through necrosis; however, it has been shown that apoptosis (as determined by terminal deoxyneucleotidyl transferase-mediated dUTP nick-end labeling assay) may also play a role in the elimination of damaged hepatocytes (3, 29, 34). We propose that the cells staining positive for cleaved caspase 3 in the intermediate and periportal areas at 24 h and then 48 h are those that are irreversibly damaged and cannot successfully divide. These results suggest that the ablation of the SSAT gene reduces the severity of oxidative injury as well as inflammatory and apoptotic responses secondary to the initial insult. The decrease in the hepatic and serum levels of MCP-1, a CC-chemokine that is involved in the mediation of both CCl4- and alcohol-induced liver injuries (19, 40), may be important in reducing the severity of early CCl4 hepatotoxicity in Hep-SSAT-Cko mice. The reduction in TNF-α expression is significant since it plays an important role in the repair process subsequent to CCl4-induced liver injury (30, 31, 40). The reduction in TNF-α levels in addition to the observation that Hep-SSAT-Cko mice also had lower numbers of proliferating cells (Fig. 4, B and C and Fig. 5) suggests that these animals have a delayed or muted proliferative/regenerative response.
Examination of the effect of SSAT ablation on the expression of ODC and SMO revealed that hepatic ODC and SMO transcripts levels increase in both WT and Hep-SSAT-Cko mice in response to CCl4-induced liver injury (Fig. 6). However, while ODC expression and activity was comparable in WT and Hep-SSAT-Cko animals, the induction levels of SMO are higher in the livers of CCl4-treated WT compared with Hep-SSAT-Cko mice. These results suggest that polyamine flux (i.e., concomitant synthesis and back conversion of polyamines) may not play a prominent role in the SSAT-mediated induction of hepatic damage.
The importance of polyamine oxidases in the mediation of a variety of organ injuries has been documented (8, 9, 27, 35). Previous studies indicate that the induction of SSAT expression in cultured cells is associated with coincidental increases in the expression of ODC and SMO mRNAs (32). The results presented here indicate that the hepatic expression of SMO transcript is lower in CCl4-treated Hep-SSAT-Cko than similarly treated WT mice (Fig. 6). These observations suggest that elevated SSAT levels, through generation of acetylated polyamines that are oxidized by APAO, and coincidental induction of SMO, enhance the oxidative breakdown of polyamines, increase production of cytotoxic molecules (e.g., H2O2 and aldehydes) and lead to tissue injury and organ dysfunction. This is supported by our results that show animals treated with MDL72527, an inhibitor of APAO and SMO, are protected against CCl4-induced liver injury (Fig. 13).
The role of increased SSAT expression and enhanced polyamine catabolism by hepatocytes in the mediation of toxic liver injuries has not been thoroughly examined. The maladaptive role of SSAT in the induction of cellular damage and tissue injury is supported by in vitro and in vivo observations (1, 4, 5, 11, 25, 32, 35–37). In vitro studies indicate that expression of SSAT in cultured cells leads to induction of oxidative stress, DNA damage, cell cycle arrest, and onset of the intrinsic/caspase-dependent apoptosis (7, 36). In vivo examination of the role of SSAT in tissue injury has been performed in both SSAT-transgenic and SSAT-ko mice. Overexpression of SSAT in transgenic mice and rats indicates that these animals develop skin lesions as well as anomalous changes in their reproductive organs, liver, pancreas, and adipose tissue (1, 2, 25, 26). These results are complemented by observations that SSAT deficiency reduces the severity of renal and hepatic ischemia reperfusion and renal endotoxic injuries (35, 37). The preceding results demonstrate the adverse effects of the enhanced SSAT expression and activity on cellular integrity and organ function.
Studies detailed in this manuscript indicate that increased SSAT expression and the attendant increase in polyamine oxidation through generation of toxic by-products such as H2O2 and aminopropanal molecules are important in the mediation of early tissue damage in CCl4-induced liver injury. The time course of SSAT induction, increased polyamine oxidation, lipid peroxidation levels, and the correlation of these with the temporal pattern of hepatic injury and dysfunction suggests that enhanced polyamine back conversion and oxidation are involved in the mediation of early tissue damage in CCl4-induced liver injury. Furthermore, our results indicate that protection afforded through SSAT deficiency is independent of alterations in CCl4-bioactivation by CYP2e1 and primarily due to reduced levels of polyamine oxidation. Interestingly, the reduction in TNF-α levels and decrease in cellular proliferation in CCl4-treated HEP-SSAT-Cko levels suggest that these animals may have a muted hepatic repair response and that chronic inhibition of polyamine back conversion may adversely effect the recovery from CCl4-induced liver injury. In conclusion, these studies suggest that the transient suppression of SSAT activity, inhibition of polyamine oxidation and/or neutralization of toxic products of polyamine catabolism can be effective therapeutic measures for reducing the severity of early tissue damage in toxic liver injuries.
GRANTS
These studies were supported by funds from Center on Genetics of Transport and Epithelial Biology and Dialysis Clinic In (to M. Soleimani) and the National Institute of Health Grants R56-DK-62809 (to M. Soleimani), CA-51085 and CA-98454 (to R. A. Casero Jr), and DK-56029 and AG-25881 (to A. B. Lentsch).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.Z. and M.S. conception and design of research; K.Z., S.L.B., J.X., N.S., and R.S. performed experiments; K.Z., H.A., and R.A.C. analyzed data; K.Z. and J.W. interpreted results of experiments; K.Z. and J.W. prepared figures; K.Z. drafted manuscript; K.Z., S.L.B., A.B.L., H.A., R.A.C., and M.S. edited and revised manuscript; K.Z. and M.S. approved final version of manuscript.
REFERENCES
- 1. Alhonen L, Parkkinen JJ, Keinanen T, Sinervirta R, Herzig KH, Janne J. Activation of polyamine catabolism in transgenic rats induces acute pancreatitis. Proc Natl Acad Sci USA 97: 8290–8295, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alhonen L, Rasanen TL, Sinervirta R, Parkkinen JJ, Korhonen VP, Pietila M, Janne J. Polyamines are required for the initiation of rat liver regeneration. Biochem J 362: 149–153, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bansal MB, Kovalovich K, Gupta R, Li W, Agarwal A, Radbill B, Alvarez CE, Safadi R, Fiel MI, Friedman SL, Taub RA. Interleukin-6 protects hepatocytes from CCl4-mediated necrosis and apoptosis in mice by reducing MMP-2 expression. J Hepatol 42: 548–556, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barone S, Okaya T, Rudich S, Petrovic S, Tenrani K, Wang Z, Zahedi K, Casero RA, Lentsch AB, Soleimani M. Distinct and sequential upregulation of genes regulating cell growth and cell cycle progression during hepatic ischemia-reperfusion injury. Am J Physiol Cell Physiol 289: C826–C835, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Baskaya MK, Rao AM, Dogan A, Donaldson D, Gellin G, Dempsey RJ. Regional brain polyamine levels in permanent focal cerebral ischemia. Brain Res 744: 302–308, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Casero RA, Jr, Marton LJ. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat Rev Drug Discov 6: 373–390, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Chen Y, Kramer DL, Diegelman P, Vujcic S, Porter CW. Apoptotic signaling in polyamine analogue-treated SK-MEL-28 human melanoma cells. Cancer Res 61: 6437–6444, 2001 [PubMed] [Google Scholar]
- 8. Dogan A, Rao AM, Baskaya MK, Hatcher J, Temiz C, Rao VL, Dempsey RJ. Contribution of polyamine oxidase to brain injury after trauma. J Neurosurg 90: 1078–1082, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Dogan A, Rao AM, Hatcher J, Rao VL, Baskaya MK, Dempsey RJ. Effects of MDL 72527, a specific inhibitor of polyamine oxidase, on brain edema, ischemic injury volume, and tissue polyamine levels in rats after temporary middle cerebral artery occlusion. J Neurochem 72: 765–770, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Golab F, Kadkhodaee M, Zahmatkesh M, Hedayati M, Arab H, Schuster R, Zahedi K, Lentsch AB, Soleimani M. Ischemic and non-ischemic acute kidney injury cause hepatic damage. Kidney Int 75: 783–792, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Ha HC, Woster PM, Yager JD, Casero RA., Jr The role of polyamine catabolism in polyamine analogue-induced programmed cell death. Proc Natl Acad Sci USA 94: 11557–11562, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hasan R, Alam MK, Ali R. Polyamine induced Z-conformation of native calf thymus DNA. FEBS Lett 368: 27–30, 1995 [DOI] [PubMed] [Google Scholar]
- 13. Hyvonen MT, Sinervirta R, Grigorenko N, Khomutov AR, Vepsalainen J, Keinanen TA, Alhonen L. α-Methylspermidine protects against carbon tetrachloride-induced hepatic and pancreatic damage. Amino Acids 38: 575–581, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Igarashi K, Kashiwagi K. Polyamines: mysterious modulators of cellular functions. Biochem Biophys Res Commun 271: 559–564, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Janne J, Alhonen L, Leinonen P. Polyamines: from molecular biology to clinical applications. Ann Med 23: 241–259, 1991 [DOI] [PubMed] [Google Scholar]
- 16. Kovalovich K, DeAngelis RA, Li W, Furth EE, Ciliberto G, Taub R. Increased toxin-induced liver injury and fibrosis in interleukin-6-deficient mice. Hepatology 31: 149–159, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Kramer D, Stanek J, Diegelman P, Regenass U, Schneider P, Porter CW. Use of 4-fluoro-l-ornithine to monitor metabolic flux through the polyamine biosynthetic pathway. Biochem Pharmacol 50: 1433–1443, 1995 [DOI] [PubMed] [Google Scholar]
- 18. Kramer DL, Diegelman P, Jell J, Vujcic S, Merali S, Porter CW. Polyamine acetylation modulates polyamine metabolic flux, a prelude to broader metabolic consequences. J Biol Chem 283: 4241–4251, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Mandrekar P, Ambade A, Lim A, Szabo G, Catalano D. An essential role for monocyte chemoattractant protein-1 in alcoholic liver injury: regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology 54: 2185–2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marton LJ, Pegg AE. Polyamines as targets for therapeutic intervention. Annu Rev Pharmacol Toxicol 35: 55–91, 1995 [DOI] [PubMed] [Google Scholar]
- 21. Matsui I, Wiegand L, Pegg AE. Properties of spermidine N-acetyltransferase from livers of rats treated with carbon tetrachloride and its role in the conversion of spermidine into putrescine. J Biol Chem 256: 2454–2459, 1981 [PubMed] [Google Scholar]
- 22. Niiranen K, Keinanen TA, Pirinen E, Heikkinen S, Tusa M, Fatrai S, Suppola S, Pietila M, Uimari A, Laakso M, Alhonen L, Janne J. Mice with targeted disruption of spermidine/spermine N1-acetyltransferase gene maintain nearly normal tissue polyamine homeostasis but show signs of insulin resistance upon aging. J Cell Mol Med 10: 933–945, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Otsuka T, Takagi H, Horiguchi N, Toyoda M, Sato K, Takayama H, Mori M. CCl4-induced acute liver injury in mice is inhibited by hepatocyte growth factor overexpression but stimulated by NK2 overexpression. FEBS Lett 532: 391–395, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Pietila M, Alhonen L, Halmekyto M, Kanter P, Janne J, Porter CW. Activation of polyamine catabolism profoundly alters tissue polyamine pools and affects hair growth and female fertility in transgenic mice overexpressing spermidine/spermine N1-acetyltransferase. J Biol Chem 272: 18746–18751, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Pietila M, Parkkinen JJ, Alhonen L, Janne J. Relation of skin polyamines to the hairless phenotype in transgenic mice overexpressing spermidine/spermine N-acetyltransferase. J Invest Dermatol 116: 801–805, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Pirinen E, Kuulasmaa T, Pietila M, Heikkinen S, Tusa M, Itkonen P, Boman S, Skommer J, Virkamaki A, Hohtola E, Kettunen M, Fatrai S, Kansanen E, Koota S, Niiranen K, Parkkinen J, Levonen AL, Yla-Herttuala S, Hiltunen JK, Alhonen L, Smith U, Janne J, Laakso M. Enhanced polyamine catabolism alters homeostatic control of white adipose tissue mass, energy expenditure, and glucose metabolism. Mol Cell Biol 27: 4953–4967, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pledgie A, Huang Y, Hacker A, Zhang Z, Woster PM, Davidson NE, Casero RA., Jr Spermine oxidase SMO(PAOh1), Not N1-acetylpolyamine oxidase PAO, is the primary source of cytotoxic H2O2 in polyamine analogue-treated human breast cancer cell lines. J Biol Chem 280: 39843–39851, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Sawant SP, Dnyanmote AV, Shankar K, Limaye PB, Latendresse JR, Mehendale HM. Potentiation of carbon tetrachloride hepatotoxicity and lethality in type 2 diabetic rats. J Pharmacol Exp Ther 308: 694–704, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Serbetci K, Uysal O, Erkasap N, Koken T, Baydemir C, Erkasap S. Anti-apoptotic and antioxidant effect of leptin on CCl4-induced acute liver injury in rats. Mol Biol Rep 39: 1173–1180 [DOI] [PubMed] [Google Scholar]
- 30. Shibata H, Yoshioka Y, Ohkawa A, Abe Y, Nomura T, Mukai Y, Nakagawa S, Taniai M, Ohta T, Mayumi T, Kamada H, Tsunoda S, Tsutsumi Y. The therapeutic effect of TNFR1-selective antagonistic mutant TNF-α in murine hepatitis models. Cytokine 44: 229–233, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Sudo K, Yamada Y, Moriwaki H, Saito K, Seishima M. Lack of tumor necrosis factor receptor type 1 inhibits liver fibrosis induced by carbon tetrachloride in mice. Cytokine 29: 236–244, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Wang Z, Zahedi K, Barone S, Tehrani K, Rabb H, Matlin K, Casero RA, Soleimani M. Overexpression of SSAT in kidney cells recapitulates various phenotypic aspects of kidney ischemia-reperfusion injury. J Am Soc Nephrol 15: 1844–1852, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol 33: 105–136, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Wu Y, Li L, Wen T, Li YQ. Protective effects of echinacoside on carbon tetrachloride-induced hepatotoxicity in rats. Toxicology 232: 50–56, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Zahedi K, Barone SL, Kramer DL, Amlal H, Alhonen L, Janne J, Porter CW, Soleimani M. The Role of spermidine/spermine-N1-acetyltransferase in endotoxin-induced acute kidney injury. Am J Physiol Cell Physiol 299: C164–C174, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zahedi K, Bissler JJ, Wang Z, Josyula A, Lu L, Diegelman P, Kisiel N, Porter CW, Soleimani M. Spermidine/spermine N1-acetyltransferase overexpression in kidney epithelial cells disrupts polyamine homeostasis, leads to DNA damage, and causes G2 arrest. Am J Physiol Cell Physiol 292: C1204–C1215, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Zahedi K, Lentsch AB, Okaya T, Barone S, Sakai N, Witte DP, Arend LJ, Alhonen L, Jell J, Janne J, Porter CW, Soleimani M. Spermidine/spermine-N1-acetyltransferase ablation protects against liver and kidney ischemia-reperfusion injury in mice. Am J Physiol Gastrointest Liver Physiol 296: G899–G909, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zahedi K, Revelo MP, Barone S, Wang Z, Tehrani K, Citron DP, Bissler JJ, Rabb H, Soleimani M. Stathmin-deficient mice develop fibrosis and show delayed recovery from ischemic-reperfusion injury. Am J Physiol Renal Physiol 290: F1559–F1567, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Zahedi K, Wang Z, Barone S, Prada AE, Kelly CN, Casero RA, Yokota N, Porter CW, Rabb H, Soleimani M. Expression of SSAT, a novel biomarker of tubular cell damage, increases in kidney ischemia-reperfusion injury. Am J Physiol Renal Physiol 284: F1046–F1055, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Zamara E, Galastri S, Aleffi S, Petrai I, Aragno M, Mastrocola R, Novo E, Bertolani C, Milani S, Vizzutti F, Vercelli A, Pinzani M, Laffi G, LaVilla G, Parola M, Marra F. Prevention of severe toxic liver injury and oxidative stress in MCP-1-deficient mice. J Hepatol 46: 230–238, 2007 [DOI] [PubMed] [Google Scholar]