Abstract
Objective
To evaluate the test-retest reliability of event-related power changes in the 30–150 Hz gamma frequency range occurring in the first 150 ms after presentation of an auditory stimulus.
Methods
Repeat intracranial electrocorticographic (ECoG) recordings were performed with 12 epilepsy patients, at ≥ 1-day intervals, using a passive odd-ball paradigm with steady-state tones. Time-frequency matching pursuit analysis was used to quantify changes in gamma-band power relative to pre-stimulus baseline. Test-retest reliability was estimated based on within-subject comparisons (paired t-test, McNemar’s test) and correlations (Spearman rank correlations, intra-class correlations) across sessions, adjusting for within-session variability. Reliability estimates of gamma-band response robustness, spatial concordance, and reproducibility were compared with corresponding measurements from concurrent auditory evoked N1 responses.
Results
All patients showed increases in gamma-band power, 50–120 ms post-stimulus onset, that were highly robust across recordings, comparable to the evoked N1 responses. Gamma-band responses occurred regardless of patients’ performance on behavioral tests of auditory processing, medication changes, seizure focus, or duration of test-retest interval. Test-retest reproducibility was greatest for the timing of peak power changes in the high-gamma range (65–150 Hz). Reliability of low-gamma responses and evoked N1 responses improved at higher signal-to-noise levels.
Conclusions
Early cortical auditory gamma-band responses are robust, spatially concordant, and reproducible over time.
Significance
These test-retest ECoG results confirm the reliability of auditory gamma-band responses, supporting their utility as objective measures of cortical processing in clinical and research studies.
Keywords: Auditory Cortex, Gamma-Band, Reliability, Auditory Processing, Variability
1. Introduction
Electrocorticographic (ECoG) recordings from human auditory cortex have shown event-related power changes in the 30–150 Hz gamma-band range within the first 150 ms after stimulus presentation (Howard et al., 2000; Crone et al., 2001; Edwards et al., 2005; Canolty et al., 2007; Sinai et al., 2009). Early gamma-band responses are elicited with tones or other auditory stimuli and can last up to several hundred milliseconds (Edwards et al., 2005; Towle et al., 2008; Steinschneider et al., 2011). The spectral composition of these responses has been characterized as predominantly non-phase-locked, high-gamma (> 60 Hz) activity (Crone et al., 2001; Edwards et al., 2005; Lachaux et al., 2007). Early gamma-band power changes are thought to reflect activation of local cortical processing networks involved in perception, based on evidence from scalp electroencephalographic (EEG) recordings (Tallon-Baudry and Bertrand, 1999; Gurtubay et al., 2001; Herrmann et al., 2004) and intracranial ECoG recordings (Crone et al., 2001; Fries, 2009; Jerbi et al., 2009). Although auditory gamma-band responses are used increasingly as indices of cortical processing, the reliability of these responses has not been investigated directly.
ECoG recordings are performed for surgical planning in patients with medically refractory epilepsy. In addition to recording ongoing ECoG activity for seizure localization, event-related ECoG studies are often performed intra-operatively or extra-operatively over multiple days. The reliability of event-related ECoG responses has not been determined and could be affected by a number of factors, including the presence of long-term seizure activity as well as short-term changes in medication and seizures. Moreover, patients with epilepsy may experience auditory processing difficulties (Henkin et al., 2003; Boatman et al., 2006; Han et al., 2011), including impaired speech recognition under adverse listening conditions such as background noise, that could contribute to increased response variability. Because attention is also frequently impaired in patients with chronic seizure disorders (Dunn et al., 2003; Reilly, 2011), the use of attention-dependent auditory recording paradigms may introduce additional variability. Inter-subject variability is frequently observed in intracranial recording studies and has been attributed, in part, to differences in electrode locations between patients (Jerbi et al., 2009). Because test-retest recordings are rare in intracranial ECoG studies, it is not known whether intrinsic response variability may be a contributing factor. Determining the reliability of event-related ECoG recordings is important for accurate interpretation of electrophysiological responses acquired from single or multi-session recordings.
Test-retest studies of evoked responses based on scalp recordings have demonstrated that the auditory N1 response, which occurs in the same time period as the early gamma-band response, is highly robust and reliable (Näätänen and Picton, 1987; Virtanen et al., 1998; Tremblay et al., 2003). Until recently (Lenz et al., 2008), methodological limitations associated with scalp recordings made it difficult to simultaneously record high-frequency responses (Nunez and Srinivasan, 2006). Although ECoG studies have focused mainly on high-gamma responses, increased low-gamma activity (30–60 Hz) has also been identified in early auditory gamma-band responses (Edwards et al., 2005). Previous studies have suggested that low-gamma and high-gamma activity are functionally distinct (Crone et al., 1998; Edwards et al., 2005; Ray et al., 2008; Crone et al., 2011) raising the possibility that they could also differ in relative robustness and reliability. These issues underscore the need for test-retest ECoG studies to determine the reliability of early auditory gamma-band responses.
In this study, we used repeat ECoG recordings to investigate the test-retest reliability of early auditory gamma-band responses. We investigated three aspects of test-retest response reliability based on previous brain mapping studies: robustness, concordance, and reproducibility. Response robustness refers to how stable a response is over time and is coded as a binary variable (± response), providing a basic measure of test-retest reliability (Boutros et al., 1991; Kiehl and Liddle, 2003; Tremblay et al., 2003). Concordance is defined here as a measure of agreement (overlap) in the topography, or spatial distributions (number, location), of test-retest responses (Gasser et al., 1995; Kiehl and Liddle, 2003; Aron et al., 2006; Tervaniemi et al., 2005). Reproducibility is a measure of how replicable the response patterns are in time and frequency (Tervaniemi et al., 1999; Kiehl and Liddle, 2003; Tremblay et al., 2003).
Test-retest reliability measurements for the early cortical auditory gamma-band responses were also compared with reliability estimates from concurrent auditory evoked N1 responses. Participants were screened for auditory processing disorders, and a passive auditory odd-ball paradigm with simple tones was used to reduce attention effects. Separate analyses were performed to investigate the reliability of high-gamma versus low-gamma activity and to identify potential effects of medication changes, seizure focus, and test-retest interval on response reliability.
2. Methods
2.1 Participants
Participants were 12 consecutive patients (10 female), ages 13–50 years (mean 29 ±13.75), who had subdural electrodes implanted over the left (8) or right (4) lateral temporal lobe to assess their candidacy for surgical treatment of medically refractory epilepsy (Table 1). Patients were admitted to the Johns Hopkins Epilepsy Monitoring Unit over an 18-month period (2008–2010). Mean age of seizure onset was 14.6 years (±12.76). Eleven patients had 8×8 or 6×8 electrode arrays implanted with additional coverage of the frontal lobe and inferior parietal cortex; one patient (Patient 4) had multiple 2×6 electrode strips implanted over the right temporal lobe. Eight patients had normal MRI scans; two had small focal cortical dysplasias; one had left mesial temporal sclerosis; and one had a left temporal lobe glioma. Eleven patients had normal hearing bilaterally (≤25 dB, 0.5–4 kHz), as confirmed by audiometric screening; one patient (Patient 2) had a high-frequency (> 3 kHz) sensorineural (moderate) hearing loss. All had normal cognitive function (IQ scores >75). Eleven patients were left-hemisphere dominant for language by intracarotid amobarbital injection (Wada test); one patient (Patient 12) had bilateral language representation. Intracranial EEG recordings localized seizures to the anterior temporal lobe (6 patients), frontal lobe (4 patients), and parietal lobe (2 patients). All patients gave informed written consent for participation in the study in compliance with Institutional Review Board requirements.
Table 1.
Patient Demographics
| Patient | Sex | Age (yrs) | Handedness | Seizure onset Age (yrs) | Hemisphere implanted | MRI | Seizure Focus |
|---|---|---|---|---|---|---|---|
| 1 | F | 18 | Right | 13 | Right | Normal | Right TL |
| 2 | M | 47 | Right | 8 | Left | MTS | Left MTL |
| 3 | F | 15 | Right | 5 | Right | CD | Right FL |
| 4 | F | 22 | Left | 14 | Right | Normal | Right TL |
| 5 | F | 28 | Right | 6 | Left | Normal | Left FL |
| 6 | F | 50 | Right | 47 | Left | Tumor | Left TL |
| 7 | M | 13 | Right | 7 | Left | Normal | Left FL |
| 8 | F | 36 | Right | 20 | Left | Normal | Left TL |
| 9 | F | 50 | Right | 30 | Left | Normal | Left MTL |
| 10 | F | 15 | Right | 10 | Right | CD | Right PL |
| 11 | F | 28 | Right | 15 | Left | Normal | Left PO |
| 12 | F | 27 | Right | 1 | Left | Normal | Left FL |
Abbreviations. TL: temporal lobe; MTL: mesial temporal lobe; CD: cortical dysplasia; FL: frontal lobe; PL: parietal lobe; PO: parietal-occipital region; MTS: mesial temporal sclerosis.
2.2 Procedures
2.2.1. Electrode implantation
Patients underwent craniotomy for placement of subdural electrodes: platinum-iridium disks of 2.3-mm exposed diameter, embedded 10 mm apart (center-to-center), in medical grade silastic arrays (Ad-Tech, Racine, Wisconsin). The placement of the implanted electrode arrays was determined for each patient based on clinical considerations. Electrode locations were determined by co-registration of each patient’s post-implantation CT scan and pre-surgical volumetric MRI and by intraoperative photographs, as previously described (Sinai et al., 2009; Boatman-Reich et al., 2010). Subdural electrodes remained indwelling for an average of six days (range 5–9), during which time intracranial EEG was recorded continuously.
2.2.2. Stimuli and auditory recording paradigm
Auditory stimuli were two steady-state tones of 1000 Hz and 1200 Hz (NCH Tone Waveform Generator, NCH Software), 50-ms duration (5-ms rise/fall times). Both tone frequencies are well above the upper limit of the gamma-band frequency range analyzed (150 Hz), consistent with other auditory ECoG studies (Edwards et al., 2005; Brugge et al., 2009; Sinai et al., 2009; Todorovic et al., 2011). All 12 patients had normal hearing thresholds at both tone frequencies and readily discriminated the 200-Hz frequency difference that was well above the lower limit of the human frequency discrimination range (~ 20 Hz).
Single-frequency, steady-state tones were selected over more complex, time-varying auditory stimuli, such as speech, to avoid potential effects of stimulus-related variability on spectral responses. The tones were presented sequentially, at 1400-ms inter-stimulus intervals (onset to onset), in a 300-trial passive auditory odd-ball paradigm. In an odd-ball paradigm, one stimulus is repeated consecutively in a series and termed the ‘frequent’ (or standard) stimulus, while a second stimulus is inserted infrequently and pseudo-randomly into the series of frequent stimuli and is termed the ‘infrequent’ (or deviant) stimulus. The auditory odd-ball paradigm is well established and has been used in ECoG and scalp recording studies to investigate a variety of auditory functions, including perception of novel stimuli and/or changes in stimulus features, attention, and memory (Escara et al., 1998; Gurtubay et al., 2001; Henkin et al., 2003; Edwards et al., 2009; Sinai et al., 2009). In this study, the frequent stimulus was a 1000-Hz tone (82% trials), and the infrequent stimulus was a 1200-Hz tone presented pseudo-randomly (i.e., nonconsecutively). Stimuli were presented binaurally through insert earphones (Etymotic ER2) at a comfortable listening level (approximately 65 dB nHL).
The timing and order of stimulus presentation were controlled using the GENTASK function of the Neuroscan STIM 2 system (Compumedics, Inc., El Paso, Texas). Patients were instructed to ignore the auditory stimuli and watch a silent animated DVD movie on a small (3×5 inch) screen to minimize eye movement; the screen was located approximately one foot in front of the patient on a stationary tray. Passive listening paradigms are used frequently to study auditory-related gamma-band activity and are useful for reducing attention to auditory stimuli by not requiring a behavioral response and/or through use of visual distractors (Kaiser et al., 2002; Edwards et al., 2005; Brugge et al., 2009; Sinai et al., 2009; Steinschneider et al., 2011).
2.2.3. Intracranial recordings
Recordings were initiated 3–4 days after electrode implantation while patients were awake and fully responsive. Patients were tested individually at the bedside in one of two identically-configured, single-occupant rooms in the Epilepsy Monitoring Unit. All patients completed a minimum of two recording sessions on different days: an initial recording and one or more repeat (retest) recordings (range 1–3 recordings/patient). Six patients also completed retest recordings in the same session to assess intra-session reliability. To avoid contaminating the recording signal with EEG artifact, recordings were not obtained earlier than six hours after a seizure or following clinical electrocortical stimulation mapping studies.
The continuous EEG signal was amplified (5×1000) and recorded digitally (Stellate Systems, Inc., Montreal, Canada) from all subdural electrodes using a referential montage, 1000-Hz A/D sampling, and a bandwidth of 0.1–350 Hz (6 dB/octave), as previously described (Crone et al., 2001; Sinai et al., 2009; Boatman-Reich et al., 2010; Cervenka et al., 2011a). The intracranial reference electrodes were located at the corners of the arrays, distal to the perisylvian region, to reduce their potential contributions to the recordings (for discussion, see Boatman-Reich et al., 2010). Stimulus onset markers were recorded simultaneously to separate EEG marker channels for infrequent and frequent tones.
2.2.4. Behavioral testing
Standardized clinical tests of auditory processing were administered to assess speech recognition in quiet (CID-W22 word lists) and under adverse listening conditions, including background noise (SCAN-A test; Keith, 1994). Tests were played from a compact disk through a two-channel audiometer (50 dB HL). Four subtests of the SCAN-A were administered: 1) low-pass filtered words (1.0 kHz cut-off); 2) words embedded in multi-speaker babble noise (+8 dB S/N); 3) dichotic words; and 4) dichotic phrases. The SCAN-A test was not administered to two patients who did not meet test criteria of normal hearing bilaterally (Patient 2) and native speaker of English (Patient 9), respectively.
2.3. Data analysis
For each patient, we analyzed two recordings from separate days and selected the first and last recordings for each patient to maximize the duration of the test-retest interval. Repeat recordings within the same session were analyzed separately to measure intra-session variability. The total number of test-retest recordings, maximum test-retest interval, repeat intra-session recordings, and changes in clinical status (e.g., medication) are listed for each patient in Table 2.
Table 2.
Test-Retest Recordings, Inter-Session Intervals, and Changes in Medication by Patient
| Patient | Total # Recordings | Maximum Test-Retest Interval (hrs) | Repeat Intra-Session Recording | Change in Medication Status |
|---|---|---|---|---|
| 1 | 3 | 32 | YES | YES |
| 2 | 5 | 71 | NO | YES |
| 3 | 3 | 25 | YES | NO |
| 4 | 3 | 46 | YES | NO |
| 5 | 2 | 41 | NO | YES |
| 6 | 3 | 53 | YES | NO |
| 7 | 2 | 24 | NO | NO |
| 8 | 3 | 51 | YES | NO |
| 9 | 2 | 46 | NO | NO |
| 10 | 2 | 24 | NO | YES |
| 11 | 2 | 44 | NO | NO |
| 12 | 3 | 47 | YES | NO |
2.3.1. Signal pre-processing
The continuous ECoG recordings were pre-processed, to exclude electrode channels and trials with artifact from analysis, and to remontage the recording data. In order to exclude channels with excessive artifact and/or epileptiform activity, the continuous recordings for each patient and session were inspected visually by an epileptologist (MCC). For each patient, only channels included in both the test and retest recording montages were analyzed. Recordings from each session were remontaged to a common average reference to control for differences in the distances between recording (i.e., active) electrodes and the reference electrode (Crone et al., 2001; Sinai et al., 2005; Boatman-Reich et al., 2010). For each channel, the continuous ECoG signal was segmented into 300 trials using a 400-ms pre-stimulus to 1000-ms post-stimulus window to analyze changes in gamma power and a 100-ms pre-stimulus to 300-ms post-stimulus window to analyze evoked N1 response. The first three trials of each run were excluded from analysis. Individual trials with excessive physiological or non-physiological artifact or spiking were excluded (< 2% trials). This conservative approach ensured that single trials with abnormally large physiological or non-physiological spectral activity (> 3 SD) did not contribute disproportionately to the spectral responses (Edwards et al., 2005). Event-related responses to infrequent (1200 Hz; N=54) and frequent (1000 Hz; N=192) tones were analyzed separately. The first trial in every series of frequent-tone trials was excluded because it was preceded by an infrequent-tone trial (Sams et al., 1985; Escera et al., 2002).
2.3.2. Gamma-band responses
Gamma-band responses were defined as statistically significant increases (p<0.05) in spectral power at 30–150 Hz, relative to baseline (pre-stimulus), occurring within 150 ms after stimulus onset. This time interval was selected based on previous auditory ECoG recording studies (Crone et al., 2001; Edwards et al., 2005; Brugge et al., 2009; Sinai et al., 2009) and allowed for direct comparison with the auditory evoked N1 response occurring in the same time interval. Only responses that were largely contained (≥75% total duration) within the first 150 ms were included. To compute gamma-band responses, we used a time-frequency matching pursuit (MP) algorithm (Mallat and Zhang, 1993; Franaszczuk et al., 1998) implemented in C under Linux. The MP algorithm combines the advantages of other time-frequency analysis methods, including short-term Fourier transform and wavelet transforms, with improved time-frequency resolution, and it is well suited for analyzing brief, non-stationary changes in ECoG signals characteristic of cortical event-related responses (Ray et al., 2008; Boatman-Reich et al., 2010; Zhang et al., 2010). MP decomposes the signal into a linear combination of Gabor functions called ‘atoms’ (Mallat and Zhang, 1993). The time-frequency power spectrum was computed by taking the Wigner-Ville distribution of the Gabor atoms. Line noise around 60 Hz (60–64 Hz) and the 120-Hz and 180-Hz harmonics were excluded from the power computations. Baseline power at each frequency was computed by averaging over all baseline time points.
To measure the statistical significance of gamma-band responses, the power at each post-stimulus time-frequency point was compared to the respective frequency-specific power at baseline by paired t-test using log transformation and assuming unequal variances (Zygierewicz et al., 2005). The resulting values were plotted to show the magnitude and statistical significance of power changes over time. Time-frequency points (pixels) representing statistically significant changes from baseline were plotted for the gamma frequency range (30–150 Hz) between 0–150 ms post-stimulus. To control for multiple within-subject comparisons that increase the likelihood of a type I error, a false discovery rate correction was applied (Benjamini and Hochberg, 1995; Boatman-Reich et al., 2010). Band pass filtering of the time-frequency plots was used to evaluate low gamma (30–59 Hz) and high gamma (65–150 Hz) separately.
2.3.3. Test-retest reliability of gamma-band responses: robustness, concordance, reproducibility
We evaluated three aspects of test-retest reliability: 1) robustness of gamma-band responses across patients and sessions (e.g., ± response); 2) concordance in the number of gamma-band responses and the location of the largest responses within patients and across sessions; and 3) reproducibility of gamma-band time-frequency response patterns within patients and across sessions (e.g., latency, size of power changes). All test-retest reliability analyses were based on two recordings from two separate days for each patient. A subset of six patients also underwent repeat recordings within the same day (session) which were used to estimate intra-session variability.
The robustness of gamma responses refers to how consistently responses were elicited across patients and sessions, and was defined as the proportion of patients who showed statistically significant increases in gamma power during both test and retest recordings. The spatial concordance of gamma responses was determined for each patient by: 1) comparing the total number of gamma responses elicited in each session using two-sided McNemar’s test for paired samples (p<0.05); and 2) comparing the locations of the largest gamma-band responses in each session to measure spatial agreement (overlap). Channels with the largest gamma responses were identified independently for each patient and session by two experienced raters (MCC, DBR). Inter-rater reliability was measured using a kappa statistic, with very good reliability defined as a k value ≥0.81 (Landis and Koch, 1977).
There are currently no standard methods for evaluating reproducibility of spectral responses in both frequency and time (e.g., response morphology). For our analysis, we derived two spectral-temporal response measurements for comparison across sessions: the average estimated event-related change in the log-transformed power density (logpower density) for 30–150 Hz; and the latency of the maximum change in logpower density. Changes in logpower density refer to differences in mean log-transformed gamma power between the pre-stimulus and post-stimulus intervals. The logpower density was calculated for each time point in the pre-stimulus and post-stimulus periods by averaging the log-transformed power values in the gamma band (30–150 Hz) across trials. The mean value of the pre-stimulus logpower density was determined by averaging over all time points in the pre-stimulus period. This mean pre-stimulus value was then subtracted from each time point in the post-stimulus period; the average of the differences yielded the estimated change in logpower density of the response (dB/Hz). The latency of the maximum change in gamma logpower density was measured on the time-frequency power spectra.
We used two correlation tests to assess response reproducibility across sessions: Spearman rank correlations and intra-class correlations, both of which have been used previously to assess test-retest response reproducibility (Segalowitz and Barnes, 1993; Gasser et al., 1995; Tremblay et al., 2003). Spearman (rank) correlation coefficients were computed to measure dependence (correlation) between repeat logpower density estimates and between repeat logpower latency measurements. The rank correlation was used to investigate potentially non-linear associations and to evaluate exact (rank-based) tests without appealing to assumptions of normal or asymptotic distributions. Intra-class correlation coefficients were also used to assess response reproducibility. Intra-class correlations take into account all of the available data using linear mixed effects models fit, adjusting for within-session variability, to compare within-patient variance with overall inter-subject variance as in previous auditory electrophysiology studies (Segalowitz and Barnes, 1993; Tremblay et al., 2003). To avoid introducing differences in electrode location as a potential confound in our estimate of test-retest response reproducibility, we included only those patients who had the largest responses at the same site in both sessions.
Separate analyses were performed to investigate the robustness of high-gamma- and low-gamma-band activity using the same methods applied to the whole gamma-band response. To investigate potential sources of test-retest variability, linear mixed effect models were fit for patient demographics (e.g., age, gender), hemisphere of implantation, seizure focus, changes in medication, and test-retest interval.
2.3.4. Auditory N1 evoked response
To derive the evoked N1 response, the ECoG time-series from each channel was averaged across trials in the time domain. Infrequent- and frequent-tone trials were averaged separately. For each patient and session, we analyzed the same number and set of channels and trials used in the gamma-band response analyses. The N1 response was identified on the averaged waveform for each channel as the largest negative deflection between 50–150 ms post-stimulus. Only negativities that were at least two standard deviations greater than the mean baseline amplitude in the same channel were included in the analysis for comparability with gamma-band responses. The robustness of the N1 response was evaluated as the proportion of patients who had N1 responses in both testing sessions. Spatial concordance of the N1 response was determined for each patient by comparing the number of channels with N1 responses and the location of the largest N1 response across sessions using a two-sided McNemar’s test for paired samples (p<0.05), with a continuity correction for small sample sizes. Channels with the largest N1 responses were identified independently by two experienced raters (PB, DBR) and inter-rater reliability was measured using the kappa statistic. We evaluated the reproducibility of peak N1 latencies and amplitudes across sessions using the same statistical tests applied to the gamma-band responses: Spearman (rank) correlation coefficients and intra-class correlation coefficients.
2.3.5. Behavioral testing
Patients’ performances on the five behavioral clinical measures of auditory processing were scored using published norms. Auditory processing was considered impaired if patients performed more than two standard deviations below the mean on two or more tests or subtests.
3. Results
ECoG recordings were acquired from a total of 722 electrodes across all 12 patients. Of these, 646 electrodes were analyzed (mean 53.83 electrodes/patient; range 24–70). Each patient completed at least two separate recording sessions on different days. The average number of recording sessions per patient was 2.75 (range 2–5 recordings/patient). For each patient, two recordings from different days were analyzed, using a matched set of electrodes, yielding a total of 24 recordings. The average test-retest interval was 42 hours (range 24–71 hours). The mean number of infrequent-tone trials analyzed for Session 1 was 42.41 (±2.39; range 40–47) and for Session 2 was 43.1 (±2.43; range: 38–47), with no differences in the total number of trials between sessions (p=0.50, paired t-test). The mean number of frequent-tone trials analyzed for Session 1 was 150.5 (±8.61; range 130–162) and for Session 2 was 152.75 (±15.05; range: 123–178), with no differences in the total number of trials between sessions (p=0.66, paired t-test). No patient had a clinical or electrographic seizure during, or less than six hours prior to, recording.
3.1. Gamma-band responses
All 12 patients showed statistically significant (p<0.05) increases in gamma-band (65–150 Hz) power in response to infrequent (1200 Hz; Figure 1) and frequent (1000 Hz; Figure 2) tones. Gamma-band power increases were evident for all patients between 50–120 ms after stimulus presentation and localized predominately to perisylvian cortex in both the right (4 patients) and left (8 patients) hemispheres, including the posterior temporal lobe, inferior parietal cortex, and inferior pre-central gyrus. Visual inspection of individual time-frequency plots showed considerable variability between patients in the onset, duration, and shape of gamma-band responses (Figures 1, 2). Seven patients showed large broadband responses (30–150 Hz) to both tones; five had transient narrow-band responses around 100 Hz; four had sustained responses (>50 ms duration); and two showed increased gamma-band activity earlier than 50 ms post-stimulus onset.
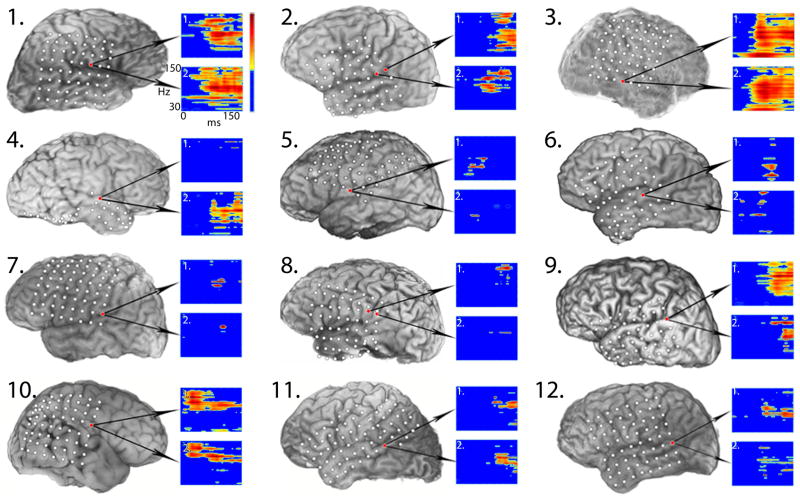
Figure 1.
Test-retest results for 12 patients showing largest gamma-band responses to the 1200-Hz infrequent tone for both sessions. Corresponding patient numbers are listed in the upper left corner. Time-frequency plots of gamma-band responses for each session are in inset boxes labeled 1 and 2 with arrows pointing from the corresponding electrode (depicted by red circles) on a left or right lateral view of the volumetric 3D MRI reconstruction of each patient’s brain co-registered with electrode locations (depicted with white circles). Electrodes covering basal temporal cortex are not shown. Plots of the time-frequency spectra are shown with time on the X-axis from 0–180 ms and frequency on the Y-axis from 30–150 Hz (gamma band). In the color spectrum, dark blue represents no significant change in logpower from the pre-stimulus baseline; all other colors represent significant changes, with red representing the maximum change (dB/Hz). Note: All patients showed statistically significant increases in gamma-band power (p <0.05), although smaller changes may not be clearly visible in the individual plots.
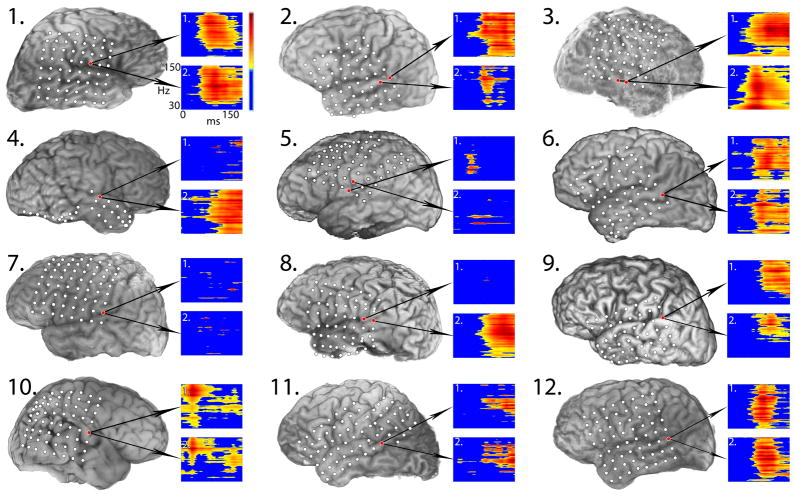
Figure 2.
Test-retest results for 12 patients showing largest gamma-band responses to the 1000-Hz frequent tone across both recording sessions. Figure layout and color coding is identical to Figure 1. Note: All patients showed statistically significant increases in gamma-band power (p <0.05), although smaller changes may not be as clearly visible in the individual plots.
3.1.1. Intra-session variability
Six patients completed repeat recordings in either the first session (5 patients) or second session (1 patient). One patient was excluded from analysis due to a transient disconnection of the ground electrode that resulted in excessive noise in all channels. Of the five remaining patients, all showed gamma-band responses and N1 responses, for both infrequent and frequent tones, at one or more channels for both intra-session recordings. The location of the largest gamma-band and N1 responses to both tones did not vary within a session.
Intra-session measures of gamma-band response reproducibility showed no obvious differences. For infrequent tones, mean logpower density estimates for the two intra-session recordings were 1.34 dB/Hz (±1.57) and 1.72 dB/Hz (±1.36), respectively; the mean peak latencies of the logpower changes were 113.85 ms (±20.26) and 120.71 ms (±21.11). For frequent tones, mean logpower densities for the two intra-session recordings were 1.05 dB/Hz (±0.89) and 0.67 dB/Hz (±0.76), respectively; mean peak latencies of the logpower changes were 112.54 ms (±20.95) and 97.85 ms (±8.81). Intra-session measures of N1 peak latencies and amplitude also did not appear to differ for either tone. For infrequent tones, the mean peak N1 latencies were 99.90 ms (±16.21) and 90.06 ms (±15.86) and the peak amplitudes were −55.37 dB (±44.39) and −47.03 dB (±31.95). For frequent tones, the mean peak N1 latencies for the intra-session recordings were 96.08 ms (±15.34) and 95.52 ms (±15.19) and the peak amplitudes were −52.43 dB (±17.99) and −39.24 dB (±23.30). Visual inspection of the data showed a within-patient trend towards decreased N1 amplitudes and latencies from the first to the second intra-session recording for both tones that was not evident for the gamma response logpower density or latency measurements.
3.1.2. Test-retest gamma response robustness
All 12 patients showed significant increases in gamma power for both infrequent and frequent tones in both sessions (p<0.05, in all cases). To test the robustness of the gamma-band responses and reduce the possibility of a type I error, we re-analyzed responses to infrequent tones from the first recording session for all 12 patients using a more conservative statistical threshold level (p<0.01) and the more conservative Bonferroni correction for multiple comparisons. Re-analysis with a more conservative threshold and correction method did not change the original finding: all patients still showed significant increases in gamma power 50–120 ms post-stimulus onset.
3.1.3. Test-retest gamma response concordance
For all 12 patients, the median number of gamma responses to infrequent tones was 2.5 (range 1–6) for the first session, and 3 (range 1–8) for the second session. The median number of gamma responses to frequent tones was 3.5 (range 1–5) for both sessions. The number of sites with gamma-band responses did not differ significantly across sessions (p≥0.27; McNemar’s test). Inter-rater reliability for identification of the largest gamma-band response was very good (k=0.83).
For 11 patients, the largest gamma responses to both tones localized to the perisylvian region in both sessions (Figures 1, 2). For one patient (Patient 3), the largest responses localized to the right middle temporal gyrus. For 10 patients (83%), the largest gamma-band responses to the infrequent tone localized to the same site in both sessions. For the other two patients (Patients 2, 8), the largest responses were at adjacent electrodes in the two sessions. For eight patients (66%), the largest gamma-band responses to the frequent tone localized to the same site across both sessions. In the other four patients (Patients 2, 3, 5, 8), the largest responses were at adjacent electrodes in the two sessions. For one patient (Patient 9), we included gamma-band responses that exceeded the 150-ms time window by more than 25% of the total response duration because it was the patient’s only response.
3.1.4. Test-retest gamma response reproducibility
Within-patient comparisons of the estimated logpower densities and corresponding peak logpower latencies were performed for those patients who had the largest gamma responses at the same sites in both sessions: 10 patients for infrequent-tone responses; eight patients for frequent-tone responses (Table 3). For the reproducibility measurements, we used a post-stimulus interval of 50–150 ms based on the observation that all patients showed increases in gamma power after 50 ms, as also recently reported (Steinschneider et al., 2011). Logpower density estimates and latencies for infrequent tones (N=10 patients) and frequent tones (N=8 patients) were plotted by session for visual comparison with peak N1 amplitudes and latencies (Figures 3, 4).
Table 3. Test-Retest Reproducibility Measurements: Gamma-Band and Evoked N1 Reponses.
Measurements based on largest gamma-band responses to infrequent tones (10 patients) and frequent tones (8 patients) that were spatially concordant across sessions. Test-retest N1 measurements are from largest responses at same site. Latencies are in milliseconds; power and amplitude measurements are in decibels.
| Infrequent | Frequent | Infrequent | Frequent | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Patient | Logpower Density | Peak Power Latency | Logpower Density | Peak Power Latency | N1 Peak Amplitude | N1 Peak Latency | N1 Peak Amplitude | N1 Peak Latency | |
| 1 | Test | 1.98 | 136.00 | 1.69 | 107.76 | −27.86 | 73.12 | −30.77 | 70.89 |
| Retest | 2.71 | 94.14 | 1.98 | 99.69 | −23.58 | 75.36 | −46.64 | 73.12 | |
|
| |||||||||
| 3 | Test | 2.90 | 120.02 | NA | NA | −124.94 | 95.52 | NA | NA |
| Retest | 3.11 | 116.08 | NA | NA | −124.50 | 100.00 | NA | NA | |
|
| |||||||||
| 4 | Test | −0.98 | 143.74 | 0.07 | 143.70 | −35.76 | 100.55 | −33.37 | 102.24 |
| Retest | 1.25 | 137.80 | 1.61 | 146.05 | −64.73 | 104.07 | −15.51 | 104.48 | |
|
| |||||||||
| 5 | Test | 0.88 | 50.31 | NA | NA | −33.77 | 117.58 | −36.47 | 117.92 |
| Retest | −0.07 | 67.09 | NA | NA | −51.48 | 128.33 | −27.32 | 129.12 | |
|
| |||||||||
| 6 | Test | 1.07 | 115.40 | 0.55 | 100.87 | −73.50 | 117.92 | −76.37 | 104.48 |
| Retest | 1.13 | 83.92 | 1.89 | 105.91 | −68.77 | 93.28 | −91.48 | 97.76 | |
|
| |||||||||
| 7 | Test | 0.83 | 116.25 | 1.02 | 65.93 | −15.88 | 73.12 | −12.77 | 77.6 |
| Retest | 0.92 | 91.97 | 0.45 | 71.98 | −1.95 | 82.04 | −5.56 | 84.32 | |
|
| |||||||||
| 9 | Test | 0.18 | 132.12 | −0.18 | 135.44 | −42.52 | 93.28 | −35.04 | 113.46 |
| Retest | −0.50 | 146.89 | −0.13 | 137.99 | −46.12 | 108.96 | −44.04 | 113.44 | |
|
| |||||||||
| 10 | Test | 2.23 | 89.95 | 0.70 | 91.29 | −28.81 | 129.12 | −22.47 | 102.24 |
| Retest | 0.14 | 71.31 | 0.80 | 81.38 | −20.57 | 108.96 | −17.27 | 106.72 | |
|
| |||||||||
| 11 | Test | 1.23 | 128.08 | −0.31 | 113.30 | −39.11 | 97.76 | −38.81 | 97.76 |
| Retest | 0.62 | 96.55 | 0.28 | 118.17 | −25.91 | 108.96 | −31.04 | 106.72 | |
|
| |||||||||
| 12 | Test | 1.02 | 95.95 | 1.59 | 105.91 | −19.06 | 110.15 | −53.32 | 104.48 |
| Retest | 0.88 | 99.86 | 1.37 | 102.04 | −24.76 | 108.17 | −29.87 | 102.24 | |
| Mean (±SD) | Test | 1.13 (1.09) | 112.78 (27.66) | 0.64 (0.76) | 108.03 (24.38) | −44.12 (32.53) | 100.81 (18.53) | −37.71 (18.27) | 99.01 (15.40) |
|
| |||||||||
| Retest | 1.02 (1.14) | 100.56 (26.16) | 1.03 (0.79) | 107.90 (25.54) | −45.24 (34.96) | 101.81 (15.16) | −34.30 (25.16) | 101.99 (16.13) | |
NA: not applicable; designates patients excluded from analysis when the largest response sites differed across sessions.
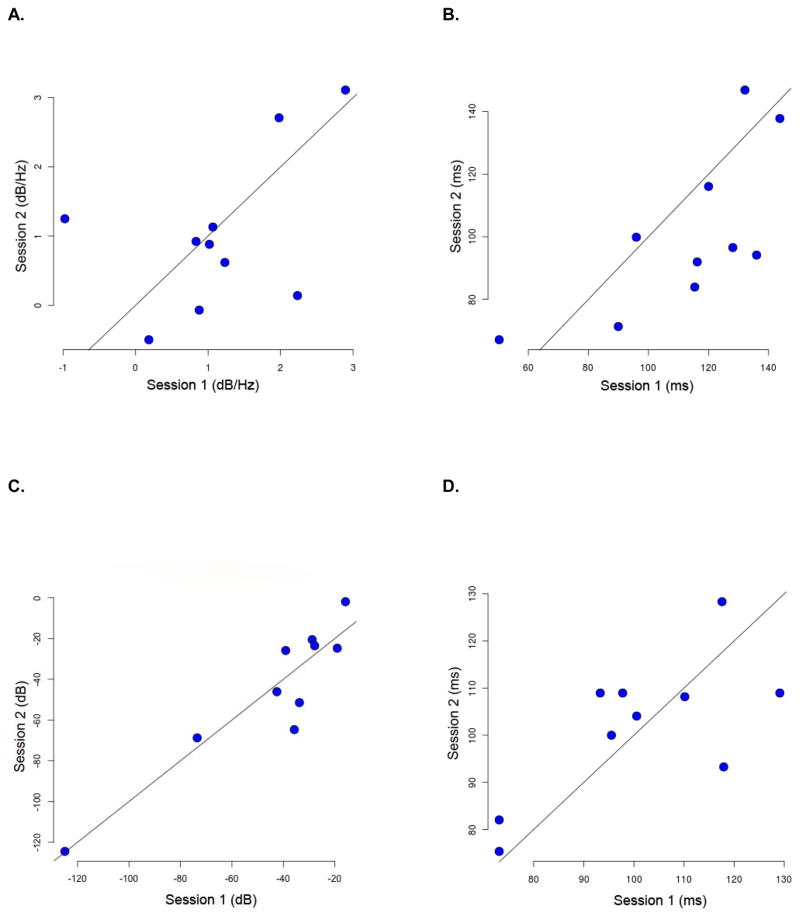
Figure 3.
Test-retest response reproducibility results for infrequent tones plotted by session for: A) Gamma-band logpower density estimates (dB/Hz); B) Gamma-band logpower latencies (ms); C) N1 peak amplitudes (dB); and D) N1 peak latencies (ms). Each patient is represented by a blue dot. Session 1 is on the x-axis; Session 2 is on the y-axis. Diagonal lines in each plot are identity lines.
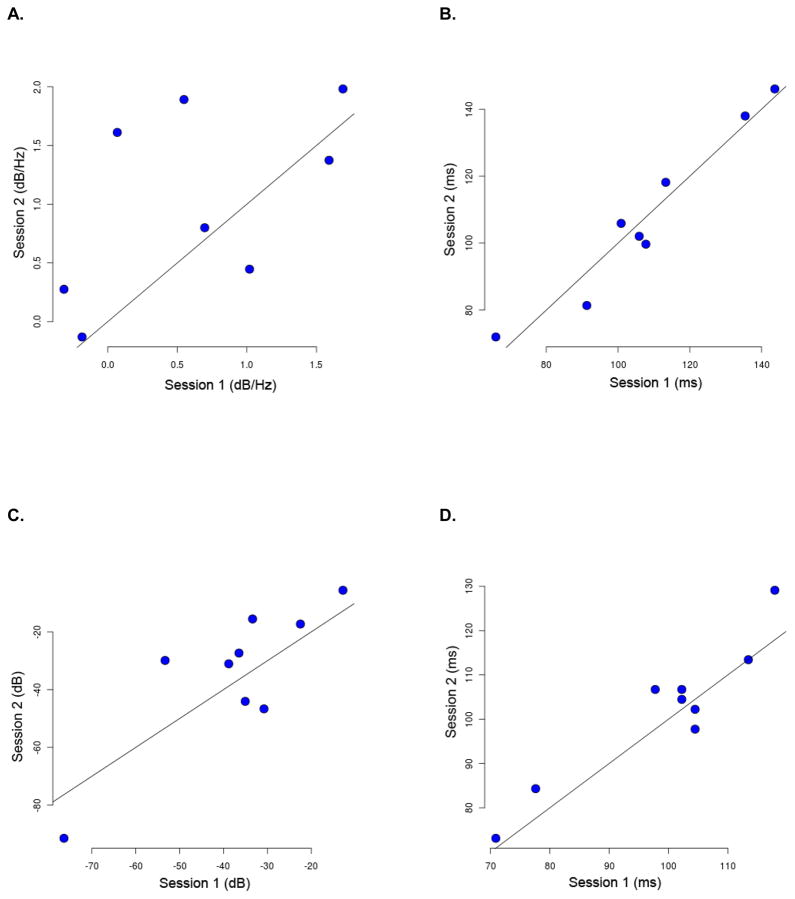
Figure 4.
Test-retest response reproducibility results for frequent tones plotted by session for: A) Gamma-band logpower density estimates (dB/Hz); B) Gamma-band logpower latencies (ms); C) N1 peak amplitudes (dB); and D) N1 peak latencies (ms). Each patient is represented by a blue dot. Session 1 is on the x-axis; Session 2 is on the y-axis. Diagonal lines in each plot are identity lines.
For infrequent tones, the mean logpower gamma-band density estimates for Session 1 (0.95 dB/Hz, ±1.09) and Session 2 (0.88 dB/Hz, ±1.14) did not differ significantly (p=0.89, paired t-test). The Spearman rank correlation coefficient for the logpower density estimates across sessions was 0.33 (p=0.349). A linear mixed effect model fit yielded an intra-class correlation coefficient of 0.53. Similarly, there were no significant differences (p=0.09) in the mean maximum change latencies between Session 1 (112.78 ms, ±27.66) and Session 2 (100.56 ms, ±26.16). The Spearman rank correlation coefficient for maximum change latencies was 0.73 (p=0.02); the intra-class correlation coefficient was 0.31.
For frequent tones, the mean logpower densities for Session 1 (0.64 dB/Hz, ± 0.76) and Session 2 (1.06 dB/Hz, ± 0.79) did not differ significantly between sessions (p=0.14, paired t-test). The Spearman rank correlation coefficient for the logpower density estimates across sessions was 0.59 (p=0.132); the intra-class correlation coefficient was 0.53. Similarly, there were no significant differences (p=0.95) in the mean maximum change latencies for Session 1 (108.03 ms, ±24.38) versus Session 2 (107.90 ms, ± 25.54). The Spearman rank correlation coefficient for maximum change latencies was 0.90 (p=0.004); the intra-class correlation coefficient was 0.20.
Visual inspection of the spectral plots suggested broader gamma-band responses to frequent tones in the time-frequency dimension (area) than responses to infrequent tones for a subset of 3–4 patients in each session (Figures 1, 2). However, the mean log power densities of gamma-band responses to frequent versus infrequent tones did not differ significantly in either Session 1 (p=0.38, paired t-test) or Session 2 (p=0.45, paired t-test).
3.2. High gamma and low gamma
Separate analyses revealed increased high-gamma (65–150 Hz) activity to both tones for all patients, maximal around 100 Hz (range 75–110 Hz), across sessions. Eleven patients (92%) showed low-gamma responses to the frequent tone in both sessions. For infrequent tones, only six patients (50%) showed increased low-gamma (30–59 Hz) activity in both sessions; three (25%) showed no increases in low gamma in either session; and three showed increased low-gamma activity in only one session. Because half of the patients failed to show low-gamma power changes consistently across sessions for the infrequent tone, additional response reproducibility analyses were not performed.
To further explore observed differences in the robustness of low-gamma activity associated with the infrequent and frequent tones, two additional analyses were performed. We first re-analyzed frequent-tone responses using the same number of trials as infrequent tones. We reasoned that if the robustness of low-gamma responses is stimulus-specific and not a function of the signal-to-noise level, then frequent-tone responses should remain more robust than infrequent-tone responses when matched by number of trials. To match the number of frequent- and infrequent-tone responses, we analyzed only the first frequent-tone trial in each series. We selected first-tone trials because they were: 1) matched in number to the infrequent-tone trials; 2) not included in the original analysis of frequent-tone responses; and 3) not as vulnerable to habituation effects as frequent-tone trials occurring later in the series. Results showed six patients (50%) had no significant increases in low-gamma power in one or more sessions when the number of frequent-tone trials was reduced to match the number of infrequent-tone trials.
To determine whether the presence of phase-locking in low gamma might account for the observed improvement in low-gamma robustness versus high-gamma robustness when the number of trials increased, the averaged evoked response was subtracted from each trial of the whole gamma-band response before computing the time-frequency power spectrum (Edwards et al., 2005; Towle et al., 2008; Brugge et al., 2009). Subtracting the evoked response from the whole-gamma response largely eliminated the low-gamma components, but had little visible effect on the high-gamma components.
3.3. Evoked N1 responses
All patients showed identifiable N1 responses to infrequent and frequent tones across both recording sessions. Inter-rater reliability for identification of the largest N1 responses was very good (k=0.92). For infrequent tones, the median number of N1 responses was 7.0 in both the first session (range 3–11) and second session (range 3–12). Nine patients (75%) had the largest N1 response at the same site in both recording sessions (Table 3). Mean N1 peak latencies in the first session (100.81 ms, ± 18.53) and second session (101.81, ms ±15.16) did not differ. Spearman rank correlation coefficient for N1 latency was 0.50 (p=0.14). Linear mixed effect model fit yielded an intra-class correlation coefficient of 0.40. Peak N1 amplitudes in the first session (mean −44.12 dB, ±32.53) and second session (mean −45.24 dB, ±34.96) did not differ significantly (p=0.28, paired t-test). Spearman rank correlation coefficient for N1 amplitude was 0.85 (p=0.003); the intra-class correlation coefficient was 0.22.
For frequent tones, the median number of N1 responses was 5.0 for both the first session (range 2–8) and second session (range 2–10). Eleven patients (92%) had the largest N1 response at the same site in both recording sessions (Table 3). Mean N1 peak latencies in the first session (99.01 ms, ±15.40) and second session (101.99 ms, ±16.13) did not differ significantly (p=0.78, paired t-test). Spearman rank correlation coefficient for N1 latency was 0.70 (p=0.034). The intra-class correlation coefficient was 0.06. Peak N1 amplitudes in the first session (mean −37.71 dB, ±18.27) and second session (mean −34.30 dB, ±25.16) did not differ significantly (p=0.098, paired t-test). Spearman rank correlation coefficient for N1 amplitude was 0.6 (p=0.096); the intra-class correlation coefficient was 0.26.
3.4. Behavioral test results
All 12 patients demonstrated excellent word recognition in quiet (≥92% accuracy). On tests of speech recognition under adverse listening conditions, two of the 10 patients tested (20%; Patients 4, 7) demonstrated impaired performances. Follow-up clinical audiologic testing was recommended for both patients to further assess their auditory processing abilities.
3.5. Patient and clinical variables
Small sample sizes precluded formal statistical testing for associations between patient demographics (e.g., age, gender) or clinical variables (e.g., implanted hemisphere, medication change, seizure focus) and gamma response variability. However, inspection of the data showed no apparent trends towards increased test-retest variability: gamma-band responses were 100% robust across all patients and recordings. Duration of test-retest interval did not appear to be associated with increased variability.
No group differences in gamma response robustness were detected for patients with temporal lobe seizures (N=6) versus extra-temporal lobe seizures (100% for both groups), or for the mean number of gamma responses (2.7 versus 2.8). For gamma response reproducibility, the mean logpower density estimates also appeared comparable across both seizure groups (1.13 ± 0.80 versus 1.89 ± 1.74). However, the two patients who did not show spatial concordance across sessions had temporal lobe seizures (Patients 2, 8). Moreover, mean logpower density latencies were also longer for patients with temporal lobe seizures (108.65 ms ±26.11) vs. patients with extra-temporal lobe seizures (79.22 ms, ±28.07) although our sample size was too small to test this difference formally.
4. Discussion
This test-retest ECoG study investigated the reliability of early auditory-related power changes in the 30–150 Hz gamma frequency range. Our results demonstrate that gamma-band responses recorded directly from cortex are highly reliable. Previous scalp EEG studies have reported reliable gamma-band responses to visual stimuli (Keil et al., 2003; Fründ et al., 2007). These studies differ from our study not only in sensory stimuli (visual vs. auditory) and recording methods (EEG vs. ECoG) used, but also in their focus on phase-locked gamma-band activity (Fründ et al., 2007), or early low gamma (< 32 Hz) or late (250–500 ms) high gamma responses (Keil et al., 2003). Our test-retest ECoG results, therefore, extend previous findings to confirm the reliability of early (< 150 ms) whole gamma-band (30–150 Hz) responses to auditory stimuli. The reliability of auditory gamma-band responses underscores their potential utility as indices of cortical processing in clinical and research studies, including recent applications in neural decoding and brain-computer interfaces (Guo et al., 2010).
4.1. Response robustness and concordance
4.1.1. Gamma-band responses
All patients showed robust gamma-band responses to the 1200-Hz infrequent and 1000-Hz frequent tones regardless of statistical threshold or correction method applied. Increased gamma-band power was evident between 50–120 ms post-stimulus onset, as reported previously (Edwards et al., 2005; Steinschneider et al., 2011). Gamma-band responses localized mainly to the posterior superior temporal gyrus and inferior parietal lobe in both hemispheres, consistent with the known generators in primary and non-primary auditory cortices (Pantev et al., 1991; Kaiser et al., 2002; Edwards et al., 2005; Steinschneider et al., 2011). The overall robustness of gamma-band responses did not vary as a function of performance on behavioral tests of auditory processing, location of seizure focus, medication changes, or duration of test-retest interval.
For all patients, both frequent and infrequent tones elicited statistically significant increases in gamma-band power across sessions. Frequent tones, which yielded three times more trials than infrequent tones, also elicited more robust low-gamma and evoked N1 responses than infrequent tones, likely reflecting differences in the signal-to-noise levels. Although gamma responses to frequent tones appeared somewhat broader in their time-frequency dimensions (area) than responses to infrequent tones, comparisons of their power density estimates showed no statistical differences. However, total power estimates may not fully capture differences in the overall time-frequency distributions of gamma-band power changes, underscoring the need for better methods to quantify such differences.
For the majority of patients, the number and location of early auditory gamma-band responses remained stable across sessions (100% spatial concordance). Two patients (Patients 2, 8) who did not show spatial concordance for the largest gamma-band responses had seizures originating from the temporal lobe (anterior temporal, mesial temporal). Studies with larger numbers of temporal lobe and non-temporal lobe seizure patients are needed to verify this observation.
4.1.2. High gamma versus low gamma
We observed differences in the robustness and concordance of high-gamma (65–150 Hz) versus low-gamma (30–59 Hz) activity. Spectral analysis of auditory gamma-band responses revealed predominantly high-gamma activity, as previously reported (Crone et al., 2001; Edwards et al., 2005; Lachaux et al., 2007; Towle et al., 2008). For the infrequent tone, high-gamma responses were relatively more robust and stable on test-retest than low-gamma responses. Only half of our patients showed increased low-gamma power for infrequent tones, and only three patients (25%) showed increased low-gamma activity in both sessions. Conversely, all but one patient showed increased low-gamma activity to the frequent tone in both sessions.
When the numbers of frequent-tone and infrequent-tone trials were matched, only half of the patients showed increased low-gamma responses to either tone. This suggests that the robustness of low-gamma activity may not be specific to a particular tone, but instead reflects the signal-to-noise level. Subtracting the averaged evoked response from the whole gamma-band response largely eliminated low-gamma activity. This supports the view that low-gamma activity is largely phase-locked and therefore likely to be more robust at higher signal-to-noise levels (i.e., larger numbers of trials).
Subtracting the evoked response had little visible effect on high-gamma activity, suggesting that high-gamma responses are largely non-phase-locked (Crone et al., 2001; Gurtubay et al., 2001; Edwards et al., 2005; Lachaux et al., 2007; Ray et al., 2008; Towle et al., 2008). High-gamma activity has been associated with activation of local cortical networks engaged in sensory processing, while low gamma has been implicated in the synchronization of distributed brain regions involved in high-level language and cognitive functions. We used a passive listening paradigm that did not require subjects to attend or respond to stimuli and, therefore, may not have recruited more distributed cortical regions.
4.1.3. Evoked N1 responses
All patients showed clear evoked N1 responses to infrequent and frequent tones in both recording sessions, with mean peak latencies between 99–102 ms after stimulus presentation. This is not surprising given that the evoked N1 response is considered an obligatory, highly robust and reliable response (Näätänen and Picton, 1987; Virtanen et al., 1998; Tremblay et al., 2003). The evoked N1 response overlapped in time with the gamma-band response, as previously reported (Edwards et al., 2009; Sinai et al., 2009; Steinschneider et al., 2011). The spatial distribution of N1 responses was greater than that of gamma-band responses for all patients, with no clear differences in the location of the largest N1 responses to the frequent versus infrequent tones, consistent with previous reports (Edwards et al., 2009; Sinai et al., 2009). For the infrequent tone, concordance estimates were comparable for the evoked N1 and gamma-band responses: 75% for the N1 and 83% for gamma-band responses. However, for the frequent tone, the concordance of the N1 response was 92%, compared with 66% for the gamma-band responses. One explanation is that the larger number of frequent trials improved the concordance of the phase-locked evoked N1 response, but did not affect the concordance of the predominantly non-phase-locked gamma-band activity, especially in the high-gamma range.
4.2. Response reproducibility
4.2.1. Gamma-band responses
The reproducibility of the estimated changes in gamma logpower density and their corresponding latencies were evaluated across sessions for both infrequent and frequent tones. For both tones, the Spearman rank correlation coefficients (≥0.33) and intra-class correlation coefficients (0.53) suggested that gamma logpower densities were not strongly correlated across sessions (p ≥ 0.13). This finding is consistent with large within-subject variability in the spectral power composition of early event-related changes in gamma-band activity. Conversely, the time course of event-related power changes, and in particular the latency of the peak power changes, was highly correlated across sessions (Spearman rank coefficients ≥0.73) for both infrequent tones (p=0.021) and frequent tones (p=0.004). This novel finding suggests that the time course of auditory-related power changes in the gamma band is highly reliable and reproducible. However, the relatively low intra-class correlation coefficients for both tones (≤0.31) appear at odds with this interpretation. One explanation for this apparent discrepancy is our relatively small sample size. In contrast to Spearman rank correlations, intra-class correlations take into account all of the data, not just paired data, and therefore may be more vulnerable to error with smaller sample sizes. Test-retest studies with larger sample sizes are needed to verify these findings.
Event-related activity in the gamma band, and especially high gamma, has been identified as a marker of auditory information processing for perception as well as higher-level auditory functions including attention, memory and comprehension (Herrmann et al., 2004; Ray et al., 2008; Towle et al., 2008). Abnormalities in neural timing at any level of the auditory system can contribute to impairments in processing complex auditory information, including speech, at the level of the cortex (Zeng et al., 1999; King et al., 2002; Warrier et al., 2004; Saoud et al., 2012). Examining the time course of cortical gamma-band responses within and between brain regions may provide useful information about normal and impaired auditory processing (Gilley and Sharma, 2010; Chang et al., 2011; Cervenka et al., 2011b). These measures could be useful as clinical tools for detecting impaired auditory function and for localizing the source(s) of auditory impairment. Additional studies combining ECoG recordings with more sensitive behavioral measures of auditory processing are needed. Similarly, methodological limitations preclude generalized use of high-gamma responses in auditory studies and clinical applications. Specifically, most studies of high-gamma responses have relied on intracranial recordings that are highly invasive and limited to neurosurgical populations. Methodological limitations previously precluded recording gamma responses higher than 60–70 Hz with scalp electrodes (Nunez and Srinivasan, 2006). However, several recent studies have reported robust high-gamma responses in scalp recordings using advanced EEG recording and signal processing methods (Ball et al., 2008; Lenz et al., 2008; Darvas et al., 2010), underscoring the possibility of recording these responses non-invasively in other populations. Simultaneous intracranial and scalp recordings are needed to correlate increases in high-gamma activity observed in ECoG recordings with event-related changes measured from scalp recordings. This could help refine the interpretation of scalp recordings for research and clinical studies, and extend the generalizability of ECoG findings. It will also be important to investigate further the large individual variability observed in auditory ECoG studies, including our study, and the extent to which differences in spatial sampling and electrode locations are contributing sources.
Observed increases in high-gamma power were most evident around 100 Hz (75–110 Hz), consistent with previous auditory ECoG studies using a similar experimental approach (Crone et al., 2001; Edwards et al., 2005; Towle et al., 2008). However, because we used only one recording paradigm and stimulus set, it is not possible to determine whether the maximal activity in this frequency band may be task- and/or stimulus-specific, as suggested in a recent ECoG study using multiple language tasks and stimuli (Gaona et al., 2011). Although our patients had long-standing seizure disorders, it is unlikely that these could account for the high-gamma power changes observed, since we focused only on event-related activity in EEG frequencies ≤150 Hz.
Inspection of individual time-frequency plots showed considerable variability between patients in onset time, duration, and spectral composition of early auditory gamma-band responses. This is likely due to differences in spatial sampling and the locations of recording electrodes across patients. Because of the relatively small sample size, it was not possible to determine whether other patient-related factors, including seizure history, may have also contributed. Despite individual variability, the majority of our patients (N=9) showed similar test-retest response profiles, consistent with our overall finding that inter-subject variability was greater than intra-subject variability. One of the exceptions (Patient 4) was also impaired on behavioral tests of auditory processing. Another patient (Patient 9) was not tested for auditory processing disorders. The potential effects of auditory processing disorders on response reproducibility warrant further investigation.
4.2.2. Evoked N1 responses
Peak N1 latencies were highly correlated across sessions for frequent tones (p=0.034), but not for infrequent tones (p=0.136). Poor test-retest reliability of N1 latencies to infrequently presented tones has been reported previously (Walhovd and Fjell, 2002). A likely explanation is that the larger number of frequent-tone versus infrequent-tone trials improved the reproducibility of N1 latencies, which are phase-locked to stimulus onset. Alternatively, N1 latencies may be especially sensitive to changes in patients’ seizure status and/or medication, requiring larger numbers of trials to stabilize (van Deursen et al., 2008). The opposite pattern was observed for N1 peak amplitudes, which were found to be highly correlated across sessions for the infrequent tone (p=0.0036) but not the frequent tone (p=0.97). Previous studies have reported that N1 amplitudes are typically larger and less variable for infrequent versus frequent tones in part because N1 amplitudes decrease with stimulus repetition due to habituation effects (Davis et al., 1939; Edwards et al., 2005; Rosburg et al., 2005). This could account for the poor test-retest reproducibility of N1 amplitudes for frequent tones observed in our study. Additional studies with larger numbers of patients are needed to investigate these differences.
5. Study Limitations
The relatively small number of patients studied (N=12) is a limitation and may have obscured sub-group effects of patient and clinical factors. Similarly, because our findings are based on ECoG recordings, the reliability of gamma-band responses cannot be generalized to scalp recordings. Although we chose simple tones in order to evaluate the intrinsic variability of gamma-band responses, more complex auditory stimuli may be associated with greater test-retest variability and will be important to investigate in future studies.
6. Conclusions
Test-retest results indicate that early auditory gamma-band responses recorded directly from cortex are highly robust, spatially concordant, and reproducible. The timing of event-related changes in high-gamma power appears to be strongly reproducible across intracranial recording sessions. These results support the use of gamma-band responses as reliable measures of cortical processing in research and clinical studies.
HIGHLIGHTS.
We investigated the test-retest reliability of early auditory gamma-band responses using intracranial ECoG recordings.
Gamma-band power increases were robust, spatially concordant, and reproducible across sessions; the timing of event-related power changes was highly reliable.
Auditory gamma-band responses can provide reliable indices of cortical processing for clinical and research studies.
Acknowledgments
This study was supported by NIH grants NIDCD DC005645 and DC010028 (DBR); NIBIB EB012547 (BC); NINDS NS40596 (NEC) and NS038493 (FAL); and by the National Science Foundation of China Project 61071003 (BH). Special thanks to Dr. Deepti Ramadoss and Juan Roberto Perilla for assistance with the manuscript and figures.
Footnotes
Disclosures: The authors have no financial or other conflicts of interest to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aron AR, Gluck MA, Poldrack RA. Long-term test-retest reliability of functional MRI in a classification learning task. Neuroimage. 2006;29:1000–1006. doi: 10.1016/j.neuroimage.2005.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball T, Demandt E, Mutschler I, Neitzel E, Mehring C, Vogt K, et al. Movement related activity in the high gamma range of the human EEG. Neuroimage. 2008;41:302–310. doi: 10.1016/j.neuroimage.2008.02.032. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- Boatman DF, Lesser RP, Crone NE, Krauss G, Lenz FA, Miglioretti DL. Speech recognition impairments in patients with intractable right temporal lobe epilepsy. Epilepsia. 2006;47:1397–1401. doi: 10.1111/j.1528-1167.2006.00562.x. [DOI] [PubMed] [Google Scholar]
- Boatman-Reich D, Franaszczuk PJ, Korzeniewska A, Caffo B, Ritzl EK, Colwell S, et al. Quantifying auditory event-related responses in multichannel human intracranial recordings. Front Comput Neurosci. 2010;4:4. doi: 10.3389/fncom.2010.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros NN, Overall J, Zouridakis G. Test-Retest Reliability of the P50 Mid-Latency Auditory Evoked Response. Pyschiatry Res. 1991;39:181–192. doi: 10.1016/0165-1781(91)90086-5. [DOI] [PubMed] [Google Scholar]
- Brugge JF, Nourski KV, Oya H, Reale RA, Kawasaki H, Steinschneider M, et al. Coding of repetitive transients by auditory cortex on Heschl’s gyrus. J Neurophysiol. 2009;102:2358–2374. doi: 10.1152/jn.91346.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty RT, Soltani M, Dalal SS, Edwards E, Dronkers NF, Nagarajan SS, et al. Spatiotemporal dynamics of word processing in the human brain. Front Neurosci. 2007;1:185–196. doi: 10.3389/neuro.01.1.1.014.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervenka MC, Boatman-Reich DF, Ward J, Franaszczuk PJ, Crone NE. Language mapping in multilingual patients: electrocorticography and cortical stimulation during naming. Front Hum Neurosci. 2011a;5:13. doi: 10.3389/fnhum.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervenka MC, Nagle S, Boatman-Reich D. Cortical High-Gamma Responses in Auditory Processing. Am J Audiol. 2011b;20:171–180. doi: 10.1044/1059-0889(2011/10-0036). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EF, Edwards E, Nagarajan SS, Fogelson N, Dalal SS, Canolty RT, et al. Cortical spatio-temporal dynamics underlying phonological target detection in humans. J Cogn Neurosci. 2011;23:1437–1446. doi: 10.1162/jocn.2010.21466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain. 1998;121:2301–2315. doi: 10.1093/brain/121.12.2301. [DOI] [PubMed] [Google Scholar]
- Crone NE, Boatman D, Gordon B, Hao L. Induced electrocorticographic gamma activity during auditory perception. Clin Neurophysiol. 2001;112:565–582. doi: 10.1016/s1388-2457(00)00545-9. [DOI] [PubMed] [Google Scholar]
- Crone NE, Korzeniewska A, Franaszczuk PJ. Cortical gamma responses: searching high and low. Int J Psychophysiol. 2011;79:9–15. doi: 10.1016/j.ijpsycho.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvas F, Scherer R, Ojemann JG, Rao RP, Miller KJ, Sorensen LB. High gamma mapping using EEG. Neuroimage. 2010;49:930–938. doi: 10.1016/j.neuroimage.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis P. Effects of acoustic stimuli on the waking human brain. J Neurophysiol. 1939;2:494–499. [Google Scholar]
- Dunn DW, Austin JK, Harezlak J, Ambrosius WT. ADHD and epilepsy in childhood. Dev Med Child Neurol. 2003;45:50–54. [PubMed] [Google Scholar]
- Edwards E, Soltani M, Deouell LY, Berger MS, Knight RT. High gamma activity in response to deviant auditory stimuli recorded directly from human cortex. J Neurophysiol. 2005;94:4269–4280. doi: 10.1152/jn.00324.2005. [DOI] [PubMed] [Google Scholar]
- Edwards E, Soltani M, Kim W, Dalal SS, Nagarajan SS, Berger MS, et al. Comparison of time-frequency responses and the event-related potential to auditory speech stimuli in human cortex. J Neurophysiol. 2009;102:377–386. doi: 10.1152/jn.90954.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escera C, Alho K, Winkler I, Näätänen R. Neural mechanisms of involuntary attention to acoustic novelty and change. J Cogn Neurosci. 1998;10:590–604. doi: 10.1162/089892998562997. [DOI] [PubMed] [Google Scholar]
- Escera C, Corral MJ, Yago E. An electrophysiological and behavioral investigation of involuntary attention towards auditory frequency, duration and intensity changes. Brain Res. 2002;14:325–332. doi: 10.1016/s0926-6410(02)00135-0. [DOI] [PubMed] [Google Scholar]
- Franaszczuk PJ, Bergey GK, Durka PJ, Eisenberg HM. Time-frequency analysis using the matching pursuit algorithm applied to seizures originating from the mesial temporal lobe. Electroenceph clin Neurophysiol. 1998;106:513–521. doi: 10.1016/s0013-4694(98)00024-8. [DOI] [PubMed] [Google Scholar]
- Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci. 2009;32:209–224. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- Fründ I, Schadow J, Busch NA, Körner U, Herrmann CS. Evoked γ oscillations in human scalp EEG are test–retest reliable. Clin Neurophysiol. 2007;118:221–227. doi: 10.1016/j.clinph.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Gaona CM, Sharma M, Freudenburg ZV, Breshears JD, Bundy DT, Roland J, et al. Nonuniform high-gamma (60–500 Hz) power changes dissociate cognitive task and anatomy in human cortex. J Neurosci. 2011;31:2091–2100. doi: 10.1523/JNEUROSCI.4722-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser T, Bächer P, Steinberg H. Test-retest reliability of spectral parameters of the EEG. Electroencephalogr Clin Neurophysiol. 1985;60:312–319. doi: 10.1016/0013-4694(85)90005-7. [DOI] [PubMed] [Google Scholar]
- Gilley PM, Sharma A. Functional brain dynamics of evoked and event-related potentials from the central auditory system. Perspect Hear Hear Disord. 2010;14:12–20. [Google Scholar]
- Guo J, Gao S, Hong B. An Auditory Brain Computer Interface Using Active Mental Response. IEEE Trans Neural Syst Rehabil Eng. 2010;18:230–235. doi: 10.1109/TNSRE.2010.2047604. [DOI] [PubMed] [Google Scholar]
- Gurtubay IG, Alegre M, Labarga A, Malanda A, Iriarte J, Artieda J. Gamma band activity in an auditory oddball paradigm studied with the wavelet transform. Clin Neurophysiol. 2001;112:1219–1228. doi: 10.1016/s1388-2457(01)00557-0. [DOI] [PubMed] [Google Scholar]
- Han MW, Ahn JH, Kang JK, Lee EM, Lee JH, Bae JH, et al. Central auditory processing impairment in patients with temporal lobe epilepsy. Epilepsy Behav. 2011;20:370–374. doi: 10.1016/j.yebeh.2010.12.032. [DOI] [PubMed] [Google Scholar]
- Henkin Y, Kishon-Rabin L, Pratt H, Kivity S, Sadeh M, Gadoth N. Linguistic processing in idiopathic generalized epilepsy: an auditory event-related potential study. Epilepsia. 2003;44:1207–1217. doi: 10.1046/j.1528-1157.2003.65402.x. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Munk MHJ, Engel AK. Cognitive functions of gamma-band activity: memory match and utilization. Trends Cogn Sci. 2004;8:347–355. doi: 10.1016/j.tics.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Howard MA, Volkov IO, Mirsky R, Garell PC, Noh MD, Granner M, et al. Auditory cortex on the human posterior superior temporal gyrus. J Comp Neurol. 2000;416:79–92. doi: 10.1002/(sici)1096-9861(20000103)416:1<79::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Jerbi K, Ossandón T, Hamamé CM, Senova S, Dalal SS, Jung J, et al. Task-related gamma-band dynamics from an intracerebral perspective: Review and implications for surface EEG and MEG. Hum Brain Mapp. 2009;30:1758–1771. doi: 10.1002/hbm.20750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser J, Lutzenberger W, Ackermann H, Birbaumer N. Dynamics of gamma-band activity induced by auditory pattern changes in humans. Cereb Cortex. 2002;12:212–221. doi: 10.1093/cercor/12.2.212. [DOI] [PubMed] [Google Scholar]
- Keil A, Stolarova M, Heim S, Gruber T, Müller MM. Temporal Stability of High-Frequency Brain Oscillations in the Human EEG. Brain Topogr. 2003;16:101–110. doi: 10.1023/b:brat.0000006334.15919.2c. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF. Reproducibility of the hemodynamic response to auditory oddball stimuli: a six-week test-retest study. Hum Brain Mapp. 2003;18:42–52. doi: 10.1002/hbm.10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith R. SCAN-A: A test of auditory function for adolescents and adults. San Antonio, TX: Psychological Corporation; 1994. [Google Scholar]
- King C, Warrier CM, Hayes E, Kraus N. Deficits in auditory brainstem pathway encoding of speech sounds in children with learning problems. Neurosci Lett. 2002;319:111–115. doi: 10.1016/s0304-3940(01)02556-3. [DOI] [PubMed] [Google Scholar]
- Lachaux J-P, Jerbi K, Bertrand O, Minotti L, Hoffmann D, Schoendorff B, et al. A blueprint for real-time functional mapping via human intracranial recordings. PloS One. 2007;2:e1094. doi: 10.1371/journal.pone.0001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- Lenz D, Jeschke M, Schadow J, Naue N, Ohl FW, Herrmann CS. Human EEG very high frequency oscillations reflect the number of matches with a template in auditory short-term memory. Brain Res. 2008;1220:81–92. doi: 10.1016/j.brainres.2007.10.053. [DOI] [PubMed] [Google Scholar]
- Mallat SG, Zhang Z. Matching pursuits with time-frequency dictionaries. IEEE Trans Signal Process. 1993;41:3397–3415. [Google Scholar]
- Näätänen R, Picton T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiol. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. Electric Fields of the Brain: The Neurophysics of EEG. Oxford Univ Press; 2006. [Google Scholar]
- Pantev C, Makeig S, Hoke M, Galambos R, Hampson S, Gallen C. Human auditory evoked gamma-band magnetic fields. Proc Natl Acad Sci USA. 1991;88:8996–9000. doi: 10.1073/pnas.88.20.8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Niebur E, Hsiao SS, Sinai A, Crone NE. High-frequency gamma activity (80–150Hz) is increased in human cortex during selective attention. Clin Neurophysiol. 2008;119:116–133. doi: 10.1016/j.clinph.2007.09.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly CJ. Attention deficit hyperactivity disorder (ADHD ) in childhood epilepsy. Res Dev Disabil. 2011;32:883–893. doi: 10.1016/j.ridd.2011.01.019. [DOI] [PubMed] [Google Scholar]
- Rosburg T, Trautner P, Dietl T, Korzyukov OA, Boutros NN, Schaller C, et al. Subdural recordings of the mismatch negativity (MMN) in patients with focal epilepsy. Brain. 2005;128:819–828. doi: 10.1093/brain/awh442. [DOI] [PubMed] [Google Scholar]
- Sams M, Paavilainen P, Alho K, Näätänen R. Auditory frequency discrimination and event-related potentials. Electroenceph clin Neurophysiol. 1985;62:437–448. doi: 10.1016/0168-5597(85)90054-1. [DOI] [PubMed] [Google Scholar]
- Saoud H, Josse G, Bertasi E, Truy E, Chait M, Giraud AL. Brain-Speech Alignment Enhances Auditory Cortical Responses and Speech Perception. J Neurosci. 2012;32:275–281. doi: 10.1523/JNEUROSCI.3970-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segalowitz SJ, Barnes KL. The reliability of ERP components in the auditory oddball paradigm. Pyschophysiol. 1993;30:451–459. doi: 10.1111/j.1469-8986.1993.tb02068.x. [DOI] [PubMed] [Google Scholar]
- Sinai A, Bowers CW, Crainiceanu CM, Boatman D, Gordon B, Lesser RP, et al. Electrocorticographic high gamma activity versus electrical cortical stimulation mapping of naming. Brain. 2005;128:1556–1570. doi: 10.1093/brain/awh491. [DOI] [PubMed] [Google Scholar]
- Sinai A, Crone NE, Wied HM, Franaszczuk PJ, Miglioretti D, Boatman-Reich D. Intracranial mapping of auditory perception: event-related responses and electrocortical stimulation. Clin Neurophysiol. 2009;120:140–149. doi: 10.1016/j.clinph.2008.10.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinschneider M, Nourski KV, Kawasaki H, Oya H, Brugge JF, Howard MA. Intracranial study of speech-elicited activity on the human posterolateral superior temporal gyrus. Cereb Cortex. 2011;21:2332–2347. doi: 10.1093/cercor/bhr014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- Tervaniemi M, Lehtokoski A, Sinkkonen J, Virtanen J, Ilmoniemi RJ, Näätänen R. Test-retest reliability of mismatch negativity for duration, frequency and intensity changes. Clin Neurophysiol. 1999;110:1388–1393. doi: 10.1016/s1388-2457(99)00108-x. [DOI] [PubMed] [Google Scholar]
- Tervaniemi M, Sinkkonen J, Virtanen J, Kallio J, Ilmoniemi RJ, Salonen O, Näätänen R. Test-retest stability of the magnetic mismatch response (MMNm) Clin Neurophysiol. 2005;116:1897–1905. doi: 10.1016/j.clinph.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Todorovic A, van Ede F, Maris E, De Lange FP. Prior expectation mediates neural adaptation to repeated sounds in the auditory cortex: an MEG study. J Neurosci. 2011;31:9118–9123. doi: 10.1523/JNEUROSCI.1425-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towle VL, Yoon H-A, Castelle M, Edgar JC, Biassou NM, Frim DM, et al. ECoG gamma activity during a language task: differentiating expressive and receptive speech areas. Brain. 2008;131:2013–2027. doi: 10.1093/brain/awn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay KL, Friesen L, Martin BA, Wright R. Test-retest reliability of cortical evoked potentials using naturally produced speech sounds. Ear Hear. 2003;24:225–232. doi: 10.1097/01.AUD.0000069229.84883.03. [DOI] [PubMed] [Google Scholar]
- van Deursen JA, Vuurman EFPM, Verhey FRJ, van Kranen-Mastenbroek VHJM, Riedel WJ. Increased EEG gamma band activity in Alzheimer’s disease and mild cognitive impairment. J Neural Transm. 2008;115:1301–1311. doi: 10.1007/s00702-008-0083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen J, Ahveninen J, Ilmoniemi RJ, Näätänen R, Pekkonen E. Replicability of MEG and EEG measures of the auditory N1/N1m-response. Electroenceph clin Neurophysiol. 1998;108:291–298. doi: 10.1016/s0168-5597(98)00006-9. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM. One-year test-retest reliability of auditory ERPs in young and old adults. Int J Psychophysiol. 2002;46:29–40. doi: 10.1016/s0167-8760(02)00039-9. [DOI] [PubMed] [Google Scholar]
- Warrier CM, Johnson KL, Hayes EA, Nicol T, Kraus N. Learning impaired children exhibit timing deficits and training-related improvements in auditory cortical responses to speech in noise. Exp Brain Res. 2004;157:431–441. doi: 10.1007/s00221-004-1857-6. [DOI] [PubMed] [Google Scholar]
- Zeng FG, Oba S, Garde S, Sininger Y, Starr A. Temporal and speech processing deficits in auditory neuropathy. Neuroreport. 1999;10:3429–3435. doi: 10.1097/00001756-199911080-00031. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Luk KDK, Hu Y. Identification of detailed time-frequency components in somatosensory evoked potentials. IEEE Trans Neural Syst Rehabil Eng. 2010;18:245–254. doi: 10.1109/TNSRE.2010.2043856. [DOI] [PubMed] [Google Scholar]
- Zygierewicz J, Durka PJ, Klekowicz H, Franaszczuk PJ, Crone NE. Computationally efficient approaches to calculating significant ERD/ERS changes in the time-frequency plane. J Neurosci Methods. 2005;145:267–276. doi: 10.1016/j.jneumeth.2005.01.013. [DOI] [PubMed] [Google Scholar]